Introduction
Polyploidy engineering is an engineering in aquaculture that aims to improve fish farming performance, such as accelerating growth, increasing resistance to disease (Zhou & Gui, 2017), enhancing adaptability, and building stronger resistance compared to parents (Arai & Fujimoto, 2018). Several studies have shown that polyploidy engineering that is often used to improve fish farming performance is triploidization, which produces infertile fish with 3 sets of chromosomes. This method is often used to overcome slow growth due to early sexual maturation. Given that triploid fish are infertile, reproductive energy is diverted to somatic body growth, thereby increasing fish farming production (Lee et al., 2023). Although triploidization can be carried out through physical, chemical, and hydrostatic pressure shock, triploid induction through shock it is difficult to implement on a mass scale, is less efficient, and produces varying triploid fish (Hartono et al., 2016).
Another method to produce triploid fish is through crossing tetraploid with diploid fish (Nascimento et al., 2020). This method can reduce the negative impacts of shock given during the triploid induction process. The tetraploid fish induction is an intermediate step to mass production of tetraploid broodstock, which is then used to produce triploid fish. Induction of tetraploid fish has been successfully developed in various species, including Mercenaria mercenaria (Yang & Guo, 2006), Pangasius hypophthalmus (Hartono et al., 2016), Acipenser ruthenus (Fopp-Bayat et al., 2022) by heat shock treatment. Cold shock treatment has also been implemented in Misgurnus mizolepis (Nam et al., 2004), P. hypophthalmus (Syahril et al., 2020), and Clarias gariepinus (Okomoda et al., 2021). In addition, hydrostatic shock has been used in trout (Salvelinus fontinalis) (Weber et al., 2015) and turbot (Scophthalmus maximus) (Wu et al., 2019). However, these methods have various limitations, such as producing high embryo and larval abnormalities, which causes decreased hatchability, and survival (Alcántar-Vázquez et al., 2016).
In addition to temperature and hydrostatic pressure shock, tetraploid induction can be carried out using electric shock. Application of electric shock for tetraploid induction has not been widely carried out, but several studies have successfully applied the method in polyploid induction, such as in oysters and mussels (Cadoret, 1992), coho salmon (Teskeredz̆ić et al., 1993), and red hybrid tilapia (Hassan et al., 2018; Okomoda et al., 2020). Teskeredz̆ić et al. (1993) reported that electric shocks (10 V/m) with a duration of 10 min in coho salmon with varying intervals resulted in 100% triploids and a high hatchability rate. In addition, the use of an electric field of 600 V/cm before the first cell division was completed led to the induction of triploidy in oysters (55%) and mussels (36%), as well as tetraploidy of 20% and 26%, respectively (Cadoret, 1992). Electric shock method has a high polyploidization rate and low deformity. Tetraploid induction by electric shock is cheaper than other methods because the equipment is more affordable, and provides more consistent results due to the ease of its application homogeneously to eggs (Hassan et al., 2018). It can also be an alternative for mass production of triploid fish, by utilizing gametes from tetraploid individuals fertilized with diploid sperm (Weber et al., 2015). This method minimizes the negative effects of the retention of the 2nd polar body, thereby increasing survival (Lebeda & Flajshans, 2015).
According to previous studies, bagrid catfish (Hemibagrus nemerus, Valenciennes 1840) is one the freshwater species with high economic value as a source of animal protein, high meat quality, low-fat content, high nutrition, and delicious and savory meat taste (Huang et al., 2023). Bagrid catfish has been widely cultivated in ponds or floating net cages, but its cultivation is constrained by slow growth due to the reproductive process in the pre-adult phase. Improving the growth performance of bagrid catfish can be carried out through breeding approach, such as genetic engineering, hybridization and polyploid engineering (Huang et al., 2023). Tetraploidization is a ploidy engineering that can be carried out to increase growth through crossbreeding with diploid fish for mass production of triploid (Weber et al., 2015). Despite the potential, study activities on the application of electric shock in the formation of tetraploid bagrid catfish have never been carried out. The success of tetraploid induction through shock is largely determined by the time of the treatment after fertilization, the duration of the shock, and the intensity (Bidwell et al., 1985; Okomoda et al., 2021). The time and duration of shock must be determined accurately to maximize the formation of tetraploid fish (Bidwell et al., 1985). Therefore, this study aims to determine the effect of shock time and duration on the formation of tetraploid bagrid catfish, fertilization rate, hatching rate, survival rate, and larval abnormalities. Tetraploid fish are identified based on the number of nucleoli, the erythrocyte size, and DNA content.
