Introduction
Some of the most harmful pathogens that cause diseases in freshwater fish, crustaceans, amphibians, and insect larvae are belonging to oomycetes and they can cause significant harm to the environment and economy in both natural and cultivated habitats (van West, 2006). Fish pathogenic Oomycetes were estimated to cause 17% of all animal-source protein, utilized for human consumption to struggle against hunger and malnutrition, which comes from aquaculture and fishing combined (FAO, 2020). These diseases are widespread and can strike fish at any stage of their life cycle (Hussien et al., 2010). Saprolegnia, Achlya, and Aphanomyces are the three genera of water molds that belong to the class Oomycetes that cause the majority of fungal infections in cultured fish, including catfish and tilapia species (Daugherty et al., 1998). However, because there is little evidence available on the pathobiology of water molds in fish, the significance and impact of Saprolegnia and other oomycetes infections are frequently unnoticed (van den Berg et al., 2013).
Only in cases of prior sickness, mechanical harm or environmental stress could Saprolegnia spp. generate disease. Among fish, Saprolegnia parasitica is the primary pathogen (Durborow et al., 2003; Pelczar et al., 2008; Roberts, 1989). Neish (1977) reported that the immunosuppressive effect initiated by environmental factors advances a mechanism for altering ordinarily non-pathogenic organisms—like Saprolegnia into pathogenic ones. Certain alterations, such stress and immunosuppression, let infections spread fast in farmed fish (Pickering, 1994). Saprolegnia is the etiologic agent of Saprolegniasis. The naked eye may easily identify fish infected with Saprolegnia spp. by their cotton-like white to greyish spots on their skin and gills (Chauhan et al., 2014; Hussien et al., 2010; Quiniou et al., 1998; Stueland et al., 2005). The infection spreads promptly, frequently resulting in death, and can result in significant losses of fish and ova. (Howe & Stehly, 1998; Ilondu et al., 2009). Many earlier investigations have indicated that saprolegniasis fungi are the most dominant pathogenic fungi in fish. (Bruno & Poppe, 1996; Bruno et al., 2013; Chauhan et al., 2014; Hatai & Hoshiai, 1994; Robert et al., 2003; Wuensch et al., 2018). Several authors observed that saprolegniosis disease of fish severely targeted and affects skin, epidermis, subepidermal muscular tissues and gills of infected fish and induced many aspects of histopathological alterations especially in vital tissues and organs in diseased fish. Sunken eyes, irregular swimming behavior, and skin lesions associated to dry mucus-depleted epidermis are some of the common indications of this disease (Ciji & Akhtar, 2021; Majhi et al., 2005). When Saprolegnia infection reaches the gills, it affects osmoregulation, respiration, and heamodilution; critical gill infections can result in immediate respiratory failure that is lethal (Aly & El-Ashram, 2000). Histopathological changes caused by saprolegnia infection include the loss of gill lamellae in severe cases and the destruction of epidermal cells in the skin and gills, which lead to death (Ashour et al., 2017; Neish & Hughes, 1980; Willoughby & Roberts, 1992; Zaki et al., 2008). However, El-Deen et al. (2018) reported that the signs appear on the fish agonized from saprolegniasis were depicted as grayish white cotton-like tufts on the mouth, dorsal, caudal, and pectoral fins; the fish became emaciated and eventually died from blindness as a result of their inability to feed.
Angahar (2016) reported ulcerative skin lesions and eroding of infected barbells on saprolegniosis infected fish sampled from fish farms in Gboko, Nigeria. Saprolegniosis superficial, white growth lesions on the injured skin, or gills may extend into dermis and the subjacent superficial musculature with time with sloughing of the epidermis (van West, 2006) Moreover, Patel et al. (2018) mentioned that fish tissue samples containing Saprolegnia infection displayed varying degrees of gill pathology and colonies morphology was circular and white cottony. In the primary gill filaments there was severe bleeding and edema along with hyperplasia in the epithelial cells and infusion in the secondary gill filaments. Between necrotic and edematous myofiber with inflammatory cell infiltration, Saprolegnia hyphae were seen.
This study was designed to test and evaluate the mode of potency and pathogenicity of two zoosporic fungi namely; Saprolegnia parasitica and Aphanomyces laevis, which were common and frequently isolated from Egyptian fish farms and hatcheries, on the most edible and popular Nile tilapia freshwater fish (Oreochromis niloticus). The study included rates of mortalities and growth pathology in Nile tilapia fish samples exposed to zoospores cysts of S. parasitica and A. laevis compared to untreated controls after 10 days of infection. In addition, the histopathological alterations of some biomarkers in Nile tilapia fishes were photographed, described and compared to that of nontreated controls.
