Introduction
The Pacific oyster (Crassostrea gigas) is an economically important bivalve species widely farmed and consumed worldwide due to its acceptable taste, high nutritional value, competitive market price, and plenty of biopharmaceutical applications. Specific to South Korea, oyster production occupies a significant portion of domestic aquaculture production owing to its popularity as a health food, causing high demand (Park et al., 2018). Domestic oyster farming began with small-scale cultivation methods such as the stilt-type, raft-type, and mass production became possible through the introduction of the hanging cultivation method in the 1960s. The oyster farming industry began to develop rapidly in the 1970s and continued to improve until the 1980s. However, afterward, no major development in farming technology caused fluctuations in aquaculture production (Kim & Lee, 2022). In the past 10 years, production has not changed significantly and has been stagnant (KOSIS, 2025). Furthermore, oyster farming deals with problems in the overall genetic degradation of the oyster population and the deterioration of the farm environment. This is brought about by intraspecific breeding, climate change, and aging of the aquaculture farm. Due to these problems, the supply of natural seeds is becoming unstable, and the artificial seed industry of shellfish acts as a solution to these problems (Kim & Lee, 2022). For this purpose, research on understanding and regulating the reproductive physiological characteristics of oysters to produce hatchery-produced seeds from broodstocks is needed.
The competitiveness of oysters in the market is primarily based on their edible portion or the so-called oyster meat mainly composed of the gonad, adductor muscle, gills, and mantle (Liu et al., 2020). During maturation season, the gonad occupies a large portion of the meat, and thus oyster quality is highly dependent on its gonadal maturity (Gómez-Robles et al., 2013). Biologically, an adult oyster possesses a gonad capable of maturation, gamete production, and spawning activity, and that creates an annual cycle of gonad early and late active, ripe, spawned, spent, and resting stages (Steele & Mulcahy, 1999). Although there are differences depending on the location of oyster production sites and species, oysters typically mature in spring, spawn in summer, and are spent and non-active during autumn and winter (Dridi et al., 2007). Studying the reproductive cycle of oysters would have several benefits such as helping in the selection of broodstock, predicting the timing of harvest, and sustaining the supply of oysters in the market without stressing the natural population or stock (Barman et al., 2024).
Oyster reproduction is highly controlled by both exogenous factors (e.g., water temperature, salinity, photoperiod, food availability; Fabioux et al., 2005) and endogenous factors (e.g., genetics, hormones; Park & Choi, 2021). In vertebrates, reproduction is highly governed by an endocrinological system composed of the hypothalamus, pituitary, and gonad axis (HPG). In addition, liver-produced, or gonad-produced insulin growth-like factors (IGFs) act on the gonad and play a supportive role in the reproductive process (Moon & Choi, 2020).
Generally, the IGF system is a neuroendocrine substance that affects cell growth, reproduction, and survival and acts as an intracellular growth factor (Duan, 1997). The IGF system regulates cell proliferation from the early stages of embryonic development and is involved in the metabolism, growth, and differentiation process of cell types, affecting normal germ cell formation (de Pablo et al., 1990; Heyner et al., 1989; Rappolee et al., 1992). Although the IGF system has not been fully defined in invertebrates, previous works suggested their roles in somatic growth and gonad maturity in oysters (Gricourt et al., 2003; Moon & Choi, 2019; Park & Choi, 2021). To date, some members of the IGF system reported in oysters include Molluscan insulin-related peptide (MIP) as a ligand, C. gigas insulin receptor-related receptor (CIR) as a receptor, and binding protein IGF binding protein complex acid labile subunit (IGFBP_ALS), which are presumed to play similar roles as that in vertebrates (Choi et al., 2018; Gricourt et al., 2003). Previously done studies on some members of the IGF system family, in oysters, were revealed to be associated with growth during winter (Choi et al., 2018), regulated adductor muscle growth in triploid individuals (Kim & Choi, 2019), and leads to gonadal maturation (Park & Choi, 2021). However, the role played by these endocrinological factors during gonadal maturation was not yet fully elucidated. Thus, this study was conducted to investigate the patterns of changes in the expression of the IGF system during reproductive maturation (both male and female) of oysters with similar shell sizes.
Materials and Methods
Around two-year-old hatchery-produced oysters grown in the grow-out farm located at Geoje, South Gyeongsang Province, Korea (34°54’18.46” N, 128°29’38.04” E) were collected, thirty (n = 30) monthly from March to September 2021 for utilization in this study. Vernier calipers were used to measure shell length (SL), shell height (SH), and shell width (SW) to the nearest 0.01 mm, and whole weight (WW) and flesh weight (FW) were obtained using a weighing balance. Fatness (F) and condition index (CI) were calculated based on previously employed formulae (Cho et al., 2023; Yoo et al., 2004),
Gonad samples were subjected to histological procedures to determine the sex and reproductive stages of oysters. Ten (10) oyster gonad was fixed in 10% neutral buffered formalin for 4 days and then stored in 70% ethanol. The tissue was dehydrated by immersion in 70% to 100% ethanol at 1-hour intervals and then cleared in xylene solution. Paraffin embedding was then carried out by affinity to paraffin. A microtome (CUT 4055, MicroTec, Walldorf, Germany) was used to cut 5 μm thick sections, which were mounted on a glass slide and dried. The dried slide was stained with hematoxylin and eosin (H&E). Stained tissue sections were observed under a light microscope (OLYMPUS SEX41, Olympus, Tokyo, Japan) and photomicrographs were taken using imaging software (Motic Image Plus 2.0, Motic, Kowloon, Hong Kong). Identification of the gonadal maturation stages was based on the work of Steele & Mulcahy (1999).
