Introduction
Siniperca chuatsi (family: Serranidae), commonly known as Chinese perch or mandarin fish, is a highly valued freshwater species native to East Asia (Yang et al., 2015). It is prized for its high-quality meat and distinctive flavor, making it a commercially important species with growing potential for aquaculture (Shim et al., 2016). Traditionally cultivated in China, S. chuatsi has been used to enhance water quality in natural bodies of water due to its role as a piscivorous predator (Li et al., 2018). However, industrial breeding and domestication of S. chuatsi face several challenges. It is a piscivorous fish with a unique feeding habit, consuming only live fish from the larval stage through adulthood (Liang et al., 2008). It is difficult to adapt them to artificial diets commonly used in aquaculture. The cannibalism of larval S. chuatsi (Peng et al., 2023), especially under crowded conditions, can result in significant losses in hatcheries and grow-out systems, posing a challenge to efficient and sustainable breeding. Therefore, the early life stages of S. chuatsi are marked by high mortality rates, often due to feeding difficulties and cannibalism among larvae. Maintaining optimal environmental conditions is crucial for their health and growth.
This issue is compounded by the fish’s exclusive consump-tion of live prey from the larval stage onwards. Consequently, breeding programs must focus on providing live feed, such as carp fry, to ensure survival and growth. Cyprinus carpio, commonly known as the common carp or Zhouhe carp, is also a widely cultivated freshwater fish species of significant economic importance (Rahman, 2015). It has been introduced globally for aquaculture due to its adaptability to various environments and long farming history (Khan et al., 2016). Research suggests that common carp (C. carpio) can potentially be used to feed S. chuatsi, since S. chuatsi naturally preys on various fish species, including Carassius auratus (crucian carp) (Li et al., 2013).
C. carpio is a unique geographical indication product of Tianjin, China. Renowned for its firm flesh texture and optimized nutritional profile—characterized by high protein and low-fat content—it is anticipated to be well-suited to meet the growth requirements of mandarin fish larvae, providing a robust nutritional foundation for their rapid and healthy development (Rahman, 2015). Furthermore, its body shape, size, and activity level closely resemble the natural prey of mandarin fish larvae in aquatic environments, making it a highly adaptable and nutritious first feed. The widespread cultivation and stable production of C. carpio ensures a reliable and sufficient supply, making it an accessible feed source. Compared to alternative feeds, C. carpio not only reduces feeding costs but was also anticipated to improve feeding efficiency, offering strong support for the large-scale cultivation of mandarin fish larvae (Khan et al., 2016). However, little is known about the appropriate stocking density for the two species, which needs to be determined.
S. chuatsi are sensitive to changes in water quality. For example, although it can live in near-freezing water, it only starts feeding when the temperature rises above 15°C and breeding above 21°C (FAO, 2009). In general, two- to three-year-old broodstock are given hormone injections followed by stripping eggs and milt in hatcheries (Kibenge, 2022). However, the dose and frequency of injections may vary depending on aquacultural conditions. Developing domesticated fish breeds through long-term breeding programs is crucial for aquaculture advancement (Yue, 2019).
In this study, we investigated two key aspects of mandarin fish aquaculture in Tianjin (China): (1) the effects of different hormone injection methods on spawning efficiency and fertilization rates and (2) the feasibility of using common carp (C. carpio) as an initial feed. By addressing these issues, we aim to improve the efficiency and scalability of mandarin fish breeding, providing insights into developing industrial-scale aquaculture models for this species.
Materials and Methods
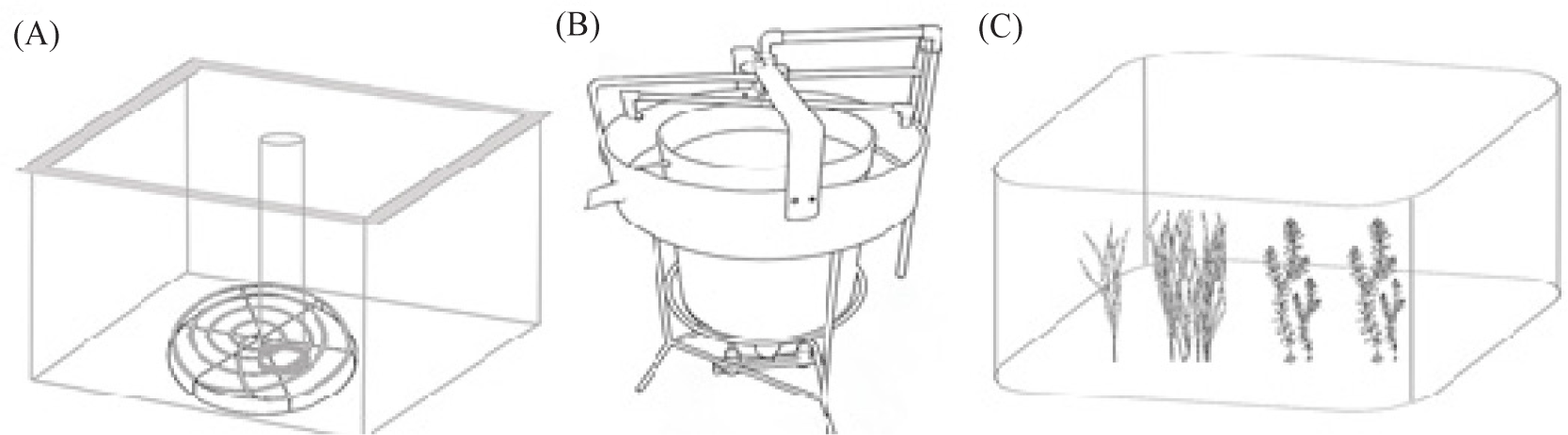
The aquaculture facility in this study is equipped with a Roots blower, aeration discs, a pump house, water storage tanks, and an electrical control room to provide oxygen, water, and power supply. Four concrete tanks, each with an area of 25 m² and a water depth of 1.5 m, were used as broodstock rearing tanks (Fig. 1A). Two 60 L hatching jars were used for egg incubation (Fig. 1B). Two additional concrete tanks, each with an area of 25 m² and a water depth of 1.5 m, were used for the cultivation of fry (Fig. 1A). Two more concrete tanks of the same dimensions were used for rearing C. carpio (Fig. 1A). Furthermore, two ponds, each covering an area of 1 acre, were used for the further cultivation of C. carpio, which were introduced into the ponds after hatching (Fig. 1C).
During the experimental period, water quality in the rearing tanks was monitored using a portable water quality analyzer (Cole-Parmer Instrument, Shanghai, China, YSI 6680) every two days. The monitored parameters included pH, dissolved oxygen, and temperature. Ammonia nitrogen, nitrite nitrogen, and total alkalinity were measured according to the literature (National Environmental Protection Agency of China, 2002). Dissolved oxygen levels were maintained at ≥ 5.5 mg/L. The water flow rate was adjusted according to the developmental stage of the fertilized eggs to ensure that the eggs remained in gentle motion in the upper to middle layers of the water.
The determination of ammonia nitrogen content in water bodies using the Nessler’s reagent spectrophotometric method required the following reagents: NaOH (Shanghai Aladdin Biochemical Technology, China, Batch No.: S111518-20), KI (Shanghai Jizhi Biochemical Technology, China, Batch No.: F-039307), HgI₂ (Shanghai Mapade Instruments, Shanghai, China, UV-2102C), KNaC₄H₆O₆·4H₂O (Shanghai Aladdin Biochemical Technology, Batch No.: P112617), NH₄Cl (Shanghai Jizhi Biochemical Technology, Batch No.: P75750), and Shanghai Mapade Instruments, Shanghai, China, UV-2102C.
The detection of nitrite content in water bodies using the N-(1-naphthyl)-ethylenediamine spectrophotometric method involved the use of ultrapure water (Nanchang Xibao Biotechnology, China, Batch No.: CC0158), phosphoric acid (Shanghai Aladdin Biochemical Technology, Batch No.: P120547), sulfonamide (Sigma Aldrich (Shanghai) Trading, China, Batch No.: 45433), C₁₀H₇NHC₂H₄NH₂·2HCl (Sigma Aldrich (Shanghai) Trading, Batch No.: 1062370025), and NaNO₂ (Hebei Jiebei Technology, Batch No.: 756169705475).
The measurement of total alkalinity was conducted using the acid-base titration method with reagents including carbon dioxide-free water (Guangzhou Shuopu Biological Technology, China, Batch No.: TMSJ0008), HCl (Shanghai Jizhi Biochemical Technology, Batch No.: 624-18), phenolphthalein indicator (Shanghai Aladdin Biochemical Technology, Batch No.: P196509), and methyl orange indicator (Shanghai Aladdin Biochemical Technology, Batch No.: M432889).
S. chuatsi were collected from the Yuqiao Reservoir in Tianjin, China, in December 2023 (Fig. 2). Healthy broodstock with a body weight of 2.5–3.5 kg, free from disease and injury, and clear coloured blotches and spots were selected. After being immersed in a 5‰ saline solution for disinfection, the broodstock was placed into two concrete tanks as described above for pre-spawning conditioning. Each tank was stocked with 500 kg of healthy feeder fish, consisting of common carp (C. carpio), with individual sizes ranging from 100 to 150 g. The quantity of feeder fish was regularly monitored and adjusted to maintain a feeder fish to mandarin fish broodstock ratio of 3:1. Water changes were performed every 10–15 days, increasing to every 3–5 days during the month before spawning.
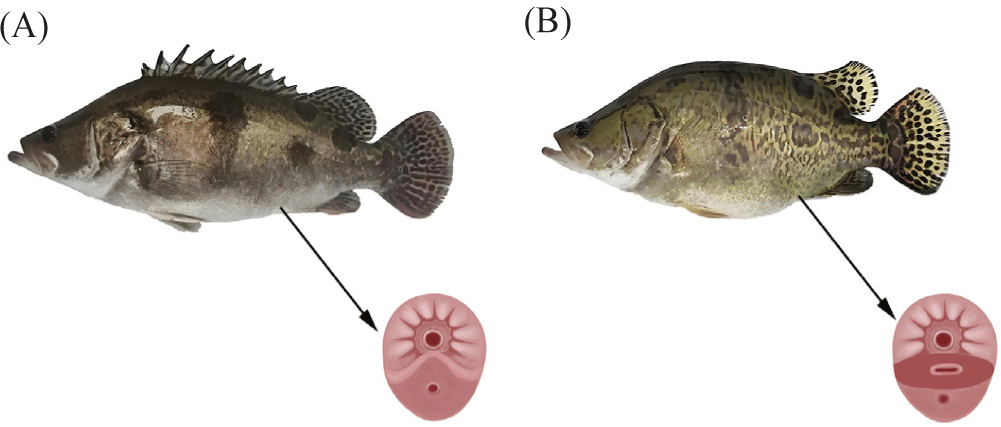
In April 2024, 2- to 3-year-old sexually mature S. chuatsi were selected for the breeding experiment. Mature males were identified by the presence of small, raised spots around the urogenital opening and the release of milky white semen upon gentle pressure on the abdomen, which disperses immediately in water (Fig. 3A). Mature females were identified by a slit-shaped urogenital opening, a swollen abdomen with clearly visible ovarian contours, a relaxed, red, and swollen urogenital area, and a soft, elastic abdomen with a slightly concave midline. When the abdomen was gently pressed, a small amount of egg fluid and pale-yellow eggs were released (Fig. 3B).
Three spawning methods were evaluated. Natural spawning was compared with single and double injection methods to determine the optimal spawning technique (Table 1). For the double-injection method, the dosage for male fish was halved during the second injection, with a 7-hour interval between the two injections. The interval between the two injections for females is also 7 hours. Broodstock were placed in spawning tanks with maintained low water flow. Water flow was increased two hours before the anticipated spawning period to stimulate spawning and induce ovulation. The water temperature in the spawning tanks was maintained at 26°C–27°C; if temperatures exceeded this range, well water was added to regulate the temperature. For the single-injection method, the dosage for male fish was halved.
After administering the ovulation-inducing hormones, females and males were paired in a 1:3 ratio and placed in the spawning tanks with continuous water flow. Depending on water temperature and the maturity of the broodstock gonads, checks were conducted every 0.5 hours during the effective period. The effective time refers to the duration from the onset of fish spawning to the time of the final injection of the spawning inducer. Once eggs and milt were observed being released from the urogenital opening with gentle pressure on the abdomen, eggs and milt were collected. For artificial fertilization, the ratio of females to males was 2:1. The collected milt was mixed with the eggs, and a 0.85% NaCl (Wuhan Xavier Biotechnology, China, Batch No.: G4702) solution was added. The mixture was stirred for 1–2 minutes, followed by the addition of fresh water to activate and fertilize the eggs.
Before using hatching jars, the filter screens were inspected, and the jars were disinfected with 20 mg/L potassium permanganate (Shangqiu Liangyuan Binhe Disinfection Products, China, Batch No.: 240403). Water flow was adjusted by valves to ensure the fertilized eggs remained in a suspended state. The hatching water was filtered through a 60-mesh screen to maintain freshwater quality and prevent the entry of harmful organisms. After rinsing with water from the culture pond, the fertilized eggs were placed in 60 L hatching jars at a density of 150,000 eggs/m³. After 5 hours of incubation, a solution of 30 mg/L povidone-iodine (Hefei Zhonglong Shenli Animal Pharmaceutical, China, Batch No.: 20231028) was added to the jars. Five hundred fertilized eggs were collected from the three induction methods (i.e., natural spawning, single, injection, and double injection), with three replicates set for each group. Fertilization status was observed under a 10-x microscope (Beijing Chengzhike Biotechnology, China, Batch No.: DM750).
C. carpio was cultivated strictly to national standards, ensuring effective disease prevention and control. C. carpio fry was cultivated to provide live feed for S. chuatsi. The timing and quantity of induced spawning for C. carpio were determined based on the spawning status of S. chuatsi, water temperature, and the developmental stage of C. carpio, which was determined by preliminary trials (Table 2). The feeding schedule for S. chuatsi fry and C. carpio is outlined in Table 3. This schedule optimized from preliminary trials ensures that the size and development of the C. carpio are suitable for the nutritional needs of the S. chuatsi fry at various stages of their growth. Spawning and hatching of C. carpio were synchronized to ensure that live feed was available when S. chuatsi fry began exogenous feeding.
Stage 1: This initial stage, lasting 5–7 days, began when the larvae have a body length of approximately 1 cm. During this period, the larvae were delicate, with low resistance to adverse environmental conditions, and had stringent requirements for feed size. Providing appropriate feed at the correct time was critical for survival and growth. Newly hatched C. carpio was used as feed, with a stocking density of C. carpio set at 3–5 times that of the S. chuatsi larvae. The overall stocking density was maintained at 30,000–40,000 larvae per cubic meter.
Stage 2: The second stage, spanning 6–7 days, occured when the larvae reach a body length of 1–1.5 cm. During this phase, the larvae showed substantial growth and increased food intake. To meet their nutritional requirements, feeding was conducted more than twice daily. Prey fish density was maintained at approximately five times that of the S. chuatsi larvae, while the total stocking density was adjusted to 20,000–30,000 larvae per cubic meter.
Stage 3: The final stage, lasting 5–7 days, began when the larvae grow to a body length of 1.8–3.3 cm. This phase was characterized by a substantial increase in food consumption and noticeable size variation among individuals. The larvae display a preference fo r bottom-dwelling behaviors, ascending to upper water layers only during feeding times, which increased the risk of contaminant accumulation at the pond bottom. Feeding was conducted twice daily, with the prey fish density maintained at five times that of the S. chuatsi larvae. The stocking density was reduced to 15,000 larvae per cubic meter to accommodate the larvae’s growth and activity levels.
Domestication of S. chuatsi begins when the fish reached a size of 2.5–3 cm. Fish were fed twice daily, at 5:00 and 19:00. During domestication, the feeding amount is slightly less than normal, and water is added three minutes before feeding begins. A combination of active and static domestication methods is used, gradually reducing the amount of live fish provided as the mandarin fish begin to surface and actively feed. The feeding schedule during the domestication process is outlined in Table 4. The fish (S. chuatsi) was gradually transitioned from a diet of live prey (C. carpio) to fish fillets and pellets. Feeding schedules were adjusted to optimize growth while minimizing the use of live prey.
Statistical analyses were performed using SPSS 26.0 software. A one-way analysis of variance (ANOVA) was conducted to evaluate the fertilization rate. If significant differences between groups were detected, multiple comparisons were performed. For homogenous variances, the Least Significant Difference (LSD) test was applied, while for heterogeneous variances, the Games-Howell test was applied.
Results
ANOVA results showed that the effect duration differed significantly among the three induction treatments (p < 0.01), following the order: natural spawning (37.2 ± 0.8 h) > single injection (24.0 ± 0.5 h) > double injection (18.5 ± 0.5 h). The fertilization rate also varied significantly among the treatments (p < 0.05), with both the single injection (81.33 ± 2.40%) and double injection (83.03 ± 2.41%) showing higher rates compared to natural spawning (73.50 ± 3.75%) (Fig. 4).
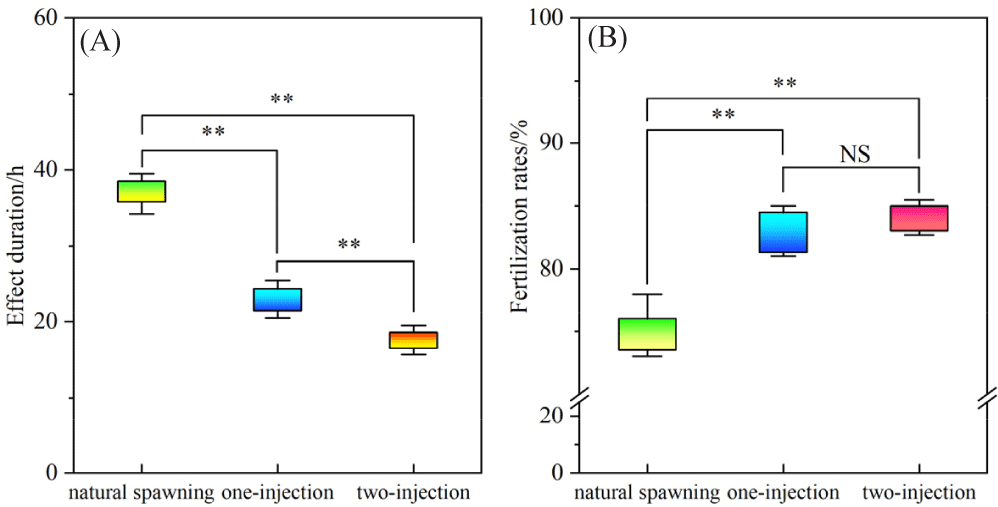
Fig. 5 shows the relationship between the total length and growth period of S. chuatsi and C. carpio. The growth patterns of both species were generally consistent, exhibiting nonlinear growth over time. Polynomial regression with a degree of 2 was selected with an R2 value closest to 1 and indicated good-fitting curves for both species (R2 = 0.9923 for S. chuatsi and 0.9899 for C. carpio).
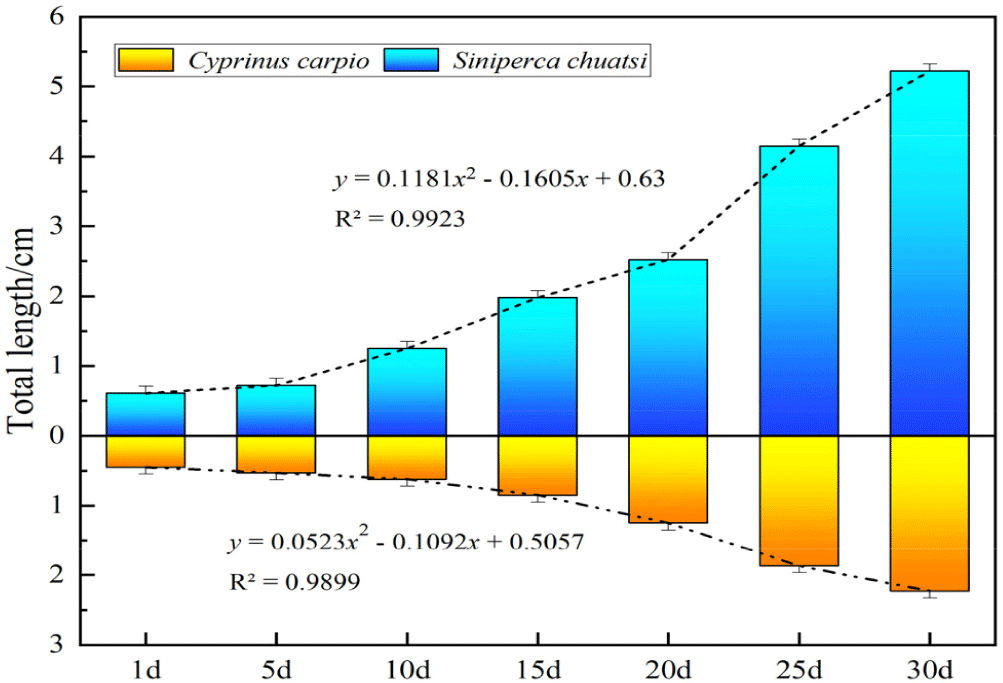
The average size of S. chuatsi larvae bred was 0.61 ± 0.11 cm, with a total quantity of 492,500. After 60 days of cultivation and domestication, the average size increased to 7.80 ± 0.15 cm, with a remaining quantity of 91,819 fish and a feed conversion ratio (FCR) of 2.2.
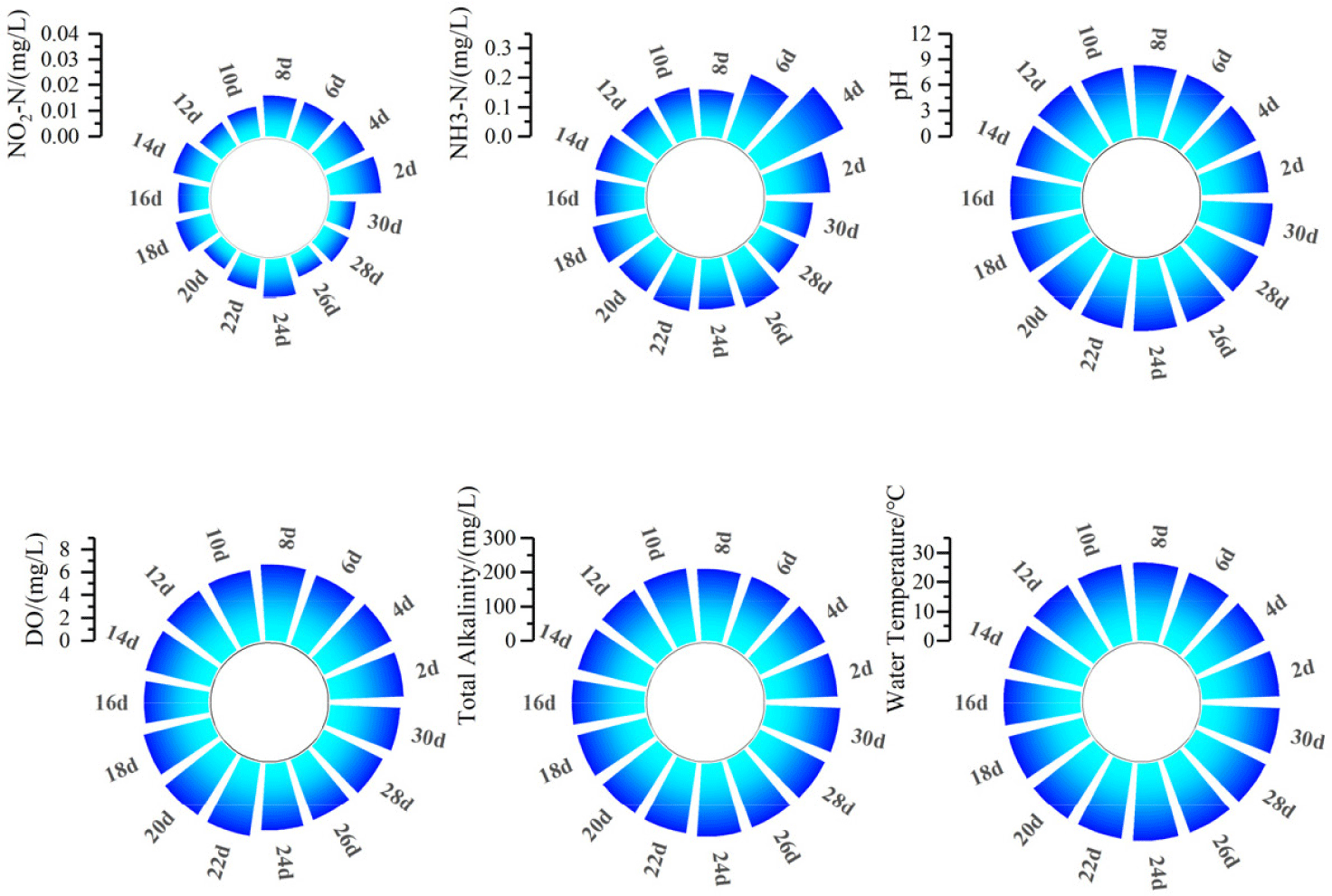
During the culture period, the nitrite concentration (NO2-N) ranged from 0.005 to 0.025 mg/L, ammonia nitrogen (NH3-N) ranged from 0.15 to 0.32 mg/L, pH ranged from 7.8 to 8.6, dissolved oxygen ranged from 5.7 to 6.7 mg/L, total alkalinity ranged from 207 to 218 mg/L, and water temperature ranged from 26.2°C to 26.8°C (Fig. 6). All the water quality parameters measured were considered within safe limits (National Environmental Protection Agency of China, 2002).
Discussion
In exploring fish reproductive biology, determining the frequency of spawning induction has become a key research focus, with the primary basis being the maturity stage of fish gonadal development. Studies have shown that fish with less mature gonads may require multiple hormone injections to stimulate ovulation effectively (Rottmann et al., 1991a). A hormone injection approach has been used in aquaculture, which helps overcome insufficient gonadal maturity (Rottmann et al., 1991b). In induced breeding protocols, especially for species with less mature gonads, the first injection initiates maturation, while the second dose, often administered hours later, helps trigger final maturation and spawning (Kumar et al., 2021). For example, Indian major carp respond better to a second injection to induce reproduction (Surnar et al., 2015). This method ensures higher fertilization rates and better outcomes than a single injection.
Several studies support the idea that the dual injection method promotes gonadal development and enhances reproductive outcomes in fish species. The double injection method works by providing two distinct hormonal stimuli. The first injection triggers the early stages of gonadal maturation, allowing the gonads to transition to a more advanced state of readiness. The second injection then accelerates the final maturation stages, promoting ovulation and ensuring a higher quality of gametes. This process is heavily regulated by hormones such as luteinizing hormone and follicle-stimulating hormone, which are released in response to injections of Gonadotropin-Releasing Hormone analogs. These hormones are crucial for initiating and controlling gonadal development, from gametogenesis to final maturation and ovulation (Mylonas & Zohar, 2000). Studies have shown that this method is effective in improving reproductive efficiency and ensuring a more synchronized and higher-quality spawning process (Adrian Rodríguez Gabilondo et al., 2022).
In addition, optimal thermal conditions could enhance the effectiveness of hormone treatments, particularly in species-specific reproductive cycles (Meng et al., 2021). For S. chuatsi, a double injection of spawning hormones was optimized to ensure proper ovulation, and the effective time—defined as the duration from the final injection to ovulation—can vary based on temperature and hormonal response. The 26°C–27°C range is ideal for inducing timely ovulation within 18–19 hours, as observed in experimental settings involving this species, indicating double injection allows for more efficient hormonal regulation, promoting quicker reproductive responses in the broodstock.
In summary, the dual injection method enhances gonadal development by providing a more structured hormonal stimulus, resulting in better reproductive performance, higher fertilization rates, and improved gamete quality in fish. The findings of this study confirm the superiority of the double-injection method in enhancing the fertilization rate of S. chuatsi.
S. chuatsi larvae, being piscivorous, exclusively feed on live prey fish from the onset of exogenous feeding, rejecting other types of food such as zooplankton. This specialized diet begins when the larvae open their mouths, typically three days post-hatching, and is essential for their growth and survival. Studies emphasize the importance of cultivating an adequate supply of palatable live prey fish, such as carp fry, to meet the nutritional needs of S. chuatsi during their early developmental stages (Xiong et al., 2013). The high costs and potential risks associated with live prey, such as pathogen transmission, make this a key challenge in mandarin fish aquaculture. However, this reliance on live prey is well-documented as a critical component of their reproductive and growth success (FAO, 2009). These insights underline the necessity of synchronized breeding and prey availability to ensure the successful cultivation of mandarin fish in aquaculture.
Ensuring that prey fish size is compatible with the growth stage of S. chuatsi is crucial for effective feeding and optimal development. Research indicates that the maximum length of prey fish should not exceed approximately 60% of the body length of S. chuatsi to facilitate smooth ingestion and meet nutritional requirements (Li et al., 2013). In this study, mathematical models were developed based on the total length indices of S. chuatsi and C. carpio. Results showed that, within the breeding cycle, the average size of C. carpio was 58.14% of the body length of S. chuatsi, aligning well with the established size ratio. Furthermore, the total lengths of both species exhibited nonlinear growth trends over time, with S. chuatsi demonstrating a markedly higher growth rate than C. carpio. These findings highlight the scientific rationale and suitability of using C. carpio as the initial prey for S. chuatsi.
Furthermore, the alignment of the growth timing between C. carpio and S. chuatsi makes carp a highly promising prey fish for the early fry stage of mandarin fish. Mandarin fish completed hatching after 72 hours, while carp completed hatching after 48 hours (Tessema et al., 2020). This overlap in timing ensures that carp fry is available at critical points when mandarin fish fry begins feeding, making them ideal live prey for successful cultivation. By comparing the growth curves of mandarin fish fry and carp fry, we found a high degree of consistency, with a fit greater than 0.95. This result confirms the scientific validity and feasibility of using carp fry as initial prey for mandarin fish fry. Based on these findings, a synchronized spawning induction strategy for carp was developed, ensuring that prey fish were available in sufficient quantity and at the appropriate developmental stage.
C. carpio, an endemic cyprinid species native to Tianjin, China, is distinguished by its delicate and smooth flesh, rich in high-quality proteins, fats, vitamins, and minerals (China National Intellectual Property Administration, 2020). These nutrients work synergistically to form a comprehensive and balanced nutritional profile, pivotal in supporting the growth and development of S. chuatsi larvae (Skibniewska et al., 2013). C. carpio serves not only as a cost-effective and practical feed option for S. chuatsi larvae but also plays an irreplaceable role in promoting their healthy growth. This finding has significant implications for optimizing the breeding structure of freshwater fish, improving aquaculture efficiency, and advancing the sustainable development of the fishery economy in Tianjin.
Regarding growth performance, S. chuatsi cultivated in the system exhibited rapid daily growth of 0.12 cm, highlighting the efficiency of this method. This stable growth environment is crucial for the breeding and cultivation of S. chuatsi. The results of this study demonstrate the advantages of the current aquaculture facilities and conditions. Research into the domestication of S. chuatsi on artificial diets has revealed that specialized feeding techniques can significantly improve survival rates and growth performance compared to traditional methods (He et al., 2021). This indicates the strong potential of the current systems in enhancing breeding success, especially when paired with tailored environmental controls and feeding regimens.
FCR is a crucial metric in aquaculture, measuring the efficiency of converting feed into animal biomass (Tacon & Metian, 2008). The industry standard FCR for various species, depending on management practices, with values close to 2 for many farmed fish species (Boyd et al., 2007), which is in agreement with our current study. Factors affecting FCR include survival rates, feed intake, weight gain, and metabolic rates (Stone et al., 2024), and further improving FCR remains crucial for reducing feed costs and environmental impacts in aquaculture (Rahman et al., 2022).
The domestication of S. chuatsi has significantly benefited aquaculture practices, economic systems, and environmental sustainability. For aquaculture practitioners, domestication has notably reduced feed costs and enhanced farming productivity. This success is largely due to the species’ adaptation from natural prey to formulated feeds, eliminating the need for baitfish cultivation. Consequently, this shift reduces land, water, and labor demands, increasing economic returns for farmers (FAO, 2009).
In conclusion, this study successfully demonstrated the benefits of using a double-injection spawning method for S. chuatsi, achieving higher fertilization rates and reducing spawning time compared to natural spawning and single injections. The use of C. carpio as initial feed proved feasible, contributing to the successful growth and domestication of S. chuatsi in an industrial-scale aquaculture setting. These findings provide valuable insights for improving the efficiency of mandarin fish breeding and domestication, with potential applications in large-scale aquaculture production. The success of industrial S. chuatsi aquaculture in Tianjin can serve as a blueprint for similar efforts in other regions and boost local economies.
While crucian carp (C. auratus) is commonly used as prey, this study focused solely on common carp due to its abundance and established use in aquaculture systems in Tianjin. Future research may include a side-by-side comparison of C. auratus and C. carpio, which would strengthen the conclusions and provide more comprehensive recommendations for practitioners.