Materials and Methods
The pathogenicity experiment was performed in fish breeding laboratory, Animal Medicine Department, Faculty of Veterinary Medicine, Assiut University by using 3 glass tanks, each using 6 mm thick, 50 cm height, 50 cm length and 45 cm width. For protection and reduction of water evaporation during the experiment, each tank had 25 liters of tap water covered in a slight steel mesh. For every water tank, two electrical air pumps equipped with air stones were utilized as oxygen providers to sustain the dissolved oxygen levels within the range of 90 to 95% saturation. The water pH was maintained between 7 and 8. Over the course of the 10-day experiment, a 12-hour light and 12-hour dark photoperiod was used to keep the temperature at 22 ± 2°C.
Two strains of zoosporic fungi namely; S. parasitica and A. laevis which were isolated from cultured fish farm located at Samalout city, El-Minia Governorate, Egypt were used as etiological agents and mycotic fish pathogens during this study (Gaber et al., 2016). Saprolegnia parasitica was cultured on glucose peptone agar medium (Willoughby & Pickering, 1977) whereas A. laevis was grown on glucose yeast extract agar medium (Hatai & Egusa, 1977) at 20 ± 2°C for 5 days. Agar pieces (1 cm) from the fresh growing edges of each tested fungal media were removed from the colony margins and aseptically inserted into 12 cm diameter Petri dishes containing sterilized distilled water and sterilized sesame seeds which used as a capture material. Incubating Petri dishes for 7 days at 22 ± 2°C until the best zoosporangial discharge and encystment of zoospores were obtained. Cysts of tested two zoosporic fungal species of Oomycetes were counted using a hemocytometer. About 6.2 × 104 cysts of S. parasitica and 10.2 × 104 cysts of A. laevis were counted in each milliliter of the zoospore suspension.
Forty five healthy fishes of Nile tilapia (Oreochromis niloticus) with approximately of the same average body weight of 120 ± 6 g/fish were obtained and collected from Samalout fish farm (El-Minia governorate) and transported alive in quantity of Nile water free from fungal propagules (as proved by mycological investigation) to our laboratory. Fish were then raised in three equal and identical glass aquaria. Aquaria were provided with daily feed of a balanced commercial diet along with continuously aerated and replenished tap water. Before the experiment, the fish were kept for one week to acclimatize to aquaria conditions.
Three equal groups were created from the fish samples where each group was encountered by 15 fishes. As a control, fish from group (1) were maintained without receiving any fungal treatments. Fish of group (2) were infected with zoospores cysts suspension of S. parasitica. Fish of group (3) were infected with zoospores cysts suspension of A. laevis. The challenge infection was done by immersing a manual wounded Nile tilapia fishes in zoospores and cysts suspensions of each of S. and A. laevis (4 Litres each) for 10 min. according to Willoughby & Pickering (1977). Then, the zoospores cysts suspensions were added to the aquaria if fish group (2) and fish group (3). During the experiment, mortalities in the experiment were taken out of the aquariums immediately. The pathological effects of the two zoosporic fungal isolates on fish were determined. At the end of the experiment, the number of dead and living fish was counted. The experiment was repeated for three times.
For the histopathological observations, pieces of liver, anterior kidney, muscle and gills were dissected and immediately immersed in 10% neutral buffered formalin as fixative solution (100 ml of formaldehyde 40%, 3.5 g NaH2PO4, 6.5 g Na2HPO4 and distilled water 900 ml) and left for 48 hours. They dehydrated in ascending series of ethanol, cleared in cedar wood oil and embedded in paraffin. Paraffin sections of 5 µm in thickness were prepared and the histology slides were stained with Harris hematoxylin and eosin (Drury & Wallington, 1980).
Results
This investigation was designed to study the effects of two zoosporic fungi namely; S. parasitica and A. laevis on the Nile tilapia fish (O. niloticus).