To analyze the expression of MIP and CIR, Twenty (20) oyster gonads were excised from the body and cut into 0.3 ⅹ 0.3 cm pieces. Total RNA was isolated using RNAiso Plus (Takara Bio, Shiga, Japan) and followed by the phenol-chloroform method. Briefly, RNAiso was added to homogenize the gonad and precipitated using chloroform and isopropanol. Then, the RNA pellet was washed using 75% and 100% EtOH, evaporated excess EtOH, and the RNA was solubilized using nuclease-free water. The RNA concentration was then quantified using a UV/VIS nano spectrophotometer (Micro Digital, Seongnam, Korea).
The PrimeScript First Strand cDNA Synthesis Kit (Takara Bio) was used for cDNA synthesis following the manufacturer’s instructions. Extracted total RNA was diluted to 1 μg and synthesized. 1 μL oligo dT primer, 1 μL dNTP mixture, and 3 μL RNase-free dH2O were added and reacted at 65℃ for 5 min, followed by the next step at 4℃. The 4 µL 5X PrimeScript Buffer, 0.5 µL RNase Inhibitor, and 0.5 µL PrimeScript were added to the reacted mixture. Then, 1 μL RTase and 4.5 μL RNase-free dH2O were added and reacted at 42℃ for 60 min, followed by RTase inactivation at 95℃ for 5 min. The synthesized cDNA was diluted with 180 μL of DNase-free water and then used as a template to perform a reverse transcription-polymerase chain reaction (RT-PCR) analysis.
Emerald AMP GT PCR Master Mix (Takara Bio) was used as the PCR mixture, and four primers were used for multiplex PCR. 25 μL Emerald Amp GT PCR Master Mix, 19 μL dH2O, 0.5 μL forward primer, 0.5 μL reverse primer, and 2 μL cDNA template were mixed to make a total of 50 μL mixture. The primers used are shown in Table 1, while the Vg and Gyc76C primers, used to distinguish between males and females, are shown on separate lines (Moon & Choi, 2020). The PCR reaction consisted of an initial denaturation at 94℃ for 5 min, followed by denaturation at 94℃ for 10 s, primer annealing at 55℃ for 30 s, and extension at 72℃ for 1 min, repeated 35 times. This was followed by a final extension at 72℃ for 7 min, followed by cooling at 10℃. The PCR reaction was performed using a Takara PCR Thermal Cycler Dice Gradient (Takara Bio) and an Applied Biosystems 2720 Thermal Cycler (Applied Biosystems-Life Technologies, Foster City, CA, USA). After the reaction, the PCR product was loaded onto a 2% agarose gel containing ethidium bromide and electrophoresed at 100 V for 20 min. Bands were captured using an imaging system (Azure Biosystem C300 analyzer, Azure Biosystems, Dublin, CA, USA), and densitometry was performed using Gene Tools version 4.03 (SYNGENE, Cambridge, UK).
Gonad was fixed in a 10% neutral buffered formalin solution for 4 days and then stored in 70% ethanol. The tissue was dehydrated from 70% ethanol to 100% ethanol and then cleared with xylene solution. Subsequently, embedding was performed by affinity to paraffin. Blocks were cut into 5 µm thick sections using a microtome (CUT 4055, MicroTec), mounted onto a slide, and dried. The dried slides were deparaffinized, dehydrated, and finally washed in 1 × phosphate buffered saline (PBS) solution. After drying in a 60℃ incubator, each tissue was washed with 1 × PBS solution in a slide staining tray, then the probe was placed onto the tissue and incubated in a 55℃ incubator for 16 hours. The FAM and BHQ1 pre-labeled probe sequence of MIP and CIR was 5’-TAAATACAAGCGGTCGGGTG-3’ and 5’-TGAGGAGGGTGATGAGGATA-3’. After the reaction, it was examined using a fluorescence optical microscope (IX51, Olympus). Control images were taken with green fluorescence while fluorescent images of MIP and CIR were taken with red-colored fluorescence. This is because of the specific wavelength absorption and emission control of the probe used in the FISH analysis.
Data were subjected to statistical evaluation using One-way Analysis of Variance (ANOVA) in SPSS version 27 (IBM, Armonk, NY, USA) with a significance level set at p < 0.05. Duncan’s multiple range test was used whenever a significant difference was detected. Results are presented as mean ± standard error of the mean (SEM).
