Introduction
Wetlands are transition zones between terrestrial and aquatic ecosystems (Ferreira et al., 2023) and thus are productive (Moges & Mebrate, 2022). Wetlands are diverse ecosystems with fluctuating water levels, periods of oxygen stress, and hydric soils with varied hydrological conditions. Wetlands provide unique functions linked too many ecosystem services essential for biodiversity conservation, climate change mitigation, and human well-being (Ferreira et al., 2023), and supporting important livelihood activities such as tourism and fisheries (Hambäck et al., 2023). Wetlands cover 570 m ha of landscapes globally and their African share is about 34.5 m ha (Wondie, 2018).
Ethiopian wetlands which cover 2.26 m ha are inland floodplains in nature and include swamps, marshes, floodplain rivers, lakes, and reservoirs (Wondie, 2018). The Ethiopian wetlands are paramount sources of ecosystem services and are home to a diverse biota. Although wetland ecosystems are greatly important in providing various ecosystem services, they are globally threatened by anthropogenic and natural drivers, such as changes in land use patterns, water availability, and climate change, including sea level rise and occurrence of extreme events (i.e., floods and droughts) and global warming (Agboola, 2017; Ferreira et al., 2023). In the developing world, brutal human activities on wetlands have been intensified since they are poorly understood with insufficient data (Fentaw et al., 2020). In Ethiopia, particularly open grazing, conversion to agricultural lands, urbanization, and water abstraction have been the main threatening factors to wetlands (Moges et al., 2018; Moges & Mebrate, 2022; Wondie, 2018). Moreover, weak policies on ecosystems, land degradation, and diversified livelihood dependency on wetlands are being continued as critical challenges facing Ethiopian wetlands (Wondie, 2018).
Now a days, anthropogenic activities and climate change are hindering wetland stability, thereby causing a loss in biodiversity (i.e., macroinvertebrates, fish, and macrophytes) (Hilsenhoff, 1988). As a common feature of the Ethiopian wetlands, the Burana Natural Wetland (BNW) and Totosie Semi-Artificial Wetland (TSAW) are common pool resources and are largely affected by excessive water abstraction. Both wetlands are also the mainstay of heavy free grazing. These human activities are extremely threatening the biodiversity and water quality of BNW and TSAW. However, no research has been done so far, despite the importance of natural resource management for sustainable utilization. Nearly all sections of the wetlands, particularly the BNW are being used as waste disposal sites which limits the effective use of the wetland. Therefore, documenting biological entities and examining the water quality status of these wetlands need to be a prioritized agenda for recommending appropriate measures to restore and conserve wetland ecosystems. In studies pertaining to examine wetlands status, biological diversities particularly macroinvertebrates, fish, and aquatic plants have been widely used as wetland quality bioindicators (e.g., Mereta et al., 2013; Moges et al., 2018; Tampo et al., 2021). Thus, determining the diversity indices of these organisms is crucial for conservation and ecosystem monitoring. In particular, macroinvertebrates with limited mobility and spending up to one year within aquatic ecosystems, which are abundant and main functional feeding groups are good indicators of local conditions and easy to sample (Mereta et al., 2013; Tampo et al., 2021).
While, macroinvertebrates are important biological component of wetland systems and their population changes and community structure affect the function of the ecosystem directly (Tampo et al., 2021). Moreover, macroinvertebrates have rapid response to water environmental change and thus increasingly important in biological monitoring of wetlands (Agboola, 2017; Ferreira et al., 2023; Wondie, 2018). Thus, the assessment of the macroinvertebrate diversity and their abundance in relation with the factors that affect its distribution in the BNW and TSAW is necessary, because macroinvertebrates are widely accepted and irreplaceable biological indicators in monitoring water quality, ecological conservation and management of wetland environments (Hilsenhoff, 1988; Mandaville, 2002; Mereta et al., 2013).
Despite their ecological importance, the biodiversity of macroinvertebrates and fishes are unknown in many in Ethiopian wetlands and no exception for BNW and TSAW wetland ecosystems. Thus, examining the diversity and abundance indices of aforementioned biological entities in relation with water physicochemical parameters is necessary to support a healthy and productive wetland ecosystem.
Diversity indices are mostly employed to measure the species richness and evenness of diversity (Magurran, 2004). Because these indices aid in the interpretation of changes in macroinvertebrate communities used in water quality status examination (Mandaville, 2002), and have advantages over other water quality assessment approaches, such as physicochemical water quality examination modalities, because of their more realistic application under field conditions (Mereta et al., 2013). For macroinvertebrate communities, diversity indices are usually estimated from counts of individuals obtained by using a real unit sampler (Hosokawa et al., 2021). These diversity indices including species richness, Shannon-Wiener index and other evenness indices are diversity indices that are commonly estimated from these variables, and they all tend to decrease with increasing water environmental contamination (Johnston & Roberts, 2009).
Besides, the use of biological composition and diversity of macroinvertebrates, properly measured physicochemical parameters of water quality can also reflect natural water condition (Hong & Chen, 2002; Shabani, 2021). In this paper, therefore, assemblages of macroinvertebrate and fish, water physicochemical parameters and environmental variables were employed and examined, aiming at providing reference for wetland water resources management and pollution control of the BNW and TSAW wetlands in central Ethiopia for proper management and advancing ecosystem health improvement strategies of the two wetlands.