The results presented in Table 1 indicate that the mass mortalities in Nile tilapia (O. niloticus) fishes treated at control and those infected with suspensions of zoospores cysts of S. parasitica and A. laevis were 3, 8 and 22 matching 20%, 53% and 73%, respectively of total fish counts at each treatment. Direct bath exposure of Nile tilapia to the inoculum of the two tested Oomycetes species S. parasitica and A. laevis caused more fish mortalities than control by 33% and 53%, respectively. The data shown in Table 1 indicate that Nile tilapia fish infected with a suspension of zoospore cysts from A. laevis experienced a higher mortality rate compared to those infected with spores from S. parasitica. Based on the observed mortalities, it can be inferred that A. laevis is more potent as a causative pathogen for Nile tilapia compared to S. parasitica.
| Group | Treatment | Number of dead fish | Number of survived fish | % Mass mortalities | % Change |
|---|---|---|---|---|---|
| 1 | Control non-infected | 3 | 12 | 20 | - |
| 2 | Saprolegnia parasitica | 8 | 7 | 53 | 33 |
| 3 | Aphanomyces laevis | 11 | 4 | 73 | 53 |
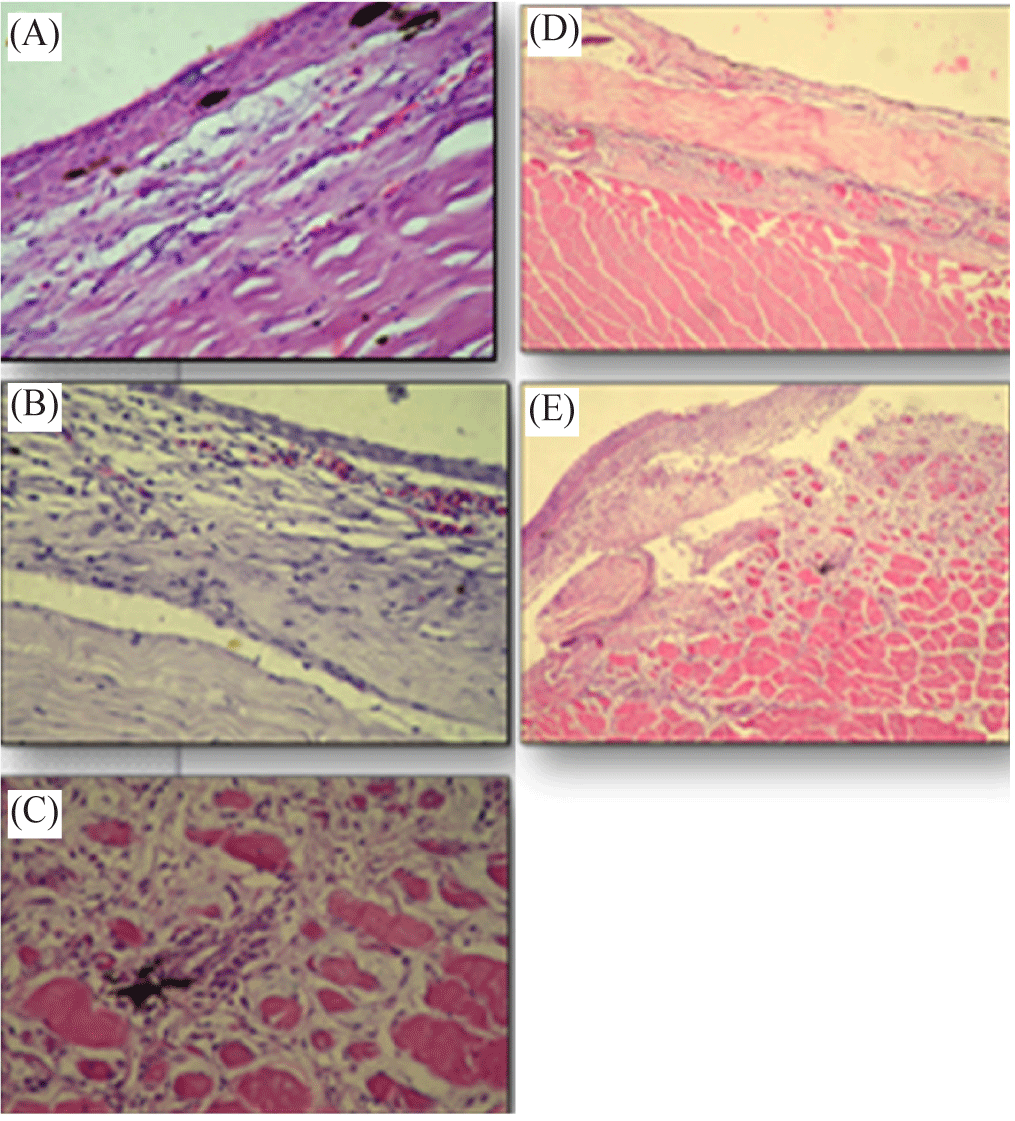
Regarding gross pathology, it was observed that the groups of tested Nile tilapia fish had distinct reactions to each treatment. Group 1 Nile tilapia fish that were not subjected to any zoospores infection of the control treatment exhibited regeneration of the artificial wound, as shown in Fig. 1A. However, fish belonging to groups 2 and 3 that were infected with the pathogenic agents of S. parasitica and A. laevis exhibited inflammatory alterations in the skin and underlying muscles in the area of the injury (Fig. 1B and 1C, respectively).