Materials and Methods
The bagrid catfish broodstock came from the Polifish Farm Production Unit of the Politeknik Negeri Lampung, Indonesia. The weight of the female and male broodfish used ranged from 0.5 to 0.8 kg per fish. The study design used a completely randomized factorial design with two factors. The first treatment factor was the time of electric shock after fertilization at minutes 26, 27, 28, 29, and 30 and the second treatment factor was the duration of the shock for 6, 8, and 10 min. Each treatment was repeated three times. The study procedures were conducted at the Politeknik Negeri Lampung, Indonesia.
Brood ovulation was induced hormonal treatment. Female and male fish were injected with Ovaprim® (Syndel Laboratories, Nanaimo, Canada) intramuscularly at a dose of 0.5 mL/kg. After injection, broodfish were incubated in a 1 × 1 × 0.3 m3 fiber tank for 10–12 h. Eggs and sperm were obtained by the stripping process, in which eggs were fertilized by mixing sperm and then spread on a plastic filter (Φ20 cm), incubated in an aquarium that was aerated, and a heater as a temperature regulator at a temperature of 28°C. This process was repeated with 3 pairs of broodfish in 3 replications, where each pair of broodfish was used for 1 replication.
Electric shock was given to bagrid catfish embryos according to each treatment and used a constant electric field shock power of 12 V/m from a Century Rechargeable Battery (12 V, Lead Acid, 34 Ah, Bolt) referring to Hassan et al. (2018). Rectangular electric probes (positive and negative ends), each measuring 40 cm connected to the battery were placed on opposite edges of the aquarium (80 × 60 × 40 cm3) at about 3 cm below the highest water surface (water depth = 30 cm). In addition, to ensure the strength of electric field in the media and the duration of shock, it was monitored using a voltmeter and stopwatch. Salt was added to shock media water to a salinity of 5 ppt and controlled using a refractosalinometer to increase the transmission of electric field. Electric shock was carried out by inserting a plastic filter into the media that was supplied with electric voltage according to the time and duration of shock. The number of embryos used for each treatment ranged from 200–300 embryos. After treatment, the embryos were incubated in a 40 × 40 × 40 cm3 aquarium filled with water with a volume of 32 liters and aerated at a temperature of 28°C until hatched.
Bagrid catfish larvae were kept in an aquarium until fish were 30 days old. Feeding in the form of artemia nauplii was carried out after the catfish were 3 to 10 days old adlibitum with a frequency of 6 times a day. After 10 days, the larvae were fed silkworms until 30 days old. In the larval maintenance media, siphoning and water exchanges of 20%–30% were carried out every 2 days to maintain high quality water condition. After 1 month, bagrid catfish seeds were kept in a round tarpaulin tub with a diameter of 1.5 m and a water depth of 70 cm with a density of 50 fish m–2. Fish are kept until they are 3 months old. Fish are fed commercial pellets containing 40% protein adlibitum with a frequency of three times a day. In the maintenance media, siphoning and water exchanges of 20%–30% are carried out every 5 days to maintain the condition of the media clean. Ploidy observations are carried out at the end of maintenance.
The success of tetraploid induction of bagrid catfish was determined based on the maximum number of nucleoli per cell (Bidwell et al., 1985), erythrocyte size (Hassan et al., 2018; Normala et al., 2016), and DNA content (Adan et al., 2017). Nucleolus preparation using silver nitrate staining (Kim et al., 2017) and blood smear preparation and blood smear staining using Giemsa (Jayaprasad et al., 2011). A total of 40 fish were examined for each treatment repetition. The observation results were tabulated to calculate the maximum number of nucleoli for each nucleus per preparation. Erythrocyte measurements were also carried out on the same fish as those used in nucleolus number analysis.