Materials and Methods
The BNW and TSAW wetlands are located in the Angolelana-Tara District of the North Shewa Administrative Zone, Amhara Regional State. These wetlands belong to the Jemma River sub-basin of the upper Abbay Basin in central Ethiopia. The Angolelana-Tara District is situated between 9°15’00’’ to 9°45’00’’N, and 39°19’30’’ to 39°35’0’’E (Fig. 1). The altitude of the district ranges from 1,700 to 3,245 m.a.s.l and is largely classified as a ‘’Dega’’ (humid) agroecological zone. The highest monthly rainfall was observed in July and August, whereas the light and short rainy season was extended from March to April. The mean annual rainfall and temperature ranges between 925–1,240 mm and 6.18°C to 19.8°C, respectively (Getahun et al., 2023). The BNW and TSAW have total areas of 7.49 ha and 499.8 ha, respectively. They host different biological diversities such as birds, fish, aquatic vegetation, and macroinvertebrates. They also support a considerable livelihood through fisheries and irrigation. The most dominant land use in the wetlands is mixed agriculture where livestock grazing in the wetlands and irrigation activities during the short rainy and long dry seasons (Getahun et al., 2023).
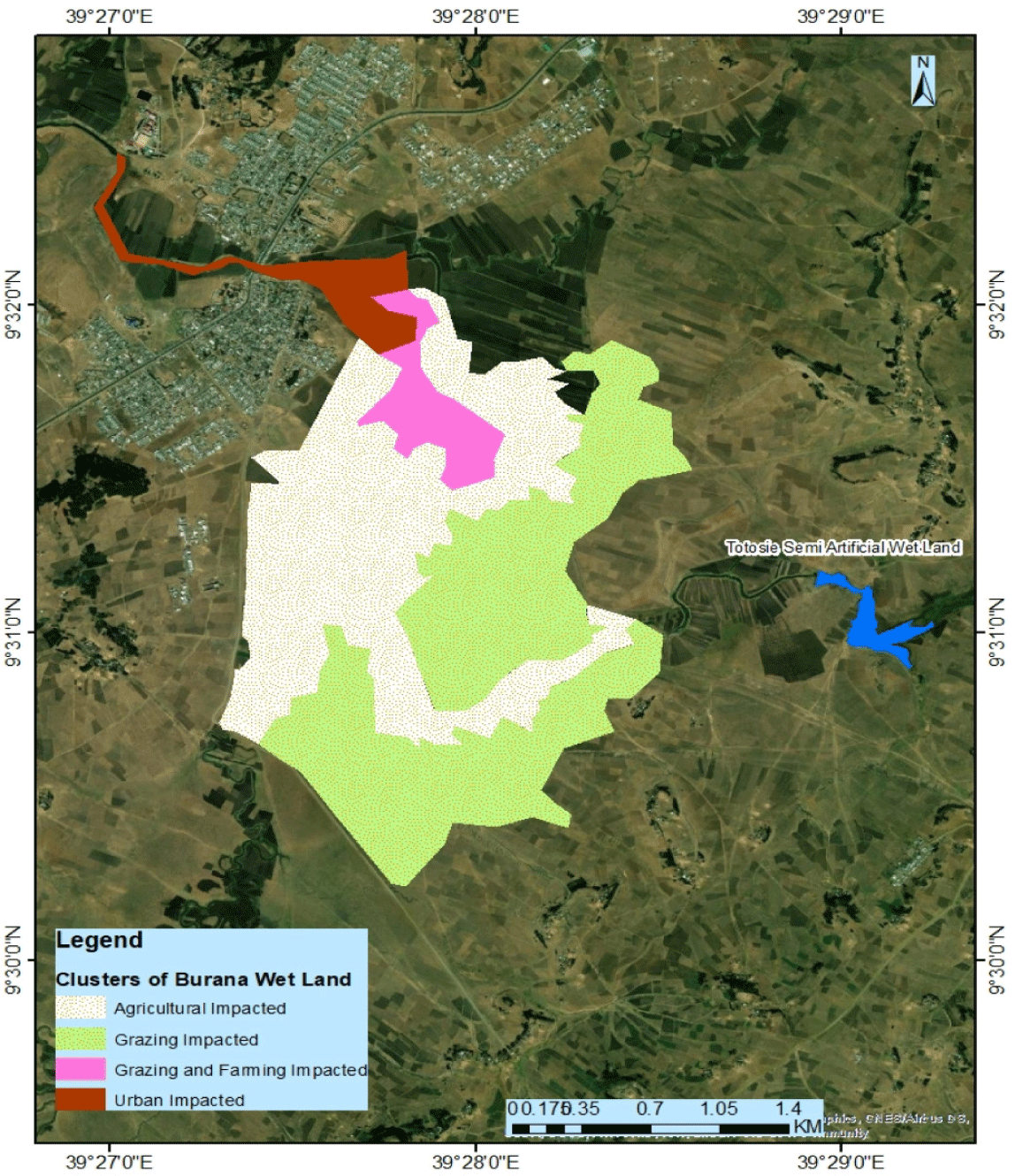
These two wetlands largely span the Burana Totosie semi-urban Kebele (lowest administrative organization). In addition, the BNW also extends towards East of Chacha Town and reaching to another urban Kebele named Kebede Michael. This Kebele is also bordered with Chafana Rural Kebele from northeast direction of Chacha. Therefore, all the local households of Burana Totosie Kebele (699 households) and Chafana Kebele (702 households) totally 1,401 households are direct beneficiaries through vegetable production during dry season irrigation. Local residents along the wetlands support their livelihoods (Belayhun irrigation expert). In our field observation, the Ajema Irrigation Dam, which is under construction on Ajema River (water source of the wetlands) in upstream catchment is expected to scaling up the District’s irrigation potential. Moreover, the BNW is useful for the urban dwellers of Chacha Town being the sources of drinking water. However, urban residents do not directly use the wetlands for irrigation with the exception of garden cultivation at back yards. As a result, the two wetlands were pressurized by human activities, especially due to land use changes (Figs. 2 and 3; pertaining to farm and grazing land via draining the BNW), overgrazing, grass harvesting, and water abstraction for irrigation, urbanization and rural settlement, which have resulted in loss of biodiversity, reduction of natural wetland and water potential, besides being polluted. Especially, the wetland water extraction for agriculture and upstream irrigation projects had reduced the flow of water to a wetland (International Water Management Institute- IWMI, 2014).