Histopathological alterations of Nile tilapia fish tissues and organs at control (Group 1)and at treatments with cysts zoospores of each of Saprolegnia parasitica (Group 2) and Aphanomyces laevis (Group 3)
The skin, muscles, gills, liver and kidney tissues of fish group (1) at control treatment showed more or less normal histological structure (Fig. 2A, 3A, 4A, and 5A. However, the anatomical and histopathological results in fish tissues and organs exposed to cysts suspensions of the two pathogenic zoosporic fungi were interesting as follows:-
Fish tissues and organs infected with each of Saprolegnia parasitica and Aphanomyces laevis
A notable histopathological alterations in various tissues and organs of Nile tilapia fishes undergoing to direct exposure of etiological agents of the oomycete S. parasitica were detected which were almost documented by photography.
Severely remarkable histopathological alterations and damages in examined tissues and organs of Nile tilapia fishes subjected to direct exposure of zoospores cysts suspension of the tested fungal species A. laevis were found. These severe hazardous and prominent alterations in various tissues and organs in Nile tilapia fishes may interpret the high mass of mortalities in fishes at this treatment compared with those at S. parasitica treatment.
Based on tissues and organs of Nile tilapia fishes, these histopathological alterations can be summarized as follows:-
Skin of Nile tilapia (O. niloticus) fishes infected with etiological agents of S. parasitica showed mild mycotic dermatitis with congestion of some epithelial capillaries and inflammatory cell reaction in the subepithelial tissue (Fig. 2B).
Severe damage in the skin tissue was observed of Nile tilapia (O. niloticus) infected with etiological agents of A. laevis. Histopathological alterations revealed obvious fungal hyphae and degeneration and sloughing of epithelium layer and inflammatory cell reaction in subepidermal layer (Fig. 2D). Also, there was heavy cellular infiltration in the dermal layer associated with mycotic myositis (Fig. 2E).
Muscles of Nile tilapia (O. niloticus) exposed to suspension of zoospores cysts of S. parasitica elaborated mycotic myositis. The presence of inflammatory cell reaction and necrosis of the muscles fibres are highly demonstrated (Fig. 2C).
Muscles of Nile tilapia (O. niloticus) fishes infected with etiological agents of A. laevis appeared mycotic myositis, presence of inflammatory cell reaction and necrosis of the muscle fibres.