Results
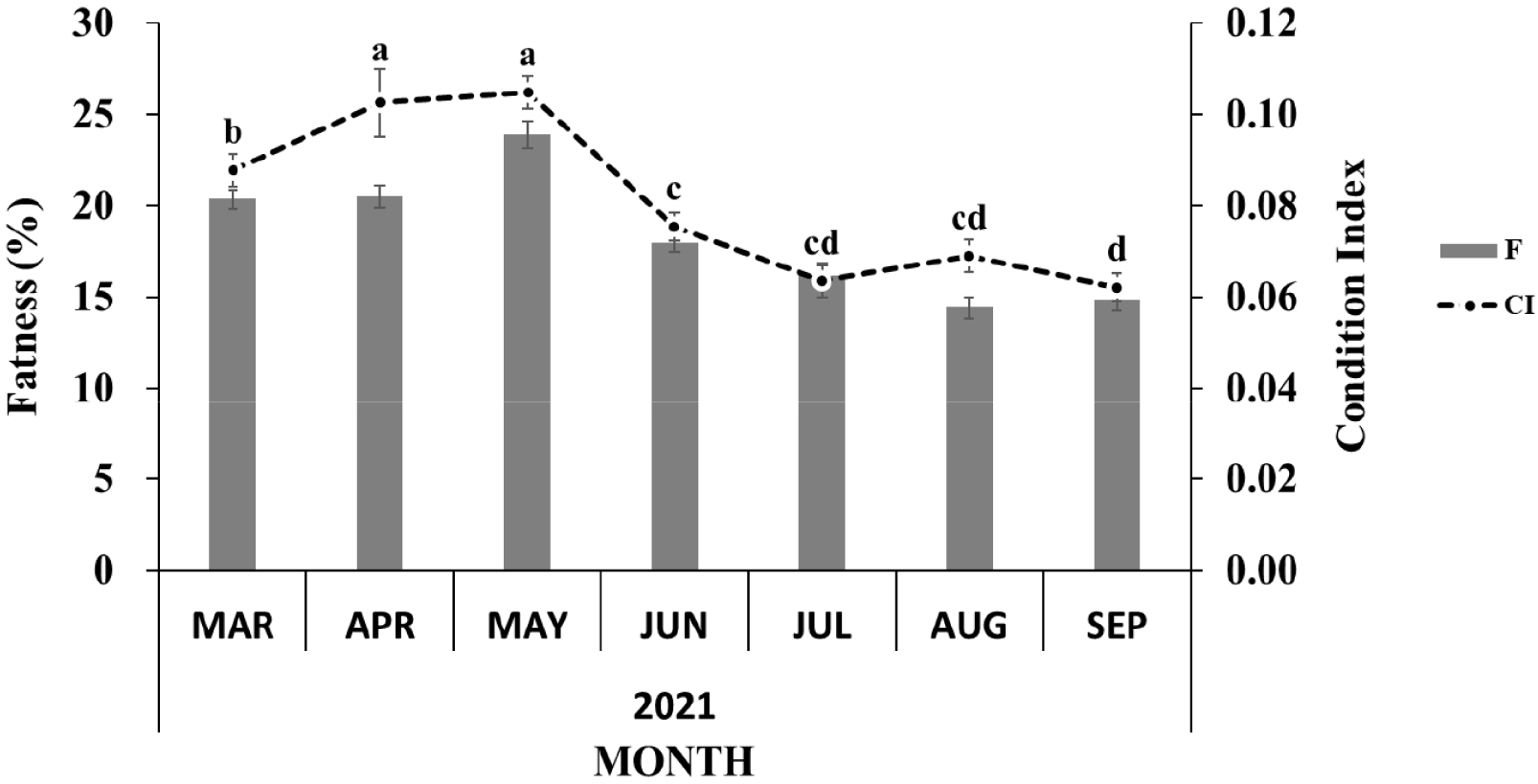
The body morphometry of the sampled oysters is shown in Table 2. The shell length was at a minimum of 57.46 ± 1.15 mm in the early active stage in early spring (March) and a maximum of 74.49 ± 3.73 mm in the resting stage in early autumn (September). In contrast, the shell height was at a minimum of 95.00 ± 5.16 mm during the resting stage in September and a maximum of 150.83 ± 4.27 mm in the partially spent stage in August. The shell width was at a minimum of 37.41 ± 0.91 mm in the early active stage in March and a maximum of 43.37 ± 1.34 mm in the partially spent stage in August. The whole weight was at a minimum of 123.37 ± 7.44 g in July during intense spawning activity and a maximum of 271.16 ± 10.72 g in August following re-maturation. The flesh weight was at a minimum of 16.73 ± 0.92 g in September after spawning activity (spent stage) and a maximum of 32.06 ± 1.67 g in August (ripe stage in female and partially spent in male; Table 2). The condition index increased from the early active stage, peaked during the ripe stage in May, and then decreased following spawning activity until spent stages from June to September. The fatness showed a similar pattern (Fig. 1).
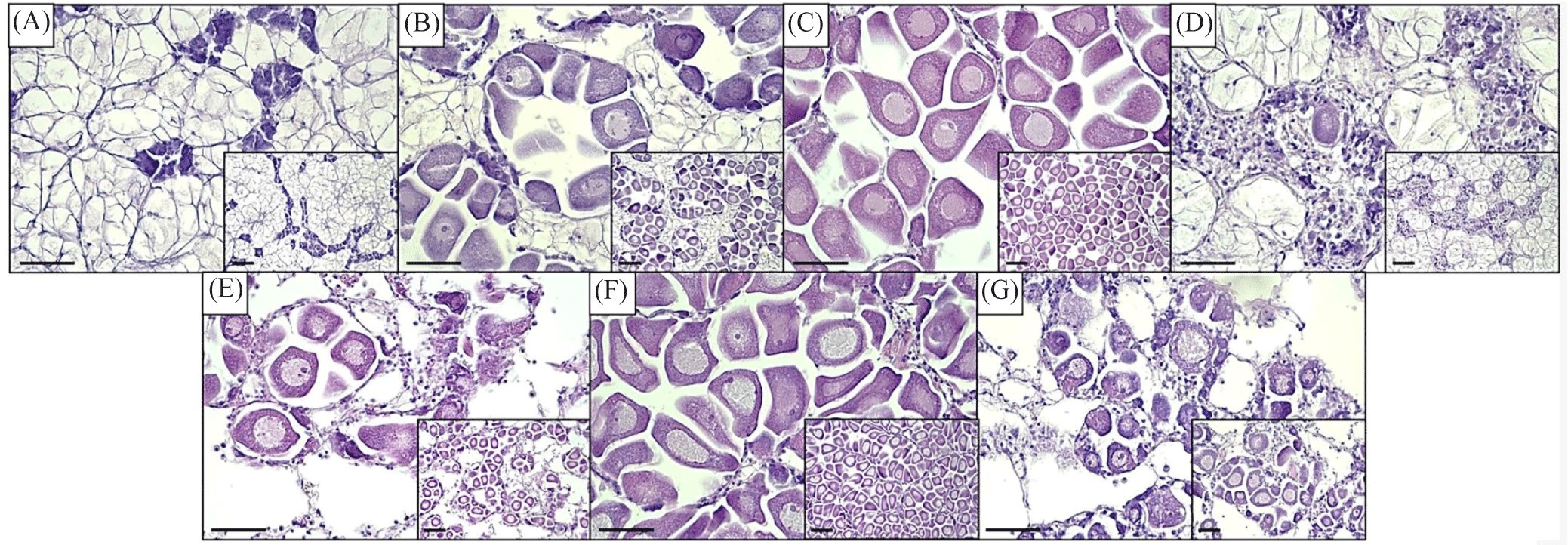
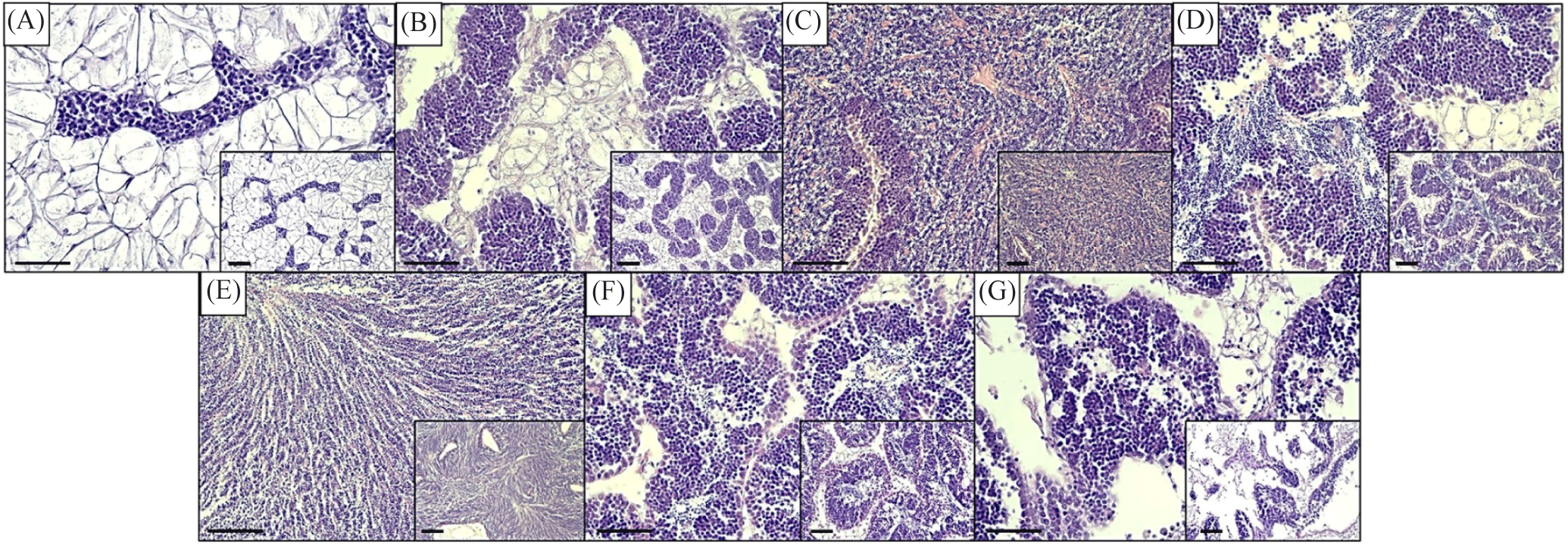
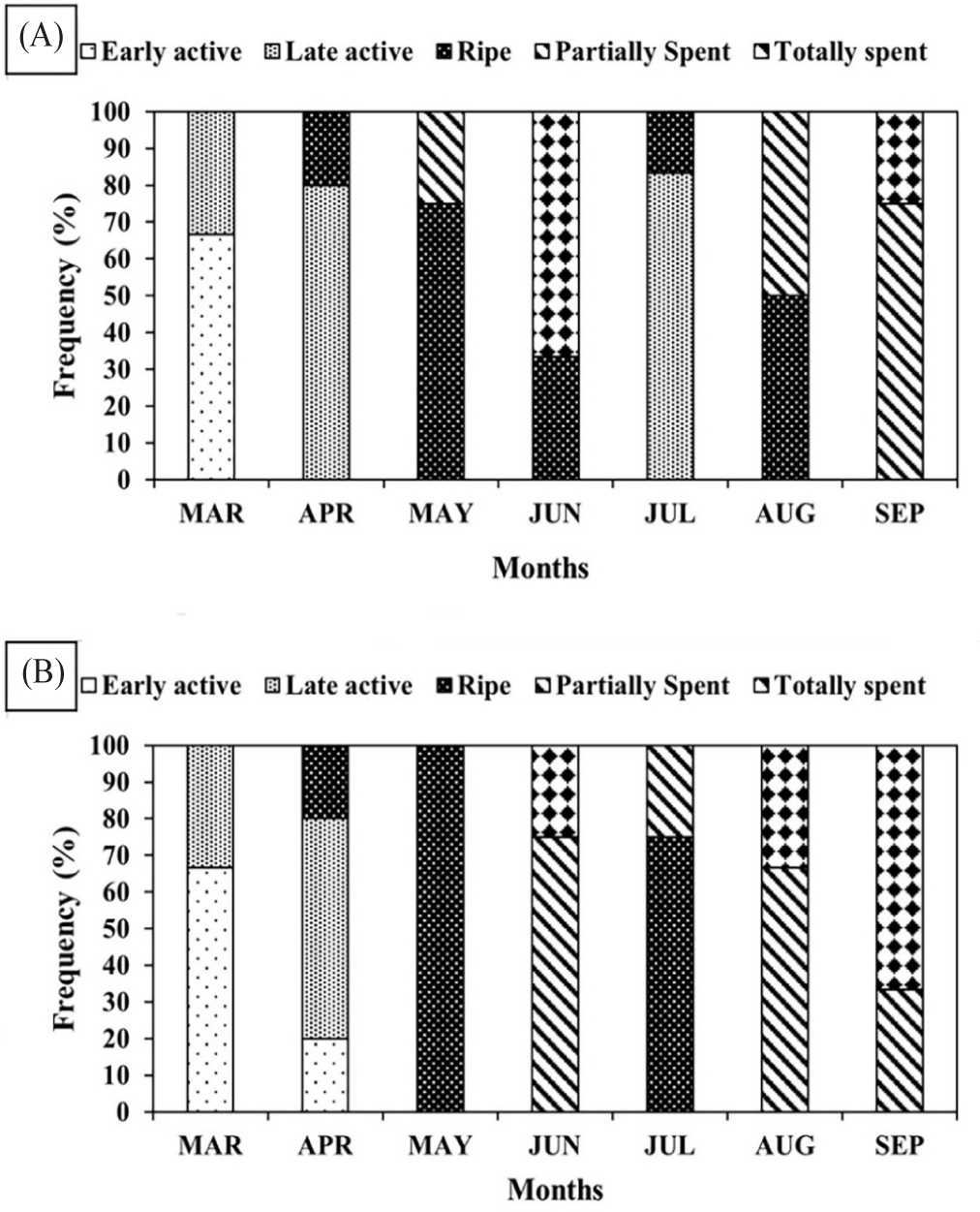