The results of ploidy analysis based on nucleolus and erythrocyte were verified by flow cytometry analysis according to the method described by Adan et al. (2017) and Allen (1983) with some minor modifications as described below. Red blood cells of 60-day-old bagrid catfish were taken from the cauda ventralis using a 1 mL syringe. A total of 0.2 mL of blood is inserted into an Ethylenediaminetetraacetic acid (EDTA) tube containing 1 μL of 10% kalium EDTA. The blood suspension is kept stable at a temperature of 4°C for about 5 min. Blood cell samples were then cultured in a condition of 70%–80% confluence for harvesting and cell dilution with culture media (Penicillin-streptomycin 1; Fetal bovine serum [FBS] 10%; Media [RPMI/DMEM] Ad 100%). Blood cells were transferred into well plates filled with 1,000 μL each, consisting of 5 × 105 cells/well then incubated for 12 h. The cell media was discarded and then washed with 500 μL of phosphate buffer saline (PBS). A single treatment requires a 1,000 μL sample concentration series to be inserted into the well and the control well is created by adding 1,000 μL of culture media subsequently stored in a CO2 incubator. Flowcytometry reagents were prepared with a composition of 25 μL of propidium iodide (PI), 1 μL of RNase, 0.5 μL of Triton-X 100 (pro GC, Merck, Darmstadt, Germany) and 500 μL of PBS. The sample was added 200 μL of trypsin-EDTA 0.25% and incubated for 3 min. Next, 1 mL of culture media is added to the suspension. The well was refilled with 500 μL of PBS and was digested at 32×g for 5 min, then the supernatant was discarded. Cell pellet washing is carried out by adding 500 μL of cold PBS, re-centrifuged (32×g for 5 min) and then the supernatant is discarded and. dripped 500 μL of 70% alcohol 1 drop per second. Next, it is stored at room temperature 37°C for 30 min. After completion, centrifuged at 32×g for 5 min, the supernatant is discarded, then 500 μL of PBS is re-centrifuged at 358×g for 3 min, the supernatant is discarded. Flowcytometry reagents are added and let stand for 30 min to suspend. The cell suspension was transferred into a flowcyto tube and prepared samples were analyzed by excitation with an argon ion laser set at 488 nm wave length, readings were performed with a BD FACS FlowTM Cytometer (BD Biosciences, Franklin Lakes, NJ, USA) to determine the cell cycle profile. Detection is carried out on 10,000 or 20,000 cells. Flowcytometry data was analyzed with the cell quest program to see the distribution of cells in the cycle phases of sub-G1 cells (apoptosis), S, G2/M, and cells undergoing polyploidy. The characteristics of the cells that can be observed directly are based on the detection received by the detector, i.e., forward side scatter-height (FSC-H); side scatter-height (SSC-H,).
The parameters of this study include tetraploid individuals, fertilization rate (FR), hatching rate (HR), survival rate (SR), and the number of abnormal larvae (AB). Observation of egg fertilization was carried out 6–8 h after fertilization. Fertilized eggs are characterized by a transparent clear color while unfertilized eggs are white and are calculated using the equation (Kurniaji et al., 2018);
Egg hatching was observed 24 h after the eggs were hatched and calculated by comparing the number of eggs that hatched with the number of eggs that were fertilized (Kurniaji et al., 2018).
Observation of survival rate was carried out every 10 days until the larvae were 30 days old. Survival rate was carried out using the Equation (3) (Kurniaji et al., 2018).
Observation of abnormal larvae was carried out by observing the body morphology of 3-day-old larvae. The percentage of abnormal larvae was calculated using the equation (Mukti, 2005).
Data on the percentage of tetraploid individuals, fertilization rate, hatching rate, survival rate, and abnormal larvae were analyzed using the two-way analysis of variances (ANOVA) method, with Least Significant Difference (LSD), using Statistical Tool for Agricultural Research (STAR) version 2.0.1.
Results
The effect of electric shock treatment on fertilization rate, hatching rate, survival rate (SR-30), larval abnormalities, and tetraploid percentage were presented in Table 1. The results showed that there was no interaction between shock time and shock duration on fertilization, hatching, survival (SR-30) and abnormal larvae (p > 0.05). Egg fertilization was not affected by the treatment of shock time and shock duration (p > 0.05). The fertilization rate ranged from 81.10% to 85.23% while the control was 90.21%. Hatching rate was not affected by the shock time (p > 0.05) but was affected by the duration of the shock (p < 0.05). The study results indicated that the longer the shock time had an impact on the decrease in hatching rate of bagrid catfish (p < 0.05). The highest hatching was found at shock duration treatment of 6 min (79.01 ± 2.04%), the lowest at 10 min (58.85 ± 1.98%) and control (84.19 ± 2.02%). Survival (SR-30) showed a difference between shock time and shock duration with control (p < 0.05). The survival rate treated with electric shock after 30 days of maintenance ranged from 63.66% to 71.36%, while the control was 79.18%. Based on abnormal larvae parameters, there was an effect of shock time and shock duration treatment on abnormal larvae (p < 0.05). The test results showed that there was a difference in abnormal larvae in the treatment of shock time and duration of shock with control. However, between the shock time treatments (minutes 26, 27, 28, 29, and 30) did not show any difference in abnormal larvae. Likewise, the shock duration treatments (6, 8, and 10 min) did not show any difference to abnormal larvae. Abnormal larvae increase with increasing embryo age and shock duration. The abnormal larvae formed in the treatment ranged from 1.82% to 3.98%, while the control was 0.29% (Table 1).