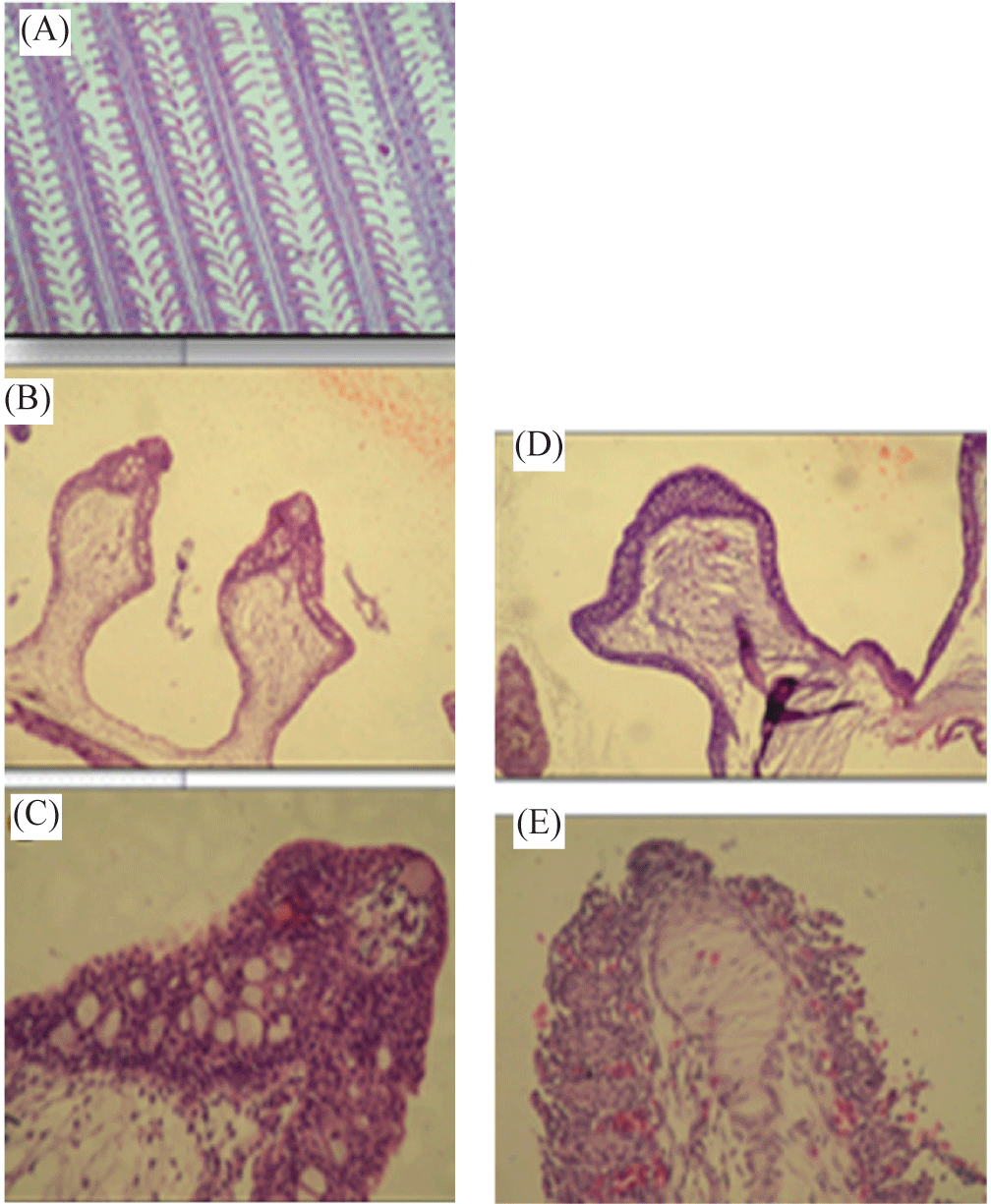
Gills of Nile tilapia (O. niloticus) treated with etiological agents of S. parasitica showed epithelial hyperplasia and increases the number of goblet cells (Fig. 3B). In addition, a remarkable congestion of gill lamella capillaries can be seen (Fig. 3C).
Gills of Nile tilapia (O. niloticus) infected with A. laevis displayed more histopathological hazardous defects than at treatment of S. parasitica. They showed presence of fungal hyphae in the subepithelial layer and increase in the number of goblet cells in the epithelial covering (Fig. 3D). Also, there was a degeneration and sloughing of epithelium and congestion, separation between surface epithelium and capillary beds, telangiectasis and fusion of the secondary lamellae (Fig. 3E).