Histological procedure (H&E) divided oysters into either male or female sexes, with infrequent cases of undivided (lack of discernable germ cells). The sex ratio was determined with a 1:1 ratio in June, while a near 1:1 ratio was observed in April, May, and July, while unbalanced in March, August, and September (Table 2). During the early stage of development in early spring, one specimen was observed to be undivided, and another one towards the end of spawning activity in early autumn (Table 2). In addition, the staging of the gonadal reproductive maturation was determined following microscopic observation of the germ cell. The reproductive cycle of oysters from March to September is shown in Figs. 2 and 3. The designation of monthly-based gonad staging was based on most of that specific stage, given that not only one stage occupies a single month due to intraspecific differences among oyster samples. Female oysters started developing in March with a designated early active stage, late active in April, ripe in May. Totally spent in June. Late active in July, Ripe in August, and, lastly, partially spent in September (Fig. 2). Meanwhile, male oysters sampled in March were in the early active stage, April was in the late active stage, May was in the ripe stage, June was in the partially spent stage, July was in the ripe stage, August was in the partially spent stage and, lastly, in September was in totally spent stage (Fig. 3). Generally, both sexes start sexual maturation in the early spring and reach the peak of maturity in late spring/early summer. Following spawning activity, another re-maturation occurs with mature oocytes found in August and mature spermatozoa a month earlier in July. In early autumn, both sexes regressed sexual activity and entered spent/post-spawning stages. The gonadal development stage frequency was similarly determined to assess what stages are prominent on a monthly basis. In female oysters, the stage with the most observed (> 50%) of all sampled oysters were early active, late active, ripe, totally spent, late active, ripe, and partially spent in March, April, May, June, July, August, and September, respectively (Fig. 4A). Meanwhile, Fig. 4B shows the gonadal stage frequency in male oysters. In March early active stage was prominent, late active in April, ripe in May, partially spent in June, ripe in July, partially spent in August, and totally spent in September. Undivided samples were excluded from the gonadal staging frequency distribution.
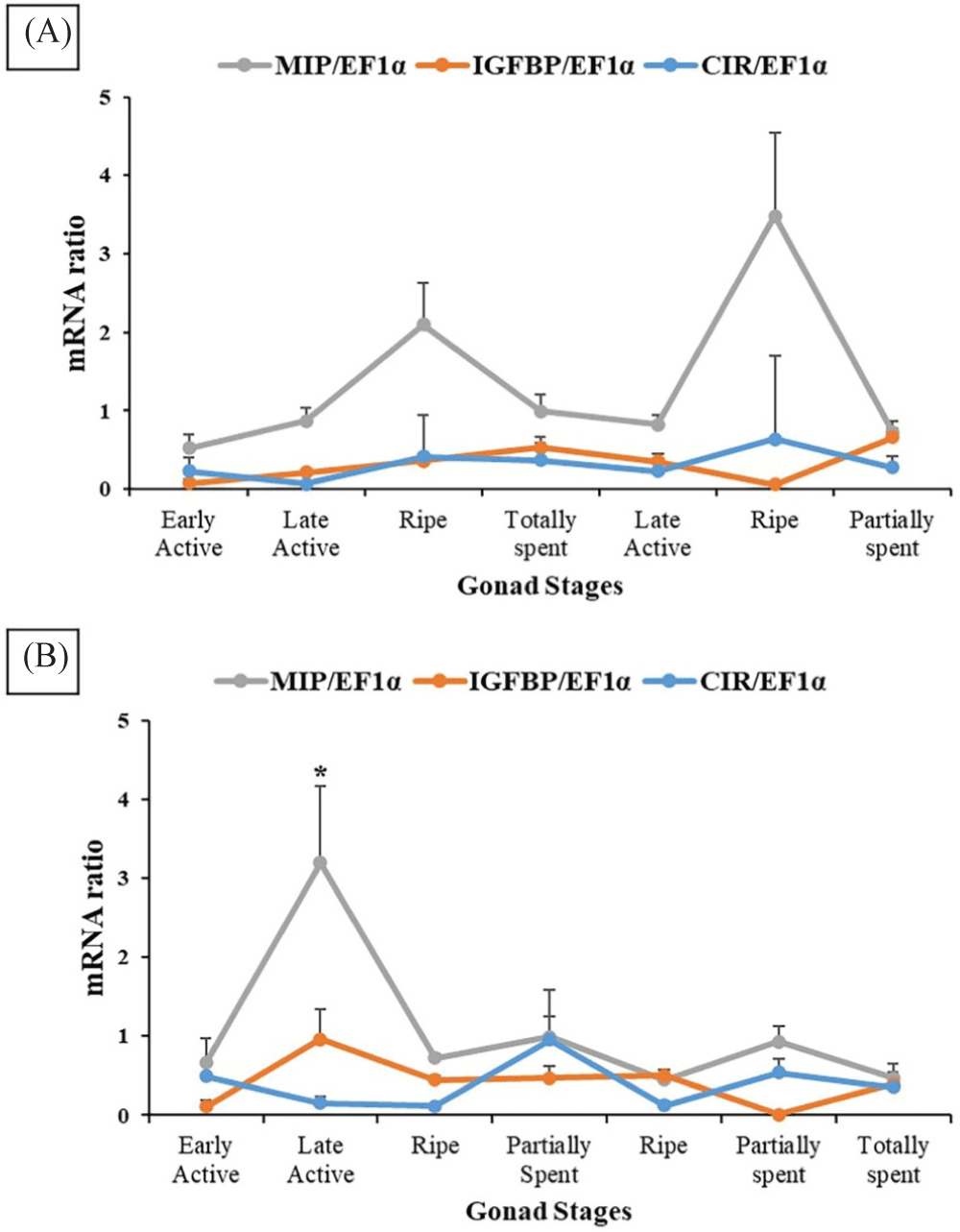
The gene expression pattern of MIP, CIR, and IGFBP_ALS was sex-specific and gonadal stage-specific based on the results of RT-PCR analysis (Fig. 5). In females, MIP continuously increased during the early stages