Tetraploid fish were identified based on the maximum number of nucleoli per cell (Fig. 1A and 1B) and the size of erythrocytes (Fig. 1C and 1D). Fig. 1 showed that the diploid bagrid catfish had a maximum number of nucleoli of 2 (Fig. 1A), while tetraploid bagrid catfish had a maximum number of 4 (Fig. 1B). Table 2 showed the difference in the size of erythrocytes of tetraploid bagrid catfish and diploid bagrid catfish. The cell volume of tetraploid bagrid catfish was 1.97 times larger than the cell volume of the diploid bagrid catfish. In addition, the volume size of tetraploid bagrid catfish nucleus was 2 times larger than the cell nucleus of diploid bagrid catfish.
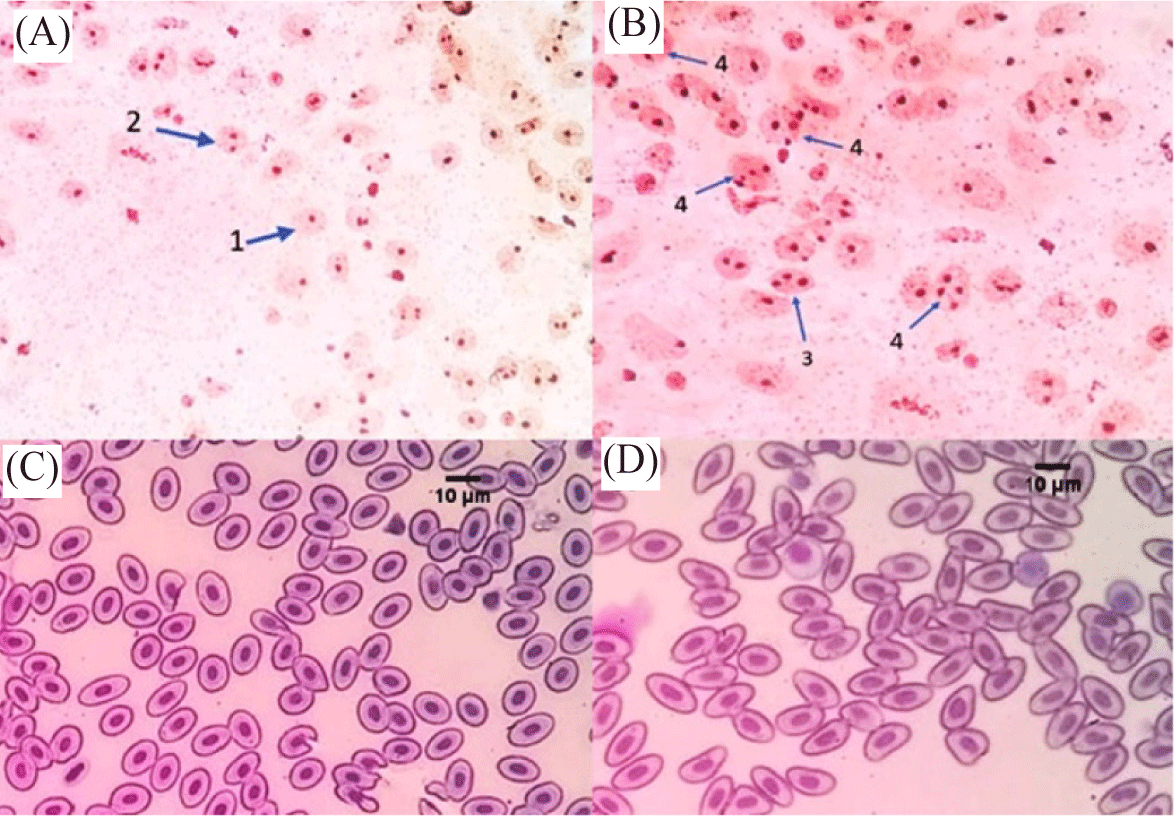
Fig. 2 shows the relative locations of the different fluorescence histogram peaks for diploid and tetraploid individuals. Based on Fig. 2, it shows that tetraploid bagrid catfish has twice the DNA content of diploid bagrid catfish.
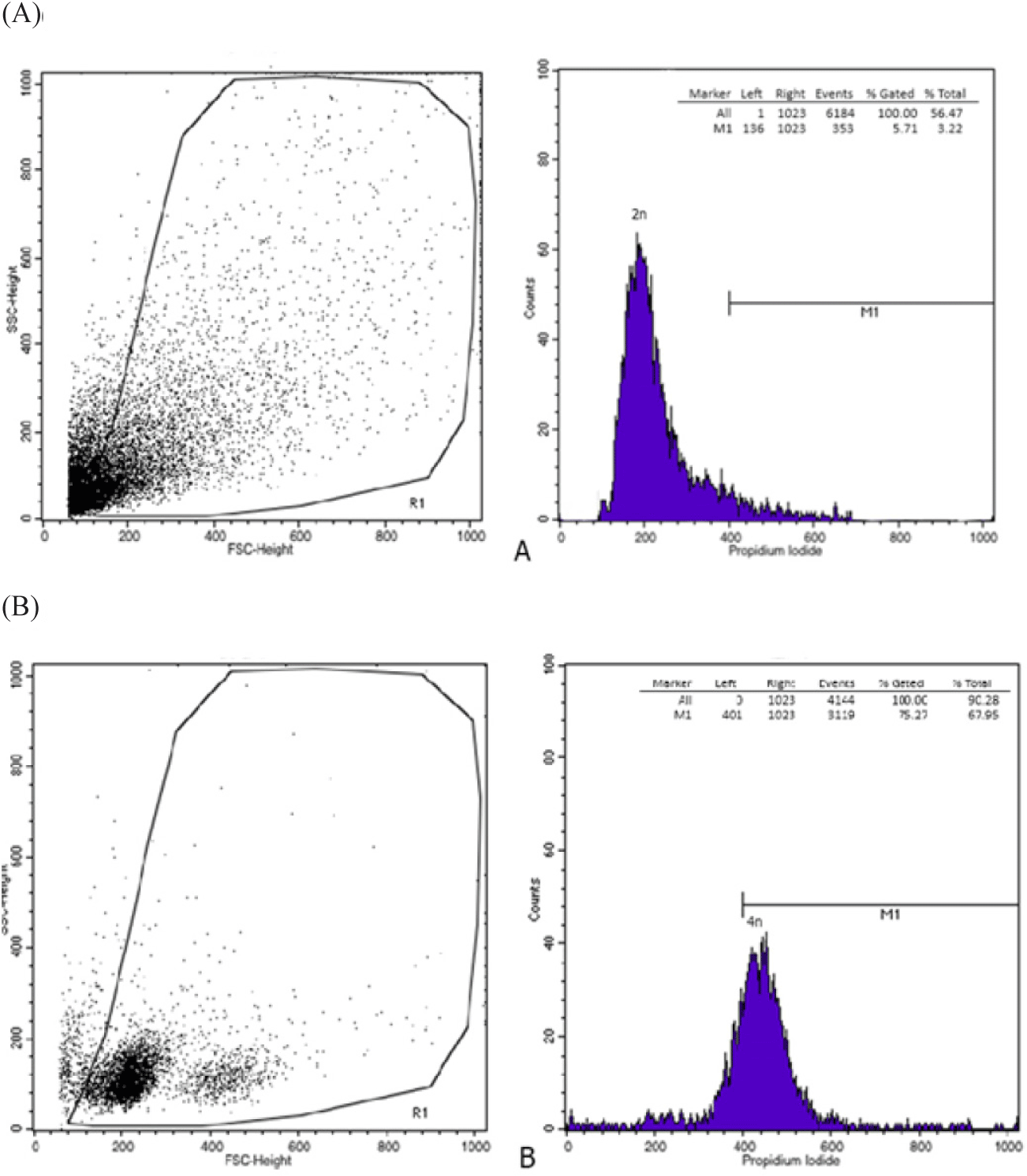
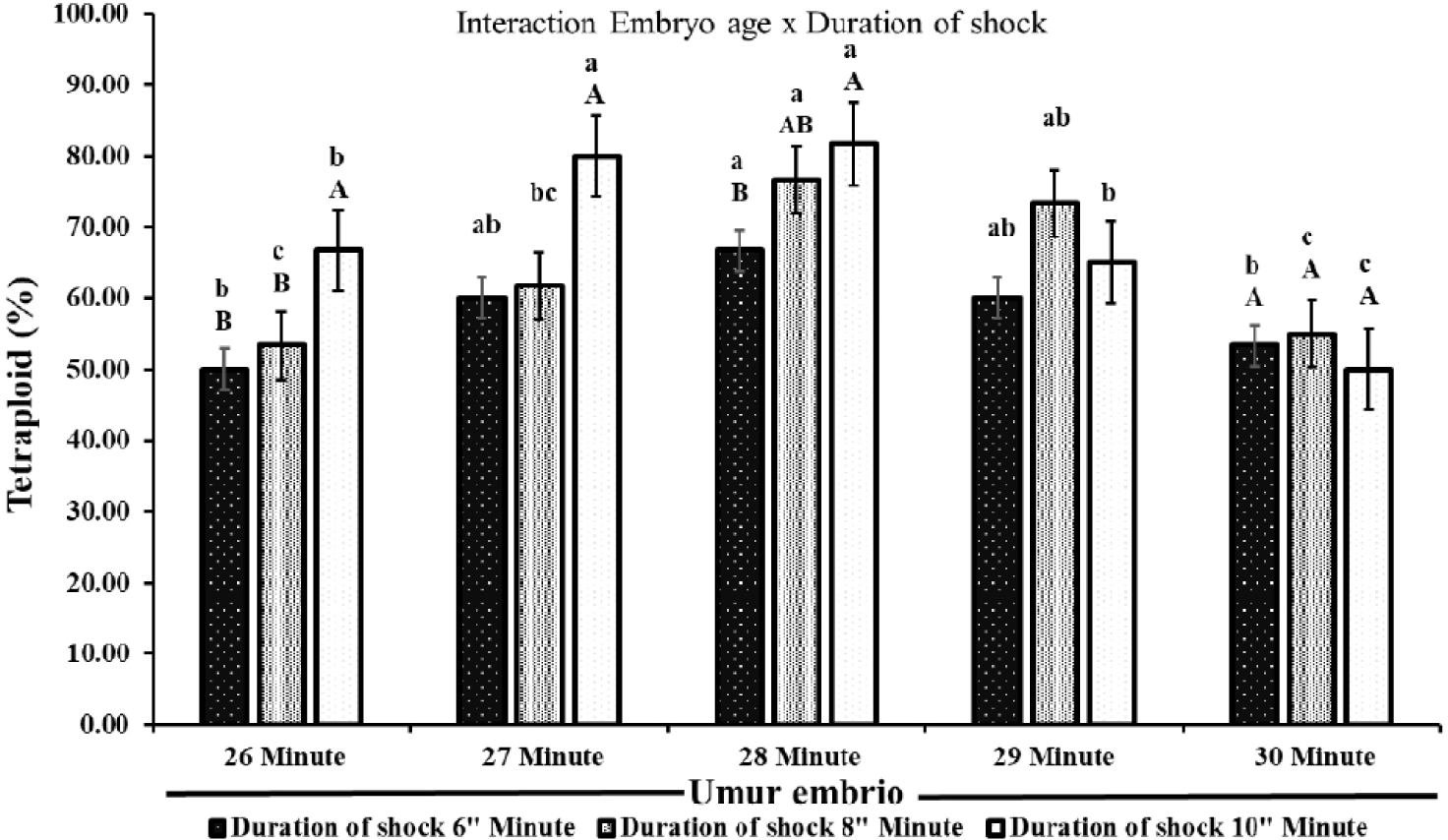
Fig. 3 showed that the treatment of shock time, duration shock, and interaction between treatments affected the percentage of tetraploid catfish formed (p < 0.05). The percentage of individuals obtained in electric shock treatment ranged from 50.00%–81.67%. The treatment results indicated that the percentage of tetraploid increased until the 28th minute of electric shock, then decreased in the 29th and 30th-minute treatments. Furthermore, the rate of tetraploid fish individuals increased with increasing duration of electric shock until the 28th minute treatment. At the 29th and 30th minutes, the longer duration shock decreases the percentage of tetraploid catfish. As shown in Fig. 3, there was an interaction between embryo age and duration of electric shock in producing tetraploid individuals. Electric shock treatment at 28 min and shock duration of 10 min resulted in the highest percentage of tetraploids at 81.67% (Fig. 3).
Discussion
The results of the study showed that electric shocks can inhibit the first mitosis division hence it can produce tetraploid fish. Tetraploid fish were found in all electric shock treatments with varying results. The highest percentage of tetraploid bagrid catfish was obtained through shocks given at 28 min after fertilization. This shows that electric shocks given just before the first mitosis effectively disrupt chromatid separation and cytokinesis, thus preventing the formation of division grooves (Ihssen et al., 1990) and producing tetraploid embryos with twice the number of chromosomes (Fopp-Bayat et al., 2022; Wu et al., 2019). Arai & Fujimoto (2018), Ihssen et al. (1990) stated that tetraploid fish can be produced through shocks just before the first mitosis division.
The results of this study indicate that electric shock treatment (time, duration of shock, and interaction between treatments) has an effect on the formation of tetraploid fish (p < 0.05). Based on the shock time treatment, the shock at 28 min produces the highest tetraploid (81.67%). Tetraploid formed in the treatment before and after the 28 min tend to decrease. This shows that it is the most effective time to give shocks to produce tetraploid individuals. In addition, the 28 min is the peak of the first mitosis in catfish. According to Yang & Guo (2006), the formation of tetraploid fish is effectively carried out during the peak period of the first mitosis. Tetraploid fish from all treatments obtained ranged from 50.00%–81.67%. However, the tetraploid fish formed did not reach 100%. The inability to achieve 100% tetraploid induction can be caused by several interrelated factors, including the time and intensity of the shock and the variability of embryo development. Shocking should be performed just before the first mitosis division. Inappropriate timing can result in induction failure or produce diploid embryos (Hershberger & Hostuttler, 2007). This is appropriate to Pandian & Koteeswaran (1998) who stated that after the first cell division, the cells enter the anaphase and telophase phases, where the chromosomes are separated causing the shock to no longer have an impact on the formation of tetraploid fish. In addition, variations in the results of tetraploid fish that do not reach 100% are caused by differences in embryo development (Hassan et al., 2018) resulting in uneven distribution of the shock effect and various tetraploid fish formed. Some eggs may not have entered the first cell division phase, while others have passed that phase when the shock is given (Teskeredz̆ić et al., 1993). If the electric shock is applied at the right time, it can effectively inhibit this extrusion, allowing the fertilized egg to maintain both sets of chromosomes.
The results showed that in all treatments, the shock duration of 10 min produced the highest percentage of tetraploids compared to the durations of 6 and 8 min. This shows that longer shock durations are more effective in producing tetraploid fish. However, increasing the shock duration can also have a negative effect on egg hatching. Hatching rate obtained in treatments with longer shock durations tends to decrease. The results kind of showed that shorter shock times made a low tetraploid percentage but with higher hatchability. On the other hand, long shock times might reduce the hatchability of eggs but will lead to more tetraploids. An increase in the shock duration will be detrimental to the survival of the treated egg (Okomoda et al., 2021; Oliver et al., 2020). In addition to the impact due to the electric field, the decrease in egg hatching is also due to the saltiness of the shock water. Salinity in the shock medium can mess up the embryo’s body functions and could even kill the embryo (Lingam et al., 2024). Bagrid catfish embryos can tolerate salt up to 2 ppt, but as the salinity increases, their survival goes down (Hadid et al., 2014). Electric shocks mess with hatching rates, but using them to induce tetraploidization of catfish is still better than other methods. For example, when heat shock was used on yellowtail tetra fish (Astyanax altiparanae), the hatching rate went down to 10.3 ± 6.2%, compared to 62.2 ± 7.7% in the control group (Nascimento et al., 2020). Heat shock when inducing carp polyploidy also made the hatching rate really low, down to 11.10 ± 8.60% (Mukti, 2005). In the study, the highest tetraploid number happened during the 28th-minute shock, with the shock lasting 10 min after fertilization. The results seem to say that electric shock is a possible method to create tetraploid fish. This study matters because tetraploid bagrid catfish might help produce triploid fish in large amounts without any negative effects from the direct shock (Lebeda & Flajshans, 2015).
The electric shock given at the age of the zygote 26–30 min after fertilization did not affect fertilization rate (p > 0.05). This is because the shock treatment was carried out after fertilization, hence, the electric shock did not affect the process. The effect of shock on the polyploidy process will have an impact on the process after the shock and will not have an impact on the process before the shock (Hassan et al., 2018). In addition, electric shock can increase the formation of abnormal larvae. The formation of larval abnormalities after electric shock treatment can be caused by aneuploidy (Felip et al., 2001). The addition of chromosome sets can disrupt the anaphase process, which then affects the alignment of the mitotic spindle in the early stages of cell division, changes the structure of microtubules, and ultimately modifies the distribution of genetic material and cytoplasmic volume among daughter cells (Voronina & Wessel, 2006). Due to shock, intracellular proteins involved in the formation of microtubules can be disrupted, causing aneuploidy or disruption of the distribution of certain proteins in dividing cells.
According to Haniffa et al. (2004), shock given to embryos can cause abnormalities in larvae. Some abnormalities found include short and curved tails, spinal abnormalities, and asymmetrical body shapes. Although electric shock in tetraploid induction causes larval abnormalities, the percentage of larval abnormalities obtained in this study was lower than other shock treatments. Heat shock treatment in catfish can produce larval abnormalities between 12.36% and 27.60% (Hartami et al., 2018) and in carp (Cyprinus carpio Linn) which reaches 24.86 ± 8.37% (Mukti 2005). In addition, electric shock also does not affect the survival of larvae (p > 0.05). The survival of larvae produced in this study ranged from 63.66%–71.36% and was lower than that of yellowtail tetra (Astyanax altiparanae), which produced a larval survival of 19.1% (Nascimento et al., 2020), and channel catfish of 40% (Bidwell et al., 1985).
The identification of the ploidy of bagrid catfish in this study was carried out by observing the number of nucleolus (NOR) and the comparison of erythrocyte size verified by flow cytometry. Tetraploid bagrid catfish individuals have a maximum number of 4 nucleoli per cell, while diploids have a maximum of 2. Also, the cell volume and the nucleus of erythrocytes in tetraploid fish are 1.97 and 2 times bigger than in diploid bagrid catfish. Tetraploid C. gariepinus have an increase in cell volume and nucleus too, by about 94.65% and 91.6%, according to Okomoda et al. (2021). In Misgurnus anguillicaudatus fish, the size of tetraploid erythrocytes is about twice as large as the diploid ones (Gao et al., 2007). The resulting fish ploidi was verified using flowcytometry. The verification results showed that the ploidy level based on the NOR and the comparison of erythrocyte size of fish were in accordance with the results of verification with flowcytometry. Identification using flow cytometry showed that tetraploid fish had 2 times greater DNA content than diploid fish. This increase in DNA content is a consequence of the addition of genetic material in tetraploid fish. In addition, tetraploid cell size shows an increase in cell volume and nucleolus as a result of the addition of genetic material at a higher rate of ploidy (Hardie & Hebert, 2003). According to Fiske et al. (2019), the identification of ploidy based on erythrocyte nucleus volume is more efficient and has a level of accuracy such as flow cytometry.
In addition to determining the number of DNA using flow cytometry, the determination of ploidy can also be done based on the NOR and the ratio of erythrocyte size. In addition, this method provides advantages by not having to kill the sample fish, thereby increasing the rearing potential of the resulting tetraploid fish. Erythrocyte size for ploidy identification in fish is an effective method apart from being easier, faster, cheaper and without the need to sacrifice fish samples (Normala et al., 2016). The results of this study show that causing erythrocyte size ratio can be used to distinguish the degree of ploidy in fish (Normala et al., 2016; Okomoda et al., 2020).
The study showed that using electric shocks before the first cell division in tetraploid induction effectively produces tetraploid catfish. Additionally, applying electric shocks can minimize negative impacts on fertilization, larval survival, and larval abnormalities. Although the best electric shock treatment has been obtained in producing tetraploid catfish, the success of tetraploid catfish induction can be further improved by standardizing the shock power and duration of the electric shock. The study results obtained tetraploid bagrid catfish broodstock with a high chance of being tested for the fertility of tetraploid bagrid catfish broodstock candidates. This study is an initial step to increase the growth of bagrid catfish. Tetraploid bagrid catfish broodstock can be used to mass production of triploid fish. The tetraploid offspring obtained can produce diploid gametes, thus avoiding the negative consequences of direct induction in triploid production.
Conclusion
In conclusion, this study showed the effectiveness of electric shock in tetraploid induction in bagrid catfish. This with a constant electric field of 12 V/m at the 28th minute for 10 min produced the highest percentage of tetraploidy, which was 81.67 ± 2.89%. Therefore, it affected egg hatching, survival rate, and larval abnormalities but did not affect fertilization rate.