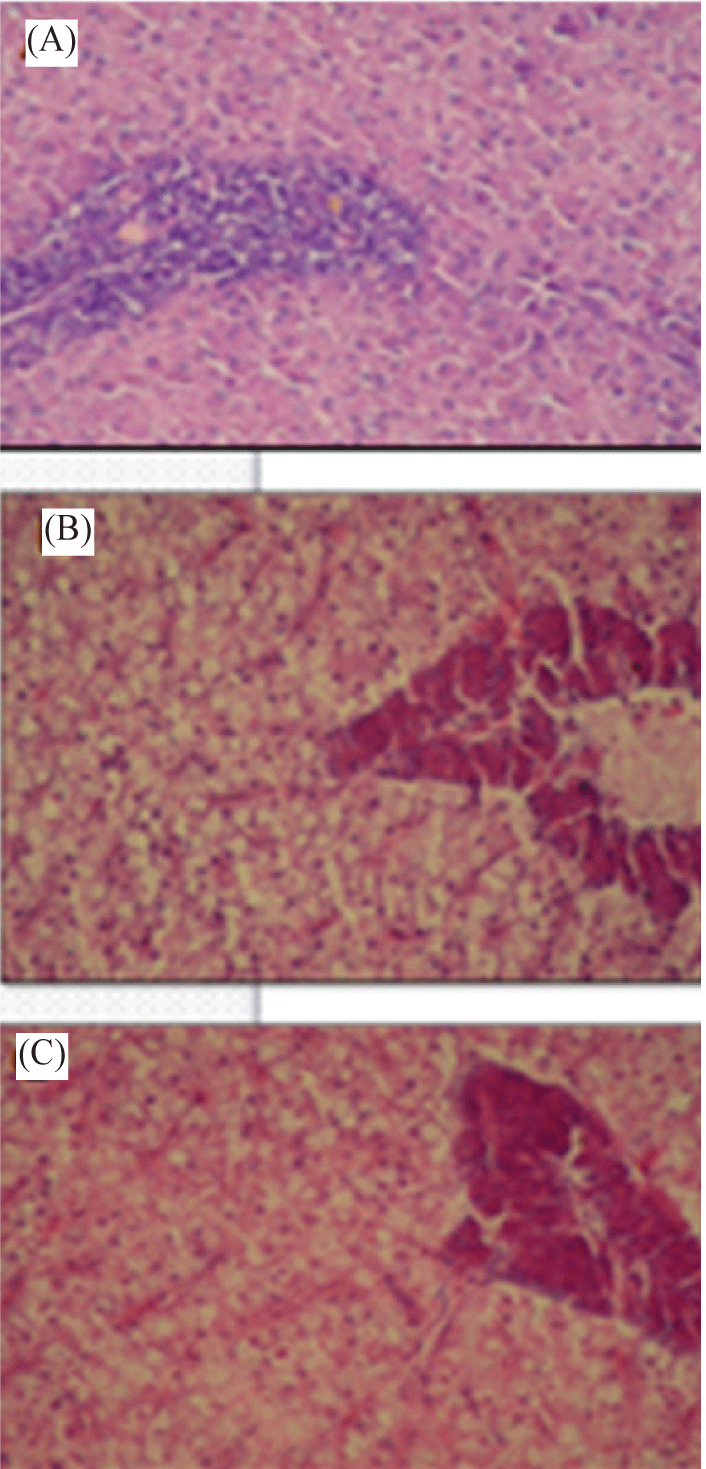
Liver of Nile tilapia (O. niloticus) infected with suspension of zoospores cysts of S. parasitica showed vacuolar degeneration of hepatocytes (Fig. 4B).
Also, histopathological alterations in liver tissue of Nile tilapia treated with etiological agents of A. laevis appeared somewhat more affection when compared with S. parasitica treatment. In this case, liver tissue of Nile tilapia infected with A. laevis showed distinct necrosis of the hepatocytes and melanomacrophage center (Fig. 4C).
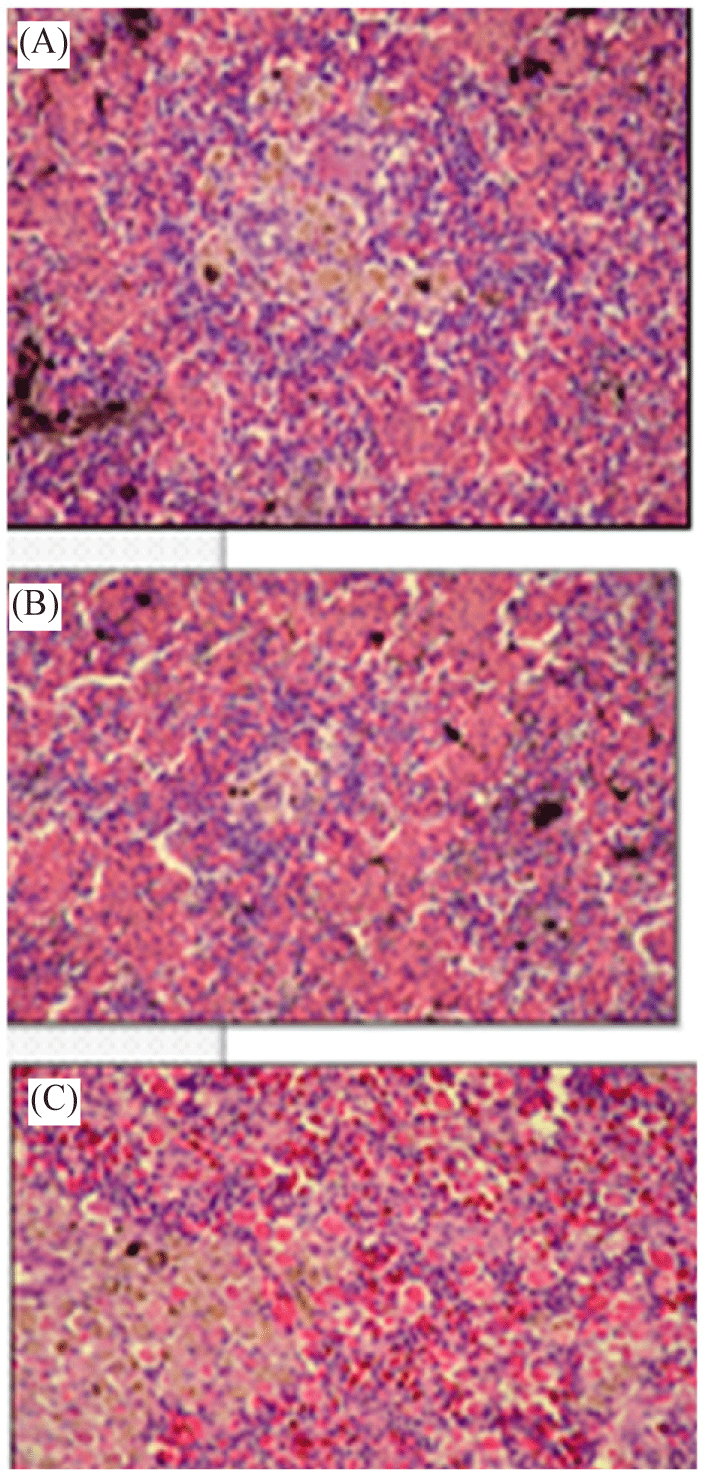
Anterior part of kidney of Nile tilapia (O. niloticus) directly exposed to suspension of zoospores cysts of S. parasitica showed multiple necrobiosis in the hemobiotic center with exhaustion of the lymphoid elements and chromafain cells (Fig. 5B).
Anterior kidney tissue of Nile tilapia (O. niloticus) infected with A. laevis showing multiple spore cysts within the hemobiotic center and at the periarterial sheet (Fig. 5C).
Discussion
Mass mortalities in Nile tilapia fishes at control treatment and those exposed to zoospores suspension of S. parasitica and A. laevis were matching 20%, 53% and 73%, respectively of total fish counts at each treatment. Direct bath exposure of Nile tilapia fishes to the inoculum of S. parasitica and A. laevis caused more fish mortalities were assessed to cause more fish mortalities by 33% and 53%, respectively than control treatment. In addition, these results of cumulative mortalities also indicated that A. laevis is more patent Nile tilapia fish plague than S. parasitica.
In this connection, the increase of mortalities in infected fishes may be attributed to the immediate effect of bath exposure on all fish organs. In this connection, Stueland et al. (2005) discovered that two of the Saprolegnia strains resulted in 89% and 31% total death rate in salmonids that were exposed to them. The infection of saprolegniasis in fish species exhibits rapid progression, resulting in significant mortality rates and substantial losses of both fish and eggs (Howe & Stehly, 1998; Ilondu et al., 2009). El-Sharouny & Badran (1995) reported that all tested fish species experienced severe infection and subsequently died due to the presence of Saprolegnia parasitica and S. ferax. Tilapia galileae exhibited greater susceptibility to fungal infection compared to Tilapia nilotica. In addition, they observed sores on the body of the fish, erosion of the fins and eyes, and darker gills as visible indications of Tilapia nilotica infected with zoosporic fungi.
Mycotic infections of fish species by zoosporic fungi was surveyed by many authors in different geographical regions of the world. In this respect, Sati (1981) documented that 80 isolates of different genera of Oomycetes, including Achlya, Aphanomyces, Dictyuchus, Protoachlya, Saprolegnia, Thraustotheca, and Pythium, infect fish in the temperate region of the Kumaun Himalaya in India. In a Nigerian freshwater fish pond Ogbonna & Alabi (1991) identified a total of 24 zoosporic fungal species from the infected fish. The following Oomycetes were found: Achlya racemosa, Aphanomyces laevis, Dictyuchus sterilis, Saprolegnia ferax, Saprolegnia litoralis, and Saprolegnia parasitica. Similarities were also discovered in the fungal species that were isolated from the infected individuals in the fish pond and those that were isolated from the hatchery. Clarias lazera and Tilapia zilli had the greatest abundance of fungal isolates. A total of 17 species of zoosporic fungi, classified into 6 genera, were obtained from the gills, fins, skin, and intestine of Tilapia (El-Sharouny & Badran, 1995).
In addition, Khulbe et al. (1995) isolated and identified Saprolegnia parasitica from four different fish species, specifically Mastacembelus armatus, Mystus vitatus, Nadus nadus, Tor putitora, and Tor tor, all found in the same reservoir in India. Also, Mondal & De (2002) identified Aphanomyces laevis as a newly discovered aggressive parasite of Aplocheilus panchax. They also observed that this parasite causes cotton-wool disease, which affects the skin and fins. Furthermore, Ali et al. (2011) identified many Oomycetous fungus as the causative agents of infections in two specific species of freshwater fish, namely Oreochromis niloticus niloticus and Clarias gariepinus.
Also, results of this investigation refer that A. laevis caused more mortalities in O. niloticus than S. parasitica. In other words, Nile tilapia fishes are more susceptible to infection by A. laevis than by S. parasitica. This finding came in agreement with Kiryu et al. (2003) who found that Aphanomyces invadans was highly pathogenic to Atlantic menhaden. Furthermore, Ali et al. (2011) claimed that Aphanomyces laevis was the commonest pathogenic Oomycetes species of Oreochromis niloticus niloticus and Clarias gariepinus.
During this study it was found that fishes exposed to zoospores cysts suspension of each of S. parasitica and A. laevis showed inflammatory changes in the skin and subcutaneous muscles in the region of mechanical injury as indicators for gross pathology. In this regard, Khulbe (1989) found that three species of Saprolegniales (Brevilegnia subclavata, Protoachlya oryzae and Scoliolegnia centrica) infected different fish species by artificial inoculation. Furthermore, Krishna et al. (1990) documented and substantiated the occurrence of mycosis caused by Saprolegnia parasitica in Indian main carps. The skin, gill, and fin exhibited cotton-like grayish white to light brown deposits, accompanied by regions of necrosis. Johnson et al. (2004) got results that were quite similar. They noticed the formation of lesions in four species of estuarine fish after injecting them with Aphanomyces invadans, and this occurred 5 days after the injection. Besides, massive fungal growth were appeared on the fins, gills and skin that associated with necrosis and ulceration in Tilapia infected with Saprolegnia parasitica. Furthermore, Johnson et al. (2004) documented that Atlantic menhaden Brevoortia tyrannus exhibited distinctive skin ulcerative lesions, referred to as ulcerative mycosis. The presence of these lesions is attributed to the Oomycete Aphanomyces invadans. A. flagellata and S. parasitica exhibited the highest level of virulence. In addition, Majhi et al. (2005) noted that fish affected by saprolegniasis had skin lesions characterized by a lack of mucous and a dry appearance. The viscosity and volume of mucus in various bodily regions may contribute to the development of saprolegniasis. Noga (1993) found that the concentration of mucus is lower on the fins compared to other areas of the body. As a result, a greater proportion of zoospores are able to attach to the fins rather than the rest of the body (Richards & Pickering, 1978). On the other hand, Ali et al. (2011) found that the growth of Oomycetes hyphae on Oreochromis nilotics niloticus was extensive across the entire body of the fish when they were exposed to inoculation in water. Contrary to this finding, Carballo et al. (1995) found that exposing rainbow trout Oncorhynchus mykiss to saprolegniosis agents in a bath did not result in significant mortality or only caused a small number of deaths.
Skin tissues of Nile tilapia fishes treated with suspension of zoospores cysts of each of the tested fungal species showed distinct histopathological alterations included mycotic dermatitis with presence of fungal hyphae, degeneration and sloughing of epithelium and inflammatory cell reaction in sub-epidermal layer. Somewhat similar results were reported by some interested authors. In this regard, Gaafar et al. (2010) found that skin of Nile tilapia exposed to pollutant edifenphos pesticide showed mild vacuolar degeneration of epidermal cells. In addition, Zaki et al. (2008) discovered that fungal hyphae and spores were present on the skin of S. parasitica infected fishes, causing significant degenerative, necrotic, and inflammatory responses. Furthermore, Aphanomