Introduction
Aquatic environments face serious issues associated with high levels of heavy metals driven by industrialization, urbanization, agricultural activities, waste management, and mining. Heavy metals that accumulate in the environment can affect the food chain and pose risks of toxicity to aquatic organisms (Nedelkoska & Doran, 2000; Rajaei et al., 2012). Heavy metals occur as free ions in water or are adsorbed on sediments, where they may persist for extended periods, later exposing aquatic organisms to heavy-metal-associated toxicity (Arai et al., 2002). Sediments often contain higher heavy-metal concentrations than the surrounding water, impacting benthic species that traverse the lower layers of the ocean and are thus susceptible to heavy-metal exposure (Goto & Wallace, 2010). Various physicochemical methods—including chemical precipitation, ion exchange, and adsorption—have been employed to remove heavy metals, despite limitations that include high cost and the potential for secondary pollution. In contrast, bioremediation offers a promising alternative due to its cost-effectiveness and minimal environmental impact (Verma & Kuila, 2019). This approach uses biological systems, including genetically modified bacteria, to absorb, detoxify, and sequester heavy metals from contaminated environments (Malik, 2004; Mejáre & Bülow, 2001). However, concerns remain regarding the environmental safety of genetically modified organisms, highlighting the need for alternative strategies that leverage naturally occurring metal-binding proteins.
Metallothioneins (MTs) are cysteine-rich proteins that play crucial roles in metal homeostasis, detoxification, and protection against oxidative stress. Since their discovery as cadmium-binding proteins in equine renal tissue (Margoshes & Vallee, 1957), various MTs have been identified in a diverse array of organisms, from bacteria to vertebrates. One structural characteristic of MTs is their high cysteine content (– 30% of total amino acids), which enables binding to both essential (e.g., Zn, Cu) and toxic (e.g., Cd, Hg) heavy metals via thiol groups, thereby reducing metal toxicity (Palacios et al., 2011; Xiang et al., 2013). MTs serve as metal reservoirs, participate in detoxification, and mitigate oxidative stress, making them important biomarkers for monitoring heavy-metal pollution in marine organisms (Blindauer, 2008; Nagamatsu et al., 2021; Palmiter, 1998).
The Pacific abalone, Haliotis discus hannai, is a benthic species that is highly valued in aquaculture, and as abalone production has increased its various bioactivities have been investigated (Cook, 2016). Furthermore, the marine environments employed for abalone production, such as cage culture facilities in coastal areas, are constantly exposed to heavy metals (Xie et al., 2020). Thus, abalone provides a unique opportunity for investigating heavy-metal accumulation and detoxification, particularly through the expression of MTs, which are crucial to the binding and neutralizing of toxic metals.
This study aimed to characterize the H. discus hannai, recombinant MT (rHdhMT) from H. discus hannai and evaluate its metal-binding capabilities. The heavy-metal tolerance of the strain and protein were characterized, as well as the accumulation of recombinant MT in Escherichia coli under conditions of Zn and Cu exposure.
Materials and Methods
The gill tissues of H. discus hannai were frozen in liquid nitrogen. Total RNA was extracted using RiboEX (GeneAll, Seoul, Korea) according to the manufacturer’s instructions. Concentrations of total RNA were measured with an ASP-2680 spectrophotometer (ACTGen, Piscataway, NJ, USA). The total RNA extract was treated with DNase and synthesized using an M-MLV cDNA Synthesis Kit (Enzynomics, Daejeon, Korea).
For the expression of recombinant HdhMT from E. coli, polymerase chain reaction (PCR) amplification of the gene encoding rHdhMT was performed using specific primers (F: ATATCATATGTCCAGTCCCCAAGGCGC, R: TATTCTCGAGTTTGCACGTGCAGG) containing Nde I and Xho I restriction sites. The PCR conditions included initial denaturation at 95°C for 3 min, followed by 30 cycles of 95°C for 30 sec, 58°C for 30 sec, and 72°C for 30 sec, and then a final extension at 72°C for 5 min. The PCR product was purified, cloned into the pTOP TA V2 vector, and transformed into E. coli DH5α.
For rHdhMT expression, subcloning was performed into the pCold I vector digested with Nde I and Xho I, followed by transformation into E. coli BL21 (DE3-codon Plus). For protein expression, E. coli harboring pCold I-rHdhMT was cultured in lysogeny broth (LB) medium with ampicillin (100 mg/mL) at 37°C overnight. The cells were subcultured until optical density at 600 nm (OD600) reached 0.6, followed by induction with 0.5 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) at 20°C for 20 h. Cells were harvested through centrifugation (6,000×g, 4°C, 10 min) and analyzed via sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and western blotting. Purification of rHdhMT was conducted as described previously (Im et al., 2024). To remove imidazole, stepwise dialysis was performed at room temperature using a 3.5-kDa dialysis membrane. The imidazole concentration was gradually reduced in seven steps, from 125 mM to 3 mM, followed by dialysis in pure low-imidazole elution (LEW) buffer. Finally, purified rHdhMT was obtained in 1× LEW buffer.
The theoretical molecular weight of the peptide region and its isoelectric point (pI) were predicted using the Expasy ProtParam tool (https://web.expasy.org/protparam/). Amino acid sequences of rHdhMT were sourced from Uniprot (https://www.uniprot.org/). The predicted protein three-dimensional model, including the locations of conserved cysteine residues, was analyzed using the Swiss-Model tool (https://swissmodel.expasy.org). Ligand prediction, including the metal binding sites of rHdhMT, was examined using the COACH tool (https://zhanglab.ccmb.med.umich.edu/COACH).
A bacterial disaggregation assay was conducted with bacterial cultures grown in tryptic soy broth (TSB) medium at 37°C overnight. The cultures were subjected to centrifugation at 6,000×g and 4°C for 5 min. The bacterial pellet resuspended in 1× TSB was diluted to obtain a cell density OD600 of 0.7, as determined using a spectrophotometer (Perkin Elmer, Shelton, CT, USA). After exposure to heavy metals and rHdhMT protein followed by incubation at 30°C for 1 h, fluorescence microscopy analysis was conducted after treatment with 2-(4-amidinophenyl)-1H-indole-6-carboxamidine (4′,6-Diamidino-2-phenylindole; 10 μg/mL) for 30 min in the dark.
For ultrasensitive radial diffusion assay (URDA) analysis, Bacillus subtilis cells were cultured overnight at 37°C in TSB medium. The bacterial culture was diluted using dilution buffer composed of 20 mM phosphate buffer (pH 6.57) and 0.03% TSB, and OD600 was measured by spectrophotometry. Subsequently, 0.4 mL of the adjusted bacterial solution was combined with 7.1 mL of radial diffusion assay buffer Type 1 (underlayer gel; 20 mM phosphate buffer, 0.03% TSB, and 1% agarose-LE) and incubated for 2 h at room temperature. Mixtures of heavy metals and rHdhMT protein were then introduced into the medium, followed by plating of 7.5 mL of overlayer gel containing 20 mM phosphate buffer, 6% TSB, and 1% agarose-LE.
A bacterial cell spotting assay was conducted to investigate the effect of rHdhMT on heavy-metal tolerance (Lesser & Guthrie, 1993). Transformed E. coli cells expressing rHdhMT were grown in LB medium supplemented with 100 μg/mL ampicillin and 0.5 mM IPTG at 20°C with shaking at 120 rpm for 24 h. After adjusting OD600 to 0.6, the cells were diluted in a 10-fold series to a final concentration of 107 cells/mL, and 5-μL aliquots were spotted onto plates containing various concentrations of heavy metals (Zn and Cu at 0, 50, 150, 300, 600, 1000, and 1500 μM). Following 16 h of incubation at 37°C, the growth rates of the cells were assessed and quantified using the threshold function of ImageJ software.
Inductively coupled plasma optical emission spectrometry (ICP-OES) was used to measure heavy-metal accumulation (Bisharyan et al., 2003). This analysis was conducted with E. coli cells grown to an OD600 of 0.6 followed by centrifugation at 6,000×g and 4°C for 10 min. The pellet was resuspended in fresh LB medium and then incubated with 300 μM Zn or Cu for 1 h, followed by a further round of centrifugation as described above and three washes of the pellet with fresh LB medium. The pellet was lyophilized, digested with HNO3 using a microwave (Multiwave 7000, Anton Paar GmbH, Austria), and analyzed through ICP-OES (Agilent 5800 ICP-OES, Agilent Technologies, Santa Clara, CA, USA). All measurements were performed in triplicate for statistical analysis. Statistical analysis was performed using the independent samples t-test. A probability value of less than 0.05 (p < 0.05) was considered statistically significant. Data are presented as means ± SE of the mean.