of maturation and peaked during the ripe stage. MIP decreased following spawning activity, increased again following re-maturation, and suddenly decreased towards spent stages. The pattern of gene expression of IGFBP_ALS and CIR were similar across gonad stages, with complementary differences during the ripe stage and partially spent stage. IGFB_ALS was higher in the former (ripe) and lower in the latter (partially spent). However, no statistical significance was detected in three genes across different gonadal stages (p > 0.05, Fig. 5A). In males, statistically higher MIP expression was observed during the late active stage compared to all other stages (p < 0.05). In contrast, IGFBP_ALS and CIR gene expression levels were statistically similar among gonad stages (p > 0.05, Fig. 5B). IGFBP_ALS was the highest in the late active stage while CIR was the highest in the partially spent stage.
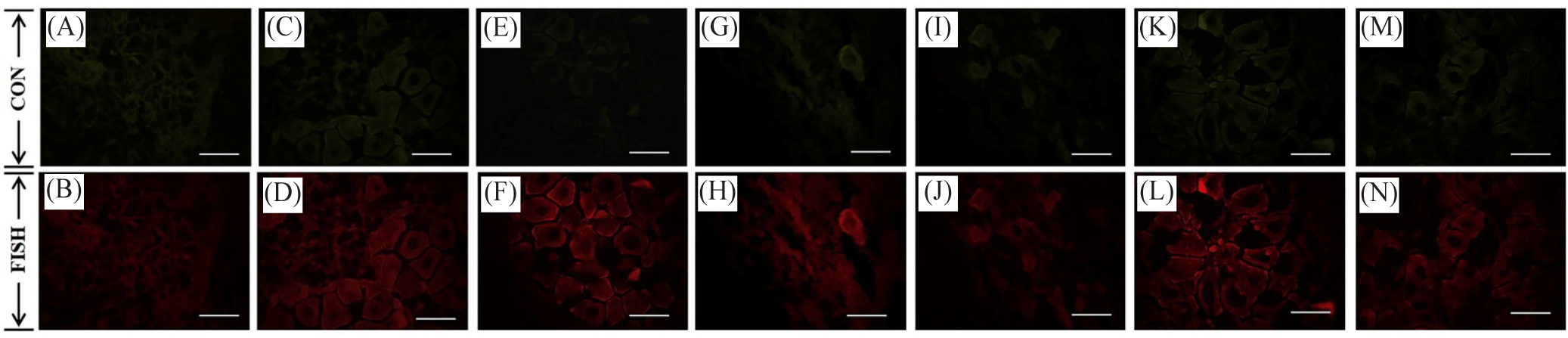
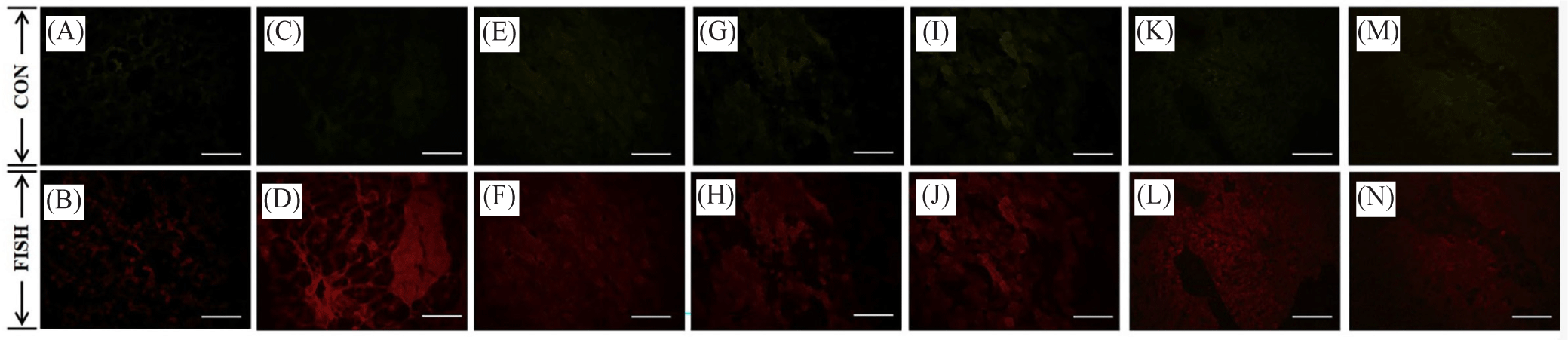
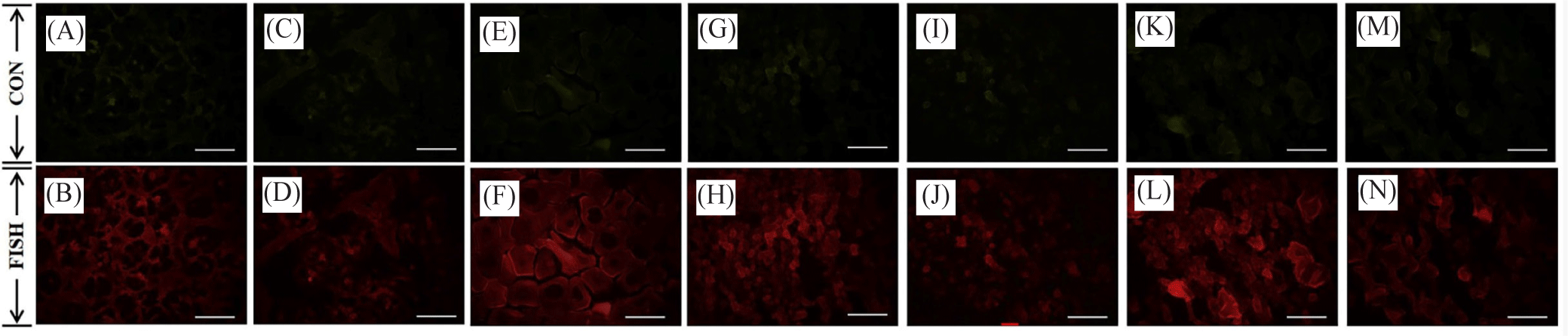
The fluorescence expression of MIP and CIR in the female and male gonad cells of oysters is given in Figs. 6–9. Based on the results of the in situ analysis, fluorescent expression was observed in the cytoplasm of the oocytes and spermatogonia. The pattern of MIP fluorescence in the female gonad is shown in Fig 6. MIP fluorescence in female oysters increased from the early active stage until the ripe stage, decreased during spawning activity (spent stage), and increased again following re-maturation (late active stage) until the second ripe stage and decreased towards the end of the spawning season (spent stage). In males, the highest fluorescence was observed in the late-active stage, while comparable in other stages (Fig. 7). The highest CIR fluorescence in female gonads was observed during ripe stages while other stages had relatively lower fluorescence (Fig. 8). In the case of male oysters, following spawning activity (spent stage) was the fluorescent at its highest. Lowest fluorescence during ripe stages (Fig. 9).
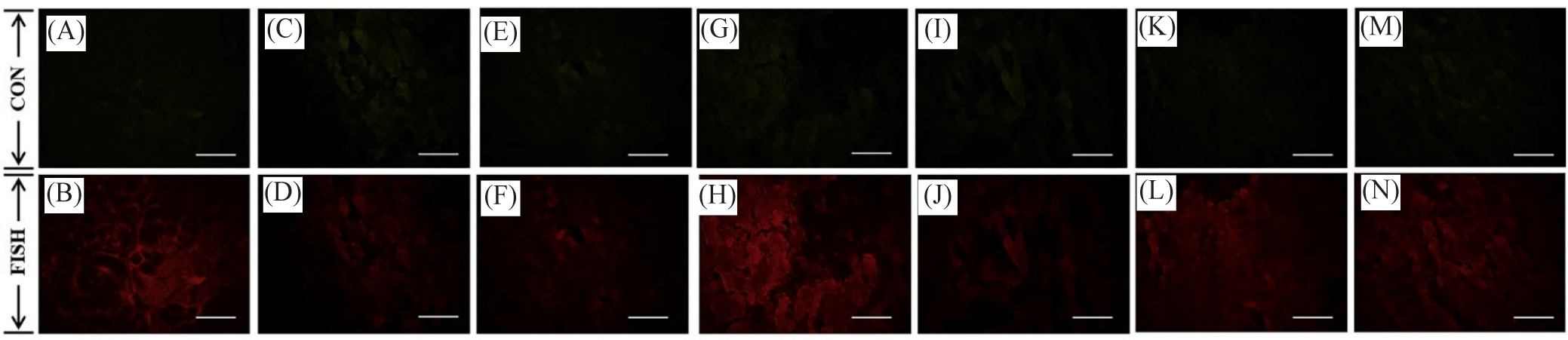
Discussion
The reproductive performance of oysters is highly affected by both internal biological factors and external environmental factors leading to the success or failure of the spawning activity. Among biological factors, the involvement of the IGF system in bivalves has gotten widespread attention in recent years (Li et al., 2021a; Moon & Choi, 2020) due to their perceived role during organogenesis and gametogenesis (Li et al., 2021b). The current study aimed to define the roles of IGF system members namely MIP, IGFBP_ALS, and CIR in the reproductive maturation stages of female and male oysters collected from the natural breeding grounds.
The body morphometry of oysters was similar across gonadal stages, however, soft tissue weight and condition index steadily increased in the early stages of maturation until ripe stages and generally decreased following spawning activity in the warm summer months. This relationship illustrates that the edible soft tissue weight and CI are closely related to the gonadal development stages of oysters, corroborating previously reported works in other bivalve species (Barman et al., 2024; Choi & Chang, 2003) and oysters collected from other localities (Hwang et al., 2023). During the breeding season, fluctuations in the sex ratio causing an imbalance in the number of male and female individuals were previously reported in oysters, nevertheless, despite these fluctuations the overall sex ratio, generally, conforms with a near 1:1 sex ratio (Eagling et al., 2017). In the present study, oysters were sampled in all months except during the early-active stage in March, and towards the end of spawning activity, there was an imbalance of sex ratio, otherwise, an almost 1:1 sex ratio was achieved in other sampled months, except in June with a 1:1 sex ratio. Due to the unstable sex ratio and limited samples used for analysis in the present study, the Geoje-based oyster farm may not necessarily represent a biologically optimal environment for growing oysters. However, such concern would be resolved if the number of sampled individuals will be increase and by making a comparison with other oyster farms in the same region (Park et al., 2012).
Monthly sampled oysters that were subjected to H&E histological procedures divided individuals into sexes, and different gonadal reproductive stages. The gonadal distribution maturity was also noted with the majority of a specific stage (most frequency) designated as the gonad stage on that specific month. In females, most oysters were in the early active stage signified by arising oogonia along the follicle, yet still connective tissue is more abundant. This stage continuously develops in April designated as a late active stage due to the increased number of free and attached oocytes showing distinct nuclei. Progressively developed oocyte showing distinct nucleus and nucleolus with an unclear boundary of the follicle and almost invisible to none connective tissue. Following spawning activity, in June, follicles became empty and broken, yet germ cells re-matured a month after in July showing a late active stage and fully matured ripe oocytes were again distinguishable in August. Partially spent oysters were observed the next month. Male oysters exhibited a similar pattern of spermatogenesis. Development starts in March with an early active stage with the presence of small follicles, spermatocytes, and abundant connective tissue. This stage developed into a late active stage exhibited by follicle cells containing spermatozoa, some spermatid, and decreased connective tissue. The ripe stage was majority in May with germ cell follicles filled with spermatozoa and inconspicuous interfollicular tissue and germinal epithelium. Some follicles were partially empty in June with not densely compacted spermatozoa indicating a partially spent stage. Then, further re-matured to a ripe stage in July and partially spent in August. In September, most of the follicles are empty with increasing connective layers yet some individuals can show remaining spermatozoa. This is designated as totally spent stage. The results of the current study conform with previous work in the gonadal maturation process in some other bivalves showcasing developing stages in the early spring, spawning and re-maturation in late spring/early summer, and spent stages in late summer/early autumn (Min et al., 2004; Park & Choi, 2021; Steele & Mulcahy, 1999). These development stages were used to correlate with the gene expression of MIP, IGFBP_ALS, and CIR to determine the role played by this IGF system in the sexual maturation of oysters.
This study focuses on the effect and correlation of the IGF system in the gonadal maturation stages of oysters. The IGF system has three components: a ligand, receptor, and IGF-binding proteins. The role of these members in aquatic vertebrate species is well-known (Wood et al., 2005), however, their roles in invertebrate species need further investigations. In oysters, previously described IGF system members MIP, IGFBP_ALS, and CIR were involved in their somatic growth regulation (Choi et al., 2018), however, their role in reproductive development is still unclear. In the present study, MIP, acting as a ligand was highly expressed during ripe stages in female oysters indicating its indispensable role in the regulation of sexual maturation. In males, MIP was higher in the late active stage presenting its intrinsic role in the development of spermatids and spermatozoa. Cherif-Feildel et al. (2019) hypothesized that MIP has a role in the reproductive process in oysters and based on the result of this study, MIP indeed is one of the endogenous factors necessary to produce mature germ cells. The one-month earlier increase of MIP in males compared to females can be a sex-specific or intraspecific variation, nevertheless, additional investigations are necessary to determine what causes such variation. On the other hand, IGFBP_ALS and CIR expression mainly did not significantly differ among stages indicating that these two factors did not affect the maturation process significantly in both sexes. This is in contrast with its role in growth regulation previously described (Choi et al., 2018; Gricourt et al., 2003). In recent years, IGFBPs characterized in oysters were suggested to have a regulatory function in the growth of animals (Li et al., 2021b), yet its specific role in reproductive maturation is not significant. Meanwhile, Gricourt et al. (2006) suggested that the presence of CIR in the gonadal area assumes a reproductive role. This CIR-specific effect is not fully exhibited as well in the current study. Full elucidation of IGFBP_ALS and CIR in gonadal maturation warrants further analysis.
The MIP and CIR fluorescent expression in the gonad followed a similar trend of its gene expression. Immunofluorescence analysis allowed ocular observation of MIP and CIR and its specific location within the egg or sperm cell. Based on the literature review, the presence of IGF system members in the oyster gonads was reported and in the present study, it is known that the fluorescence was situated within the cytoplasm of the oocyte and the spermatogonia.
In conclusion, the gonadal maturation of oysters collected along the natural breeding ground of Geoje, Korea exhibited sex-specific maturation stages. In general, maturation starts as early spring, spawns in early and late summer, and regresses into spent stages in autumn. The positive effect of IGF system member MIP was observed in the late active and ripe stages of maturation, whereas no IGFBP_ALS and CIR-specific correlation was observed. However, further analysis from different reproductive perspectives such as developmental stage analysis, whole-year round sampling, different breeding grounds, and correlation with exogenous factors are necessary to fully elucidate the regulatory effect of IGF system members on the sexual maturation of oysters.