A reconnaissance survey was carried out in September 2022 to potentially approach local administrative and officers, observe wetland landscapes, and select sampling sites. Water, macroinvertebrates, and fish samples were collected from each sampling site. Throughout our study period, information from residents confirmed the severity of human pressure on BNW. For this particular study, therefore, BNW was clustered into grazing, agriculture, urban, and less impacted (reference) categories (Fig. 1). Thus, sites in BNW were randomly selected covering each cluster according to the extent of their area size. Thus, we considered clustering systematic random sampling design at about 100m intervals. However, the TSAW was not clustered as its size and disturbance were relatively small and sampling sites followed a systematic sampling design approach.
Water samples for physicochemical parameter analysis were taken along with macroinvertebrate and fish collections. Accordingly, 58 sampling sites (51 from BNW and 7 from TSAW) were considered for water sampling. Among others, pH, electrical conductivity (EC), dissolved oxygen (DO), transparency, and water temperature were measured in-situ using a portable digital Multimeter probe (HQ 40d; Hach, Ames, IA, USA). The concentrations of total nitrogen (TN), ammonia (NO4–N), nitrate (NO3–N), soluble reactive phosphate (SRP), total phosphorus (TP), and total suspended solids (TSS) were determined in the laboratory using a spectrophotometer based on the APHA standard methods (APHA, 1999, 2005). Accordingly, the TN was determined by the Kjeldahl method through digesting 0.5g samples in 10ml concentrated H2SO4, using a catalyst mixture (CuSO4, K2SO4 and Na2SO3) and distillation. The concentration of phosphorus (P) was also determined using the ascorbic acid method, which is the most common method. This method involved using ammonium molybdate ([NH4]6Mo7O24 × 4H2O) and antimony potassium tartrate (K [SbO] C4H4O6 × ½H2O) to react in an acid medium with dilute solutions of orthophosphate to form an intensely colored antimony-phospho-molybdate complex. Soluble chloride ion (Cl−), EC, sodium ion (Na+), and potassium ion (K+) were also considered by this study to examine irrigation water quality. Acid-washed polyethylene bottles with a volume of 1 to 2 L were used for water collection and transportation. The water samples were packed in an ice-box and transported to the laboratory of Debre Berhan University (DBU) for analysis. Besides water parameters, other physical variables such as intensity of grazing/grass harvesting, waste dumping, and farming practices in each sampling station were recorded.
Gravimetric methods (APHA, 1999) was employed to determine the TSS and TDS. Water samples were filtered through pre-weighed glass fiber filter paper to obtain TSS, which was then dried in an oven at 105°C to eliminate any residual water. The resultant sample was placed in a desiccator, until it reached room temperature, and the change in weight was regarded as TSS. TDS was obtained by evaporating the filtrate to dryness in a pre-weighed dish, and subsequently drying it to a constant weight at 180°C. The increase in the weight of the dish after drying was taken as the total dissolved solids. The ascorbic acid method was employed to analyse TP, with a direct reading on a spectrophotometer following persulfate oxidation (APHA, 1999). One-way ANOVA and Tukey’s pairwise comparisons with a 5% significance level were used to compare water quality parameters at different depths within the reservoir.
From all water sampling sites, macroinvertebrate were collected using a D-frame dip net (500 µm mesh). Sweeps were made at each sampling locality, covering different microhabitats (including emergent vegetation and open water areas) to consider all micro-habitats. After collection, macroinvertebrates were sorted in the field, labeled and preserved in 50 mL vials containing 80% ethanol and transported to the laboratory for identification. Identification was made using a stereomicroscope, dissecting microscopes and standard keys (Bouchard, 2012; Gerber & Gabriel, 2002).
Fish samples were collected from five sampling sites (3 from BNW and 2 from TSAW) in April and November 2022. Gillnets, hooks, and long lines were used for fishing. Gillnets had stretched mesh sizes of 4 to 10 cm with a panel length of 25–75 m and a width of 1.5–2 m per mesh size. Gillnets were positioned in the late afternoon (3:00 PM) and inspected the following morning (7:00 AM). Observations of fishers’ collection at fishing sites were also used as supplementary data for this study. All fish specimens were identified to species level based on keys developed by Getahun (2017). Voucher specimens were soaked in 5% formalin and transported to the DBU biology laboratory for further scrutiny. After proper identification was made, representative voucher specimens of each fish species were tagged with important information (e.g., localities, date of collection, and name of collector) and deposited at DBU.
The data collected using primary sources were summarized using descriptive statistics (mean, tables, percentage, and figures) through the application of SPSS version 20 software (IBM, Armonk, NY, USA). To investigate the relationship between macroinvertebrate metrics and environmental variables, Spearman’s rank correlation was chosen because the macroinvertebrate data was not normally distributed and it has been reported that most ecological multivariate data tend not to follow a normal distribution (Moges, 2016; Moges et al., 2017). Analysis of variance (ANOVA) at a 5% level of significance was used to compare the water quality at each site of the wetlands as the assumption of normality was achieved. The analytical results from the water analyses were evaluated against recommended limits and standards set by World Health Organization (WHO) and Food and Agriculture Organization of the United Nations (FAO).
Results and Discussion
The use of physicochemical parameters as a means of evaluating water quality or habitat status is commonly used globally (Abebe et al., 2021; Corry, 2012; Enawgaw & Lemma, 2019; Mereta et al., 2013; Moges, 2016; Tampo et al., 2021; Tekile, 2023). In the present study, water samples were collected at different sites and depths across the sampling sites for physicochemical analysis. Accordingly, some of the water quality parameters of this study were measured on the spot including water temperature, pH, DO, and EC. The mean values of water temperature of BNW and TSAW were 20.43°C and 17.39°C, respectively with a significant difference between the two (Spearman, r = 0.002, each) at p < 0.01 (Table 1). This might be due to the high concentration of heat-absorbent water pollutants in BNW as a result of considerable anthropogenic activities like grazing, grass harvesting, irrigation, and urbanization, which in turn result in silt and nutrient sedimentation, cow dung intrusion, and urban waste dumping. Abebe et al. (2021) and Alsubih et al. (2022) also reported that the aforementioned factors cause a rise in the water temperature of wetlands. In other studies, without leaving the role of climate change, an increase in EC and TSS could contribute increment in water temperature (Moges, 2016).
Descriptive summary statistics of the water quality parameters across the sampled points are presented in Table 1. DO varied between 7.60 and 7.64 mg/L with a small standard error of 0.25. EC varied between 42 to 674 and 46.3 to 96.1 µS/cm, in BNW and TSAW, respectively with the standard error of 14.91 and 8.34 with no statistically significance difference between the two wetlands (Table 1). pH ranged from 5.9 to 8.6, with a mean value of 8.25 and 7.11 in BNW and TSAW, respectively. The variation trends of most of the major and minor ions and nutrients such as Na+, K+, Cl–, TP, NH4-N, TN, and TSS were similar to EC and in the range of natural freshwater quality (ANZECC & ARMCANZ, 2000; WHO, 2004).
The mean pH values of BNW and TSAW were found 8.25 and 7.11, respectively (Table 1), which were laid in the normal water pH ranges (Corry, 2012; Tampo et al., 2021) with a slightly alkalinity nature in BNW. According to Phocaides (2017), the pH as an indicator of water quality should be between 6.5 and 8.4 for normal ecosystem functioning. The mean DO concentration in BNW and TSAW were 7.64 and 7.60 mg/L, respectively, which were optimal for the survival of aquatic organisms in need of this parameter. Moreover, the level of DO can be taken as an indicator of water quality and aquatic ecosystem health (Abebe et al., 2021; Tampo et al., 2021). Thus, FAO (1985) and ANZECC & ARMCANZ (2000) also argued that the level of DO should be above 6 and 5 mg/L, respectively, for performing the reproductive activities of most aquatic organisms, although the mean DO level of 6.42 mg/L indicating the moderate water pollution status of the surface water (Alsubih et al., 2022). The high level of DO in both wetlands could be due to continual aeration from emerging and submerging aquatic plants and from the flowing water nature of rivers crossing these wetlands.
The EC, which is a common parameter that can be used for estimating dissolved ions in water was measured in BNW and TSAW wetlands. The mean values of EC in BNW and TSAW measured for 51 and 7 sampling localities were 110.80 and 63.80 µS/cm, respectively but not differ significantly between the two systems (p > 0.05). These values were laid in the range of natural freshwater systems which could vary between 0.5 and 1,500 µS/cm (Tekile, 2023). The relatively higher EC values in BNW might be attributed to the higher concentrations of ions including Na+, K+, SRP, and NH4-N originating from nearby agricultural activities and wastes dumped into this wetland (Table 1).
The ex-situ water quality parameters were analyzed in the present study. Accordingly, the overall Cl- concentration (29.93 mg/L) was low compared to the standards (106.5 mg/L) set by FAO (1985). The concentration of Cl- was slightly higher in TSAW (30.38 mg/L) but not statistically significant with the results obtained from BNW. In contrast to the present study, the concentration of Cl– was higher in the Modjo River wetland (Tekile, 2023). According to FAO (1985) guidelines, the Cl– concentration in water greater than 353.26 mg/L could pose a serious problem while applying it for irrigation. The concentration of TP was higher in TSAW than in BNW (Table 1). This might be due to weathering of rocks and silt sedimentation as a result of flooding by rivers crossing the TSAW. However, the average concentration levels of K+, Na+, SRP, NO3-N, NH4-N, TN, and TSS were higher in BNW than TSAW with only the K+ statistically differed among the sampling sites of both wetlands (Table 1). This might be attributed to the exclusive engagement of humans in BNW. In a closer comparison, the concentration of Na+ and K+ were very small as compared to standards set for irrigation and drinking water supply, respectively (FAO, 1985; WHO, 2004). The low concentration levels of Na+ and K+ in the studied wetlands might be due to low evaporation and weathering rate as there was the low temperature in the studied area. However, in the Modjo River wetland, these ions were found more concentrated as a result of the growing industries in the Modjo area that discharge untreated or partially treated wastes (Tekile, 2023). In Saudi Arabia, however, an excessive amount of Na+ can occur due to natural sources such as high evaporation and weathering processes, besides intensive fertilizer application (Alsubih et al., 2022). The leaching of salty wastes from the soil by precipitation may also contribute to the water’s chemical composition. In areas with intensive agriculture, fertilization is a major cause of aquifer salinization (Phocaides, 2017).
The NO3-N and SRP were estimated in the studied wetlands. According to Eidson (1993), surface water can be considered as eutrophic when the NO3-N concentration is greater than 0.2 mg/L. The mean values of NO3-N concentration in BNW (2.25 mg/L) and TSAW (1.81 mg/L) were higher than the standards (USEPA, 2004). In this study, the SRP concentration in BNW (0.45 mg/L) and TSAW (0.42 mg/L) were found higher than international standards (USEPA, 2004). The studied wetlands are highly exposed for erosion and river and agricultural runoffs that contribute phosphorus flux from nearby land to wetlands. This implies that, wetlands are continued to accompany state of eutrophication (Phocaides, 2017). Moreover, the results of NO3 and SRP in the studied wetlands indicate a high accumulation of organic and inorganic materials because of the use of phosphate-based fertilizers and cattle-urban waste intrusion from the catchment to the studied wetlands during the main rainy season through flooding.
The mean values of TSS concentration of BNW and TSAW were 313.61 and 268.00 mg/L, respectively. The higher values of TSS in BNW might be because of the extensive human interference in urbanization and settlement, grazing, farming, and water abstraction. Similarly, Alsubih et al. (2022), from Saudi Arabia, reported that besides natural processes, anthropogenic activities including agricultural and farming operations, urbanization, and wastes were the main factors impacting surface water. Hence, unless, wetlands are protected well, they could provide water with poor quality for irrigation, which affects agricultural crop productivity and troubles the health of local people (Alsubih et al., 2022). In conclusion, the results of most physicochemical parameters of BNW and TSAW currently showed poor and good water quality status, respectively as compared to international standards of freshwater ecosystems.
A total of 5,307 individuals of macroinvertebrates are ascribed to 24 families and 10 orders were collected during the entire sampling program of this study (Fig. 4A). Of which, 4,795 and 512 were collected from BNW (impacted site) and TSAW, respectively (Fig. 4A). This finding was in line with the findings reported by Enawgaw & Lemma (2019), but contradicted the reports made by Abebe et al. (2021), who reported the highest abundance of macroinvertebrates in less disturbed sites of the upper Gumara River (Lake Tana sub-basin). The order Isopoda, which accounted for 38.44% of the total order, was the most dominant in BNW while absent in TSAW (Fig. 4B). The 2nd most dominant order was Hemiptera, which accounted for 29.95% of the total dominance, followed by rather Diptera (10.84%), Odonata (9.93%), and Coleoptera (7.80%) in BNW. These orders were represented by a considerable number of individuals in BNW than TSAW (Fig. 4B). The contribution of the remaining orders ranged from 0.08 to 2.21%. In the present study, the least dominant order in its number of individuals was Megaloptera, which only accounted for 0.08% and was not noticed in TSAW (Fig. 4B). The present finding revealed that pollution resistant orders such as Isopoda, Hemiptera, Diptera, and Coleoptera were dominant in BNW, indicating the ecological modification and instability of this ecosystem. Enawgaw & Lemma (2019), also reported the dominance of pollution-resistant families such as Hemiptera, Diptera, Coleoptera, and Gastropoda from Lake Tinishu Abaya (1,835 m altitude) in southwestern Ethiopia. The presence of water stress-resistant orders (e.g., Hemiptera) in wetlands indicates the presence of pollution (Gooderham & Tsyrlin, 2002).
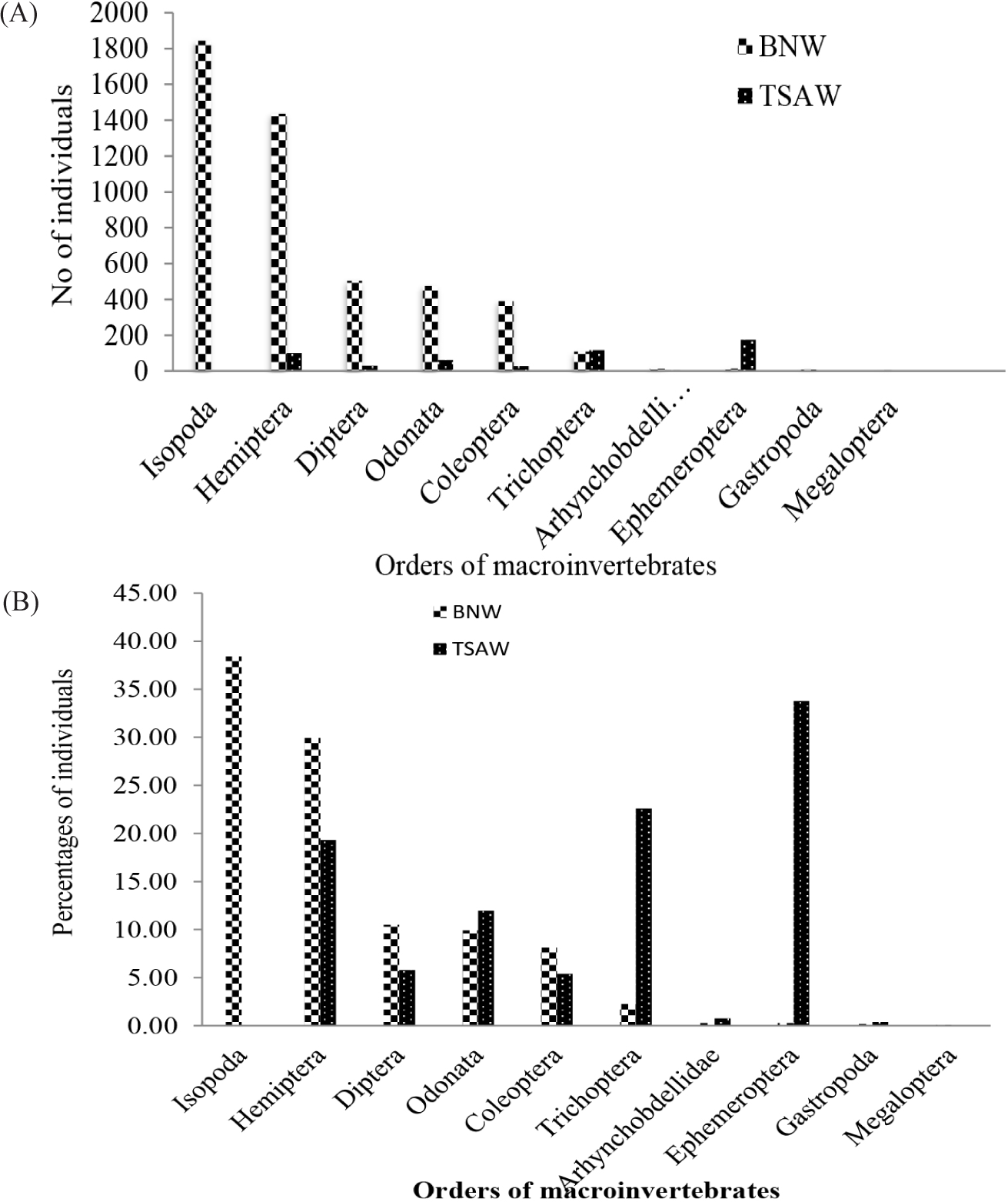
On the contrary, the orders Ephemeroptera and Trichoptera were dominant in TSAW contributing 34.18% and 23.63% of the total macroinvertebrates of the wetland, respectively. Additionally, Hemiptera and Odonata were co-dominant orders and accounted for 17.58% and 12.11% of the total macroinvertebrates sampled from TSAW (Fig. 4B). Hence, close to 70% of the macroinvertebrate individuals collected from TSAW were accounted by Ephemeroptera, Trichoptera, and Odonata, which are pollution-sensitive macroinvertebrates, indicating relatively good ecological status of the TSAW. The occurrence of sensitive taxa such as the Ephemeroptera (Enawgaw & Lemma, 2019), Ephemeroptera, Plecoptera, and Trichoptera (EPT) (Moges, 2016), and Odonata (Tampo et al., 2021) are good indicators in the detection of wetlands water quality in Ethiopia and South Africa, respectively.
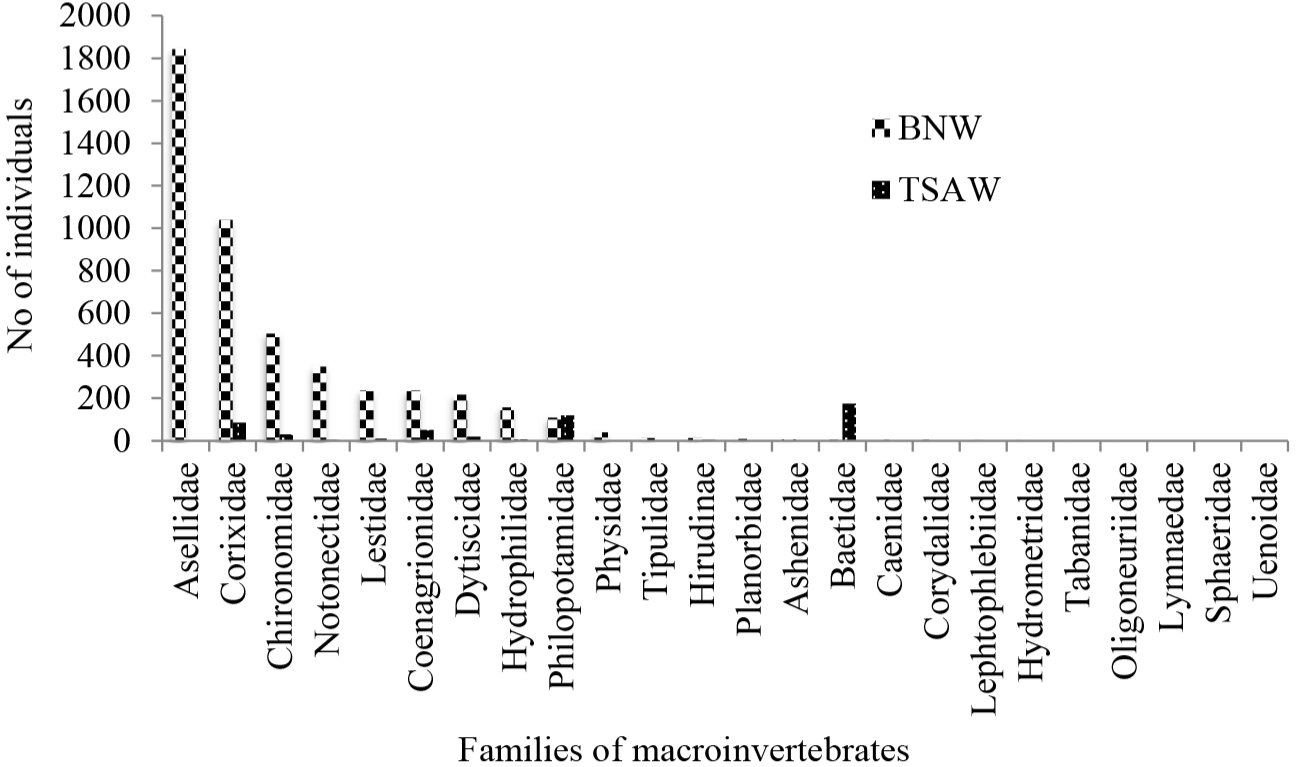
The most dominant family in BNW was Asellidae followed by Corixidae and Chironomidae (Fig. 5). The families of Notonoctidae, Lesticidae, Coenagrionidae, Dysticidae, and Hydrophilidae were found relatively in large numbers in BNW, which might be due to its ecological disturbance and their being pollution resistance (Fig. 2s and 5). The individuals of each remaining families were small, ranging from 0 to 43 in BNW and very degenerate and even many of them were absent in TSAW. In contrast, the TSAW was highly dominated by Baetidae, philopotamidae, and Physidae families, which are pollution-sensitive and good indicators of ecological stability of the wetland ecosystem despite having less taxa richness and total individual abundance of macroinvertebrates as compared to BNW. Based on the results of this study, the TSAW macroinvertebrates’ population was less in family richness and abundance than the BNW, which might be due to the very small area sampling plots considered in TSAW (n = 7) compared to BNW (n = 51). Agboola (2017) argued that the low biodiversity of macroinvertebrates at some of the sites could not be necessarily due to pollution, but rather due to other natural factors such as limited habitat. Enawgaw & Lemma (2019) also reported that pollution-resistant families were more rich and more abundant than, pollution-sensitive ones in Lake Tinishu Abaya, indicating its environmental stresses.
In the present study, a total of 192 fish specimens identified into 3 species were collected from both wetlands. The identified species include Hypophthalmichthys molitrix (Valenciennes 1844), Cyprinus carpio (L. 1758), and Carassius carassius (L. 1758). In TSAW, a total of 147 individuals of fish were collected, while the remaining 45 specimens were collected from BNW. The collection of the highest number of specimens from TSAW might be attributed to its being relatively a less impaired. A similar report was obtained by Abebe et al. (2021), who reported the occurrence of diverse fish fauna in the Wanzaye site due to its being less disturbed site among the wetlands of the Lake Tana sub-basin but did not even catch a single fish from the Shesher wetland located in a similar sub-basin due to its being highly disturbed. In this study, H. molitrix was the most abundant species, accounting for 51.04% of the total collection. C.carpio was the second most abundant species, contributing 32.8% of the total catch, whereas Carassiuscarassius only accounted for 16.2% of the total abundance in the studied wetlands. Moreover, there was a disparity in terms of fish species diversity and species types identified in the studied wetlands and wetlands in the Lake Tana sub-basin (Abebe et al., 2021), which might be attributed to their altitudinal and agroclimatic differences.
In the present study, 22 and 14 families of macroinvertebrates were sampled from BNW and TSAW, respectively (Table 2). However, the mean richness of the two wetlands was approximately equal (– 6) and there was no statistically significant difference between the wetlands (Table 2). The Shannon-diversity index (H’) of macroinvertebrates in BNW and TSAW was 1.07 and 1.22, respectively, despite no significant difference between the two wetlands. Yet, the H’ values of wetlands from southern Ethiopia (with an altitudinal range of 1,656 to 2,028 m.a.s.l.) (Moges, 2016) were generally higher than the current studied wetlands’ (2,756 to 2,780 m.a.s.l.), which might be due to altitudinal difference as altitude increases, species diversity decreases (Golubtsov & Mina, 2003). The evenness values of macroinvertebrates in BNW and TSAW were 0.52 and 0.65, respectively with no significant differences between the two wetlands (Table 2). These all implied that the H’ and distribution (evenness) of macroinvertebrates were higher in TSAW than in BNW despite no significant difference between the two, which might be due to less human impacts on the former. The TASW has a rocky substratum and is surrounded with a nearby grazing landscape and herbaceous riverine vegetation (Fig. 2). This notation is also expounded by the clear connections between an abundance of macroinvertebrates and water quality with the decrease in macroinvertebrate diversity indices’ values along pollution gradients (Agboola, 2017). The similarity (SSC) of the macroinvertebrate composition between BNW and TSAW was also low (41.27%).
Concerning fish taxa diversity indices, the richness (S) and evenness (E) of the BNW and TSAW were the same, but their mean value of BNW and TSAW were 2.67 ± 0.58 and 3 ± 0.00, and 0.91 ± 0.07 and 0.91 ± 0.02, respectively, with no significant difference in terms of richness and evenness between the wetlands (rs = 0.200) at p < 0.05 (Table 2). Yet, the H’ of TSAW was 1.0 somewhat higher than in the BNW (H’ = 0.86), indicating the TSAW had relatively better water and hence suitable for fish, despite still no significant difference (p > 0.05). Generally, the H’ of fish and macroinvertebrates was lower in BNW, owing to higher anthropogenic practices including farming, grazing, waste dumping, and water extraction in the BNW. Abebe et al. (2021), also reported that the H’ ranged from zero (no fish catch) at the largest Shesher wetland to 1.58 at Wanzaye of the Lake Tana sub-basin, Ethiopia, due to extensive agricultural and water drainage practices in the former wetland. Still, the SSC of the fish composition between BNW and TSAW was 100%, indicating the same fish species availability in both wetlands, which might be due to relatively similar ecological setups and enough DO concentration for fish in both wetlands (ranging from 7.60 to 7.64 mg/L).
The relative importance index values of each fish species were calculated and presented in Table 3. Accordingly, in terms of number percentage (%N), H. molitrix (51.04%) was the most abundant, followed by C. carpio (32.8%) and Carassius carassius (16.2%). In the case of weight percentage contribution (%Wt), C. carassius (44.5%) dominated the catch, followed by H. molitrix (31.8%) and C. carpio (23.7%) (Table 3). In terms of frequency occurrence, H. molitrix (100%), C. carpio (75%), and C. carassius (50%) were the most frequently occurring species in the total settings. Based on the rank of percentage importance relative index (%IRI), the present study revealed that C. carassius (47.94%) was the most important and abundant fish species, followed by C. carpio (34.34%). However, H. molitrix had relatively less contribution, which consisted of only (17.72%) of the total catch in the wetlands.
In addition to water physicochemical parameters, macroinvertebrates were used in assessing the habitat quality of the BNW and TSAW wetlands. Physicochemical parameters measure only the water quality of the aquatic ecosystem at the time of sampling, whereas biological indicators measure water’s ecological condition over a long period. For evaluating the present study wetlands, macroinvertebrates at family levels were used based on recommendations made by Mandaville (2002).
Based on the diversity indices, there were differences in terms of richness and H’ between BNW and TSAW with 22 and 14 numbers of taxa, and 1.07 and 1.22 diversity index values (H’), respectively. A higher number of taxa recorded in BNW might be due to the formation of various microhabitats within the wetland itself resulting from anthropogenic disturbances, which could support diverse types of macroinvertebrate taxa, but not in terms of taxa abundances. As the number and distribution of taxa within a community increases, the H’ value also tends to go up similarly (Corry, 2012). Generally, the H’ was low in both wetlands, but with relatively higher H’ was calculated in TSAW, indicating less ecological disturbances in this wetland. The SSC of the macroinvertebrate family composition between the two wetlands (41%) also indicated their low similarity and a big difference in water quality. Tampo et al. (2021) also confirmed that macroinvertebrate indices are sensitivity tools to measure water quality differences and level of anthropogenic disturbance.
The Hilsenhoff family biotic index (H-FBI) is determined by multiplying the number of individuals in each family with the tolerance value for that family, summing up the products, and then dividing by the total macroinvertebrate individuals of all families of the study site (Hilsenhoff, 1988). Accordingly, the H-FBI values for TSAW and BNW were 5.44 and 7.57, respectively, indicating their fair and very poor water quality, respectively, in terms of the extent of organic pollution in water. In other words, the water of BNW was severely polluted, whereas the water of TSAW was fairly polluted with organic matter. This is because the BNW was highly disturbed by human activities such as farming, grazing, and irrigation activities in the nearby rural kebeles (lowest administrative setup), and waste releasing and dumping from settlers of Chacha town. The H-FBI could differentiate the water quality status of impaired from unimpaired wetlands of Ethiopia (Moges, 2016).
The ratio of EPT/Chironomidae was also determined for evaluating the water quality of the wetlands. Accordingly, the results of EPT/Chironomidae in TSAW was 9.87, indicating a high water quality in the TSAW. In contrast, the results of EPT/Chironomidae in BNW was 0.24, which indicates the EPT abundance and distribution was very low in this natural wetland, which in turn indicated that the water quality of the wetland was highly impaired. The EPT including Odonata, can detect some water quality parameters (Tampo et al., 2021). Corry (2012) also reported water quality status based on the EPT/chironomidae ratio as a biological indicator.
The Percent dominant family (%DF) index can be calculated as the total number of individuals of the dominant family relative to the total number of organisms in the sample (Mandaville, 2002). According to Barbour et al. (1999), when a community is dominated by relatively few families, it would have a high %DF, which shows that the community is pressurized under environmental stress. Based on this principle, the first three most dominant families in the BNW were Asellidae, Corixidae, and Chironomidae, in order, and the total individuals accounted by these three dominant families together, were 3,387, of the total 4,795 individuals of all 10 families in the wetland. Thus, these three dominant families took the lion’s share (70.64%) to the total individuals of macroinvertebrates found in BNW. Generally, these three dominant families are pollution-resistant (Mandaville, 2002) and thus, indicating poor water quality state of the BNW. Contrarily, the most dominant families in TSAW were Baetidae and Philopotamidae which accounted for 295 individuals out of the total 512 individuals of macroinvertebrates identified from TSAW. This means that only these two families largely contributed (57.62%) to the total individuals of macroinvertebrates found in TSAW, indicating the community of arthropods was dominated by few pollution-sensitive families, resulting from relatively pure water quality of the wetland, which is still contrary to the conclusion made by Barbour et al. (1999). Thus, this result pointed out that when the wetland/water quality is good, the aquatic ecosystem is mostly dominated by a few pollution-sensitive families of macroinvertebrates, and vice versa.
This study assessed the water quality and biodiversity state of BNW and TSAW in Angolelana-Tara District using water physicochemical parameters and macroinvertebrates as a bioindicator. As the results revealed, there was a significant difference in some water quality parameters such as water temperature, K+, and TN recorded from BNW and TSAW, at p < 0.05. The water quality of BNW was relatively impaired due to extensive human activities. On the other hand, the overall H’ for both macroinvertebrates and fish in both wetlands were low, indicating low biodiversity, which in turn shows the ecological and hydrological modifications resulting from human activities. However, before we reach a bold conclusion, a full ecological image of the wetlands should be documented. Therefore, studies like land use and land cover change and soil nutrient analysis need to be conducted to produce comprehensive data about wetlands for policymakers to make informed decisions for the restoration and sustainable utilization of the wetland resources. Moreover, alternative income-generating opportunities for the local people, particularly the young, and establishing bylaws and guidelines on sustainable uses of wetland resources should be designed and implemented. Still, awareness and training on the benefits of the wetland resources and strategies used to minimize brutal human-made activities should be given to the local community and other stakeholders.