Results
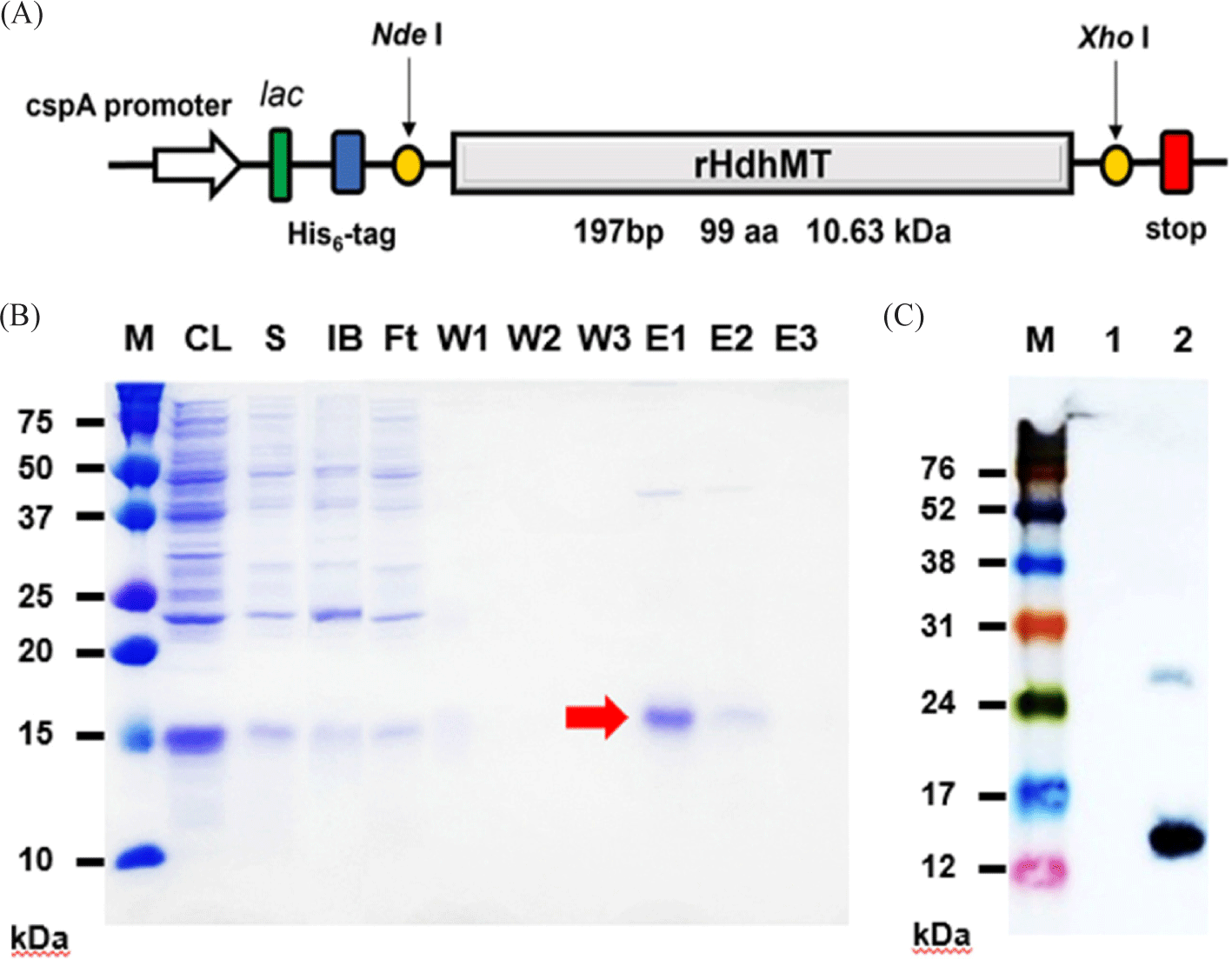
To investigate the functional properties of MT from the Pacific abalone, Haliotis discus hannai (HdhMT), the gene encoding its recombinant form (rHdhMT) was cloned into the pCold I vector using the NdeI and XhoI restriction enzyme sites (Fig. 1A). The recombinant HdhMT construct consists of 99 total amino acids, including the N-terminal His6 tag and 69 amino acids in the HdhMT structural region containing 18 cysteine residues. These residues constitute 26% of the total protein, which has a predicted molecular weight of 10.6 kDa and pI of 6.67. This protein contains eight Cys-X-Cys or Cys-X-X-Cys motifs (data not shown) that are important for metal binding. On induction of recombinant protein expression in E. coli, purification of rHdhMT was conducted via immobilized metal ion affinity chromatography. SDS-PAGE profiles of the fractions collected during purification followed by western blotting indicated successful purification of rHdhMT (Fig. 1B), as demonstrated by anti-His-tag antibody at the position corresponding to the expected molecular weight of 10 kDa (Fig. 1C).
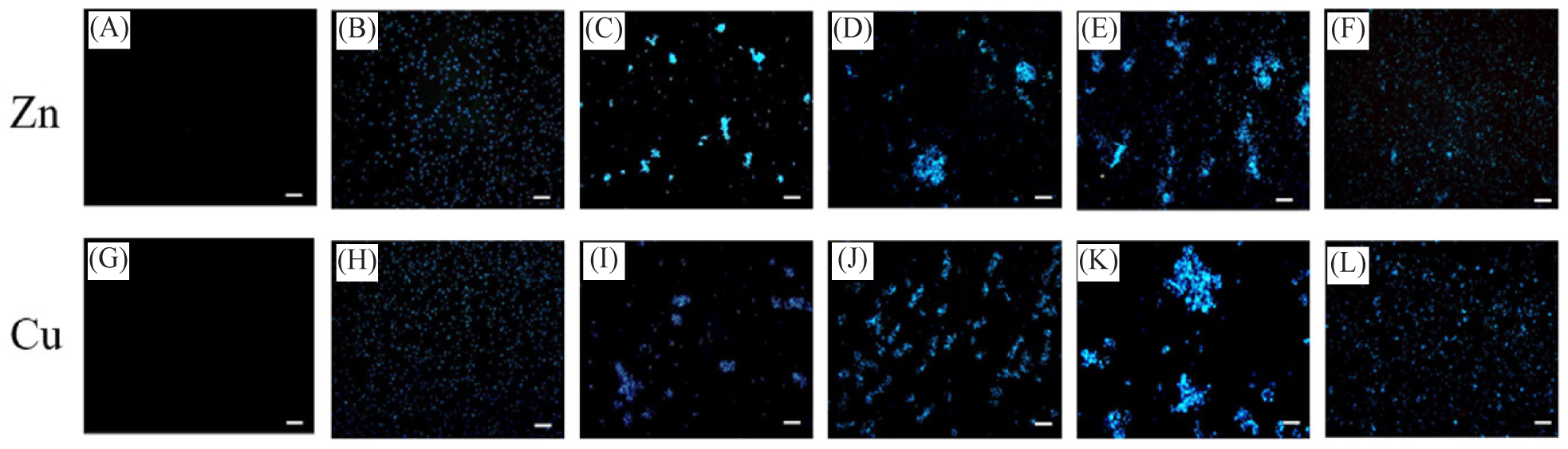
Various bacterial cultures appear to aggregate in the presence of high concentrations of heavy metals. To examine whether rHdhMT can mitigate the bacterial aggregation effect induced by heavy metals, fluorescence microscopy analysis was conducted using bacterial cells, aggregation of which was induced in the presence of Zn and Cu (Fig. 2). While no fluorescence signals were detected from samples containing heavy metals but without B. subtilis cells (Fig. 2A and 2G), significant levels of fluorescence from B. subtilis (Fig. 2B and 2H), showing both single cells and significant aggregations, were observed on exposure to Zn (Fig. 2C) and Cu (Fig. 2I). The results show that further addition of 1 × LEW buffer (Fig. 2D and 2J) and a similar amount of bovine serum albumin (BSA) protein (Fig. 2E and 2K) did not prevent bacterial aggregation. In contrast, when purified rHdhMT was added, heavy-metal-induced bacterial aggregation was prevented (Fig. 2F and 2L). These results indicate that rHdhMT mitigated the aggregation of bacterial cells induced by heavy metals, possibly through disruption of the interaction between the bacterial cells and heavy-metal ions.
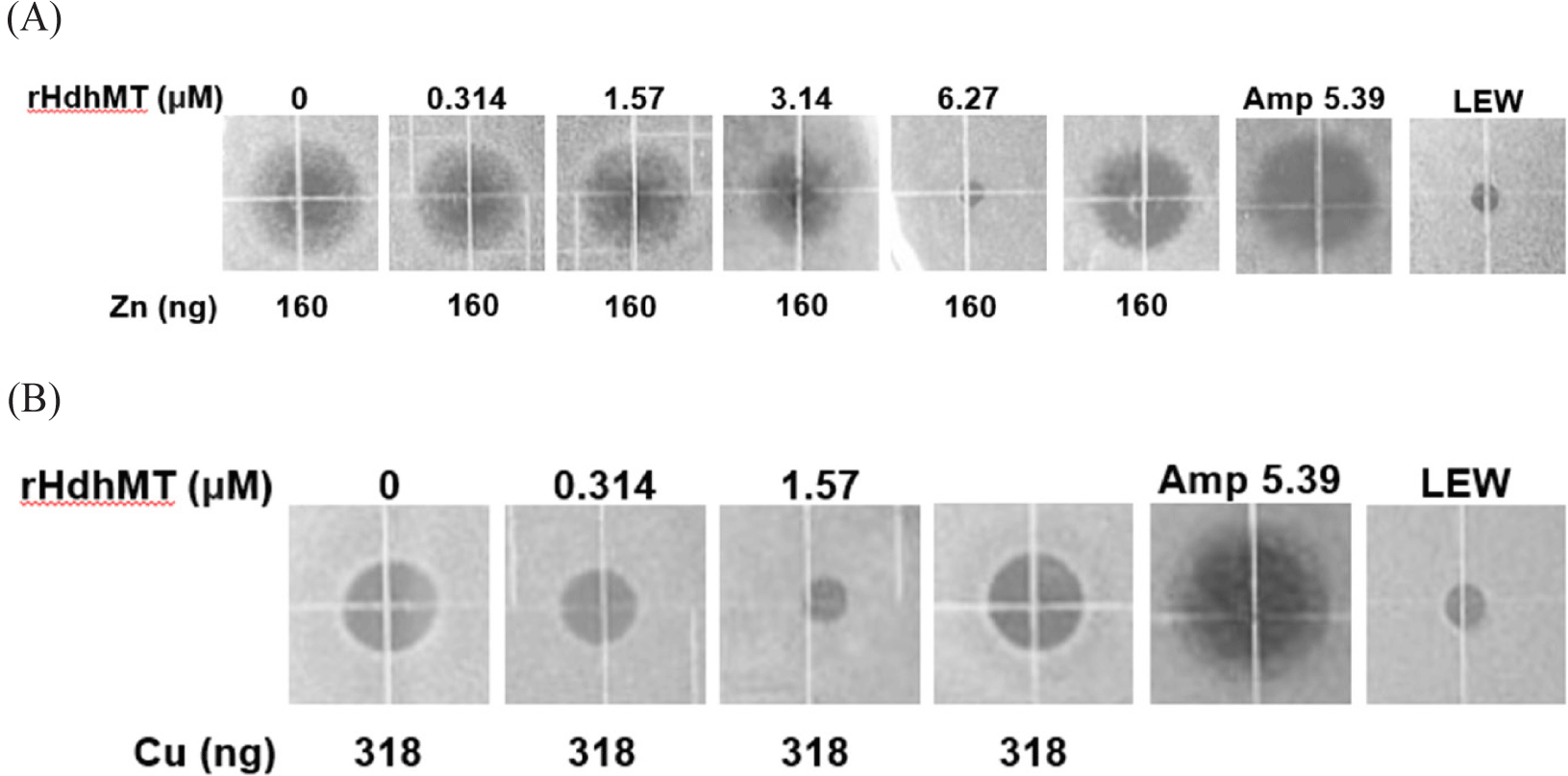
To examine whether rHdhMT confers bacterial resistance to heavy metals, URDA was conducted using B. subtilis (Fig. 3). The results clearly show a toxic, bactericidal effect of Zn and Cu, as demonstrated by the formation of clear rings in the medium where heavy-metal-induced cytotoxicity occurred. This result is in striking contrast to the lack of rings in non-cytotoxic areas and negative controls, including the treatment containing 1 × LEW buffer without recombinant protein. A clear decrease in the size of the clear zones was detected as the concentration of rHdhMT increased. The protein concentration at which no ring formation was observed was considered the threshold for MT-conferred heavy-metal cytotoxicity tolerance. The results indicate that addition of 6.27 μM rHdhMT compensated for the toxic effect induced by 160 ng Zn (Fig. 3A). Furthermore, addition of 1.57 μM rHdhMT compensated biological toxicity in B. subtilis exposed to 318 ng Cu (Fig. 3B). Overall, the results indicate that the cytotoxic effects of heavy metals were mitigated by addition of rHdhMT.
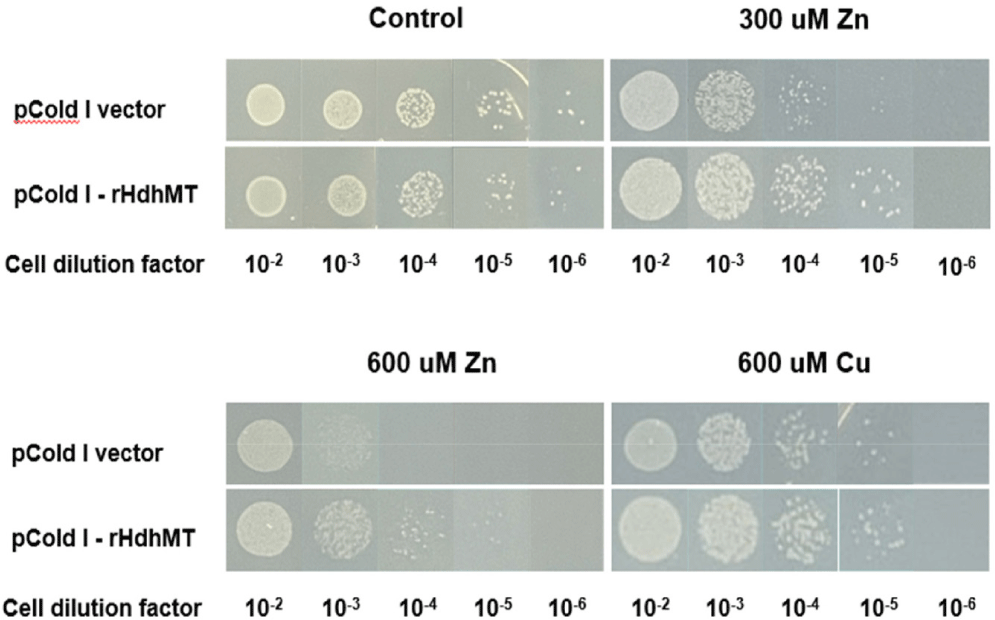
To validate the effect of rHdhMT on heavy-metal tolerance at the cellular level further, growth rates were compared between MT-expressing cells and control E. coli cells harboring the pCold I vector with no rHdhMT gene. Growth of the cells after plating at various dilution ratios in LB medium with or without heavy metals was monitored (Fig. 4). The results show no significant differences in growth between the two groups grown on plates without heavy metals. However, at dilution ratios of 10−3, 10−4, and 10−5, survival of E. coli cells expressing MT was higher than that of control cells harboring only the vector. These findings indicate that MT-expressing cells exhibited enhanced heavy-metal tolerance compared to the control cells, implying that recombinant MT increased the heavy-metal resistance of these cells.
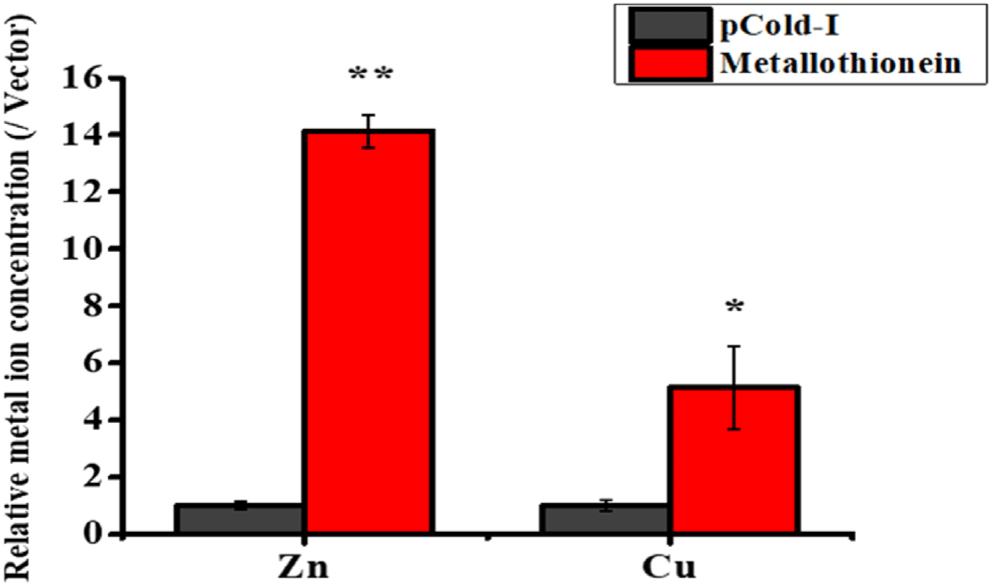
To assess the impact of rHdhMT on metal accumulation further, ICP-OES was used to measure concentrations of Zn and Cu directly in rHdhMT-expressing cell cultures. The results reveal that E. coli cells expressing rHdhMT exhibited markedly elevated levels of both Zn and Cu compared to control groups harboring only the vector (Fig. 5). Specifically, cells overexpressing rHdhMT had approximately 14-fold greater Zn levels and 5-fold greater Cu accumulation, demonstrating slightly higher efficacy for Zn compared to Cu. The observed increases were statistically significant (p < 0.05), providing further evidence that rHdhMT plays a role in facilitating heavy-metal accumulation. The results were consistent among all tests employed in this study, indicating that rHdhMT may have strong binding affinity or regulatory capacity for heavy metals, including Zn and Cu.
Discussion
Marine organisms are continuously exposed to aquatic environments containing heavy metals, which pose a threat to their survival and physiological functions (Arai et al., 2002). Biological systems that can overcome the harmful effects exerted by heavy metals have developed in response to that threat. MTs, a cysteine-rich protein family, mitigate heavy-metal toxicity through binding metal ions, thereby playing crucial roles in detoxification and metal homeostasis (Mejáre & Bülow, 2001; Palacios et al., 2011). Therefore, MT expression has been extensively studied in various marine species, including abalone, in which MT mRNA levels increase under metal stress (Lee & Nam, 2016).
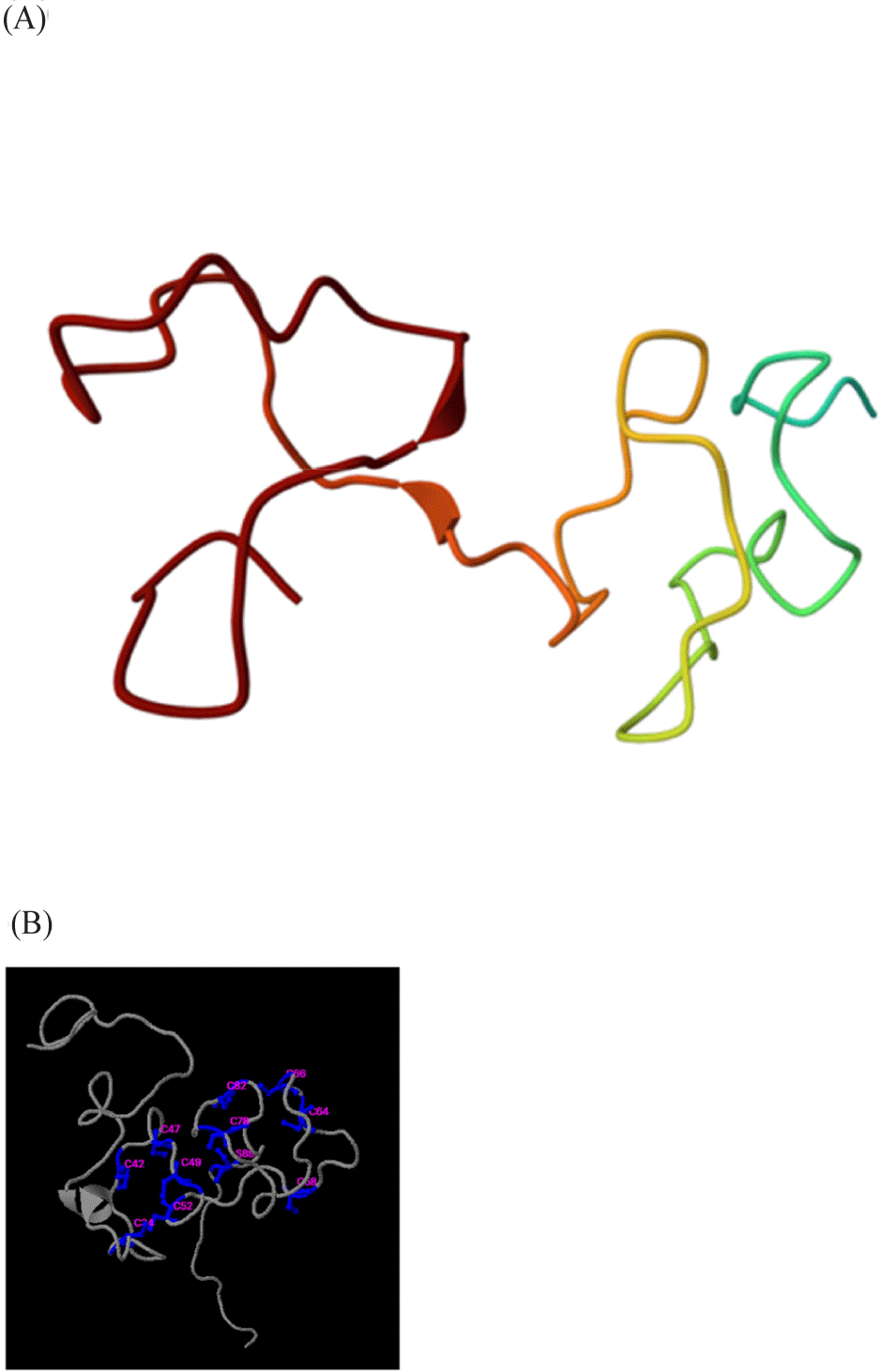
To explore the functional roles of recombinant MT from H. discus hannai, its structural characteristics and the possible implications of its primary binding with heavy metals must be examined. After purification, rHdhMT was tested for its functional activities associated with heavy metals. Structural prediction indicated that rHdhMT possesses seven Cys-X-Cys motifs and one Cys-X-X-Cys motif, which are essential to metal-ion binding (Amiard et al., 2006). The presence of these motifs in rHdhMT implies potential for metal binding and a possible role in metal detoxification. The predicted structure of rHdhMT (Fig. 6) exhibited a distinct conformation that was consistent with other MT structures. The model’s conserved cysteine residues indicate high affinity for metal ions, aligning with the observed metal-binding properties of rHdhMT (Lukács et al., 2021). Additionally, ligand-binding site prediction identified Zn, Cu, Ag, and Ca as potential binding partners, supporting the hypothesis that rHdhMT plays a role in metal homeostasis regulation. These structural features corroborate its functions in binding and neutralizing heavy metals, which were subsequently validated through in vitro experiments.
Various biological approaches were employed to obtain evidence of the functional roles of rHdhMT associated with heavy metals. A bacterial disaggregation assay demonstrated that rHdhMT inhibited metal-induced bacterial aggregation, implying its potential to mitigate heavy-metal toxicity (Fig. 2). This finding aligns with previous studies indicating that MTs play crucial roles in heavy-metal detoxification through metal-ion binding and reduction of metal bioavailability (Blindauer & Leszczyszyn, 2010). Bacterial aggregation in response to heavy-metal exposure is typically associated with stress-induced biofilm formation, which is a protective mechanism against metal toxicity (Harrison et al., 2007). The capacity of rHdhMT to prevent aggregation implies that it may effectively sequester heavy metals, thereby reducing cellular stress and negating the necessity of biofilm formation. Other MTs, such as ZntA, have exhibited similar protective effects, enhancing metal resistance through metal efflux and sequestration (Rensing et al., 1998). In contrast to efflux pumps that actively expel metals from the cytoplasm, rHdhMT appears to function primarily through intracellular metal binding, thereby reducing metal-induced aggregation.
Our findings demonstrate that rHdhMT significantly improves bacterial resistance to heavy metals, as evaluated through a radial diffusion assay. The results of cell spotting tests indicate higher survival rates for rHdhMT-expressing cells plated on Zn- and Cu-supplemented media compared to control cells (Fig. 4). These results support the notion that MT plays crucial roles in metal detoxification through binding of metal ions and minimizing their cytotoxic effects. This conclusion was also supported by analysis of heavy-metal bioaccumulation, which indicated stronger affinity of rHdhMT for Zn than for Cu and aligned well with the preferential binding characteristics of MTs (Krȩżel & Maret, 2007; Fig. 5). These findings imply that rHdhMT can selectively regulate metal-ion accumulation, which is vital to maintaining cellular metal balance. The disaggregation effect of rHdhMT may be attributed to the structural characteristics of MTs, which contain cysteine-rich regions that function as high-affinity binding sites for metal ions (Krȩżel & Maret, 2007). By chelating metals, rHdhMT likely prevents their interactions with extracellular components that would otherwise trigger clumping. This mechanism has also been observed in bacterial metallochaperones, which facilitate metal homeostasis by preventing uncontrolled metal interactions (Festa & Thiele, 2011).
Taken together, these findings imply that rHdhMT confers enhanced heavy-metal tolerance to cells and mitigates metal-induced bacterial aggregation. The findings of this study have significant implications for environmental protection and aquaculture. Additionally, recombinant MTs such as rHdhMT could serve as a biotechnological tool for mitigating heavy-metal contamination in aquatic ecosystems and have possible applications for reducing heavy-metal-induced toxicity. In conclusion, this study highlights the effective metal binding of rHdhMT and its potential role in metal tolerance. Our findings provide a foundation for further exploration of recombinant MTs as tools in environmental and aquaculture applications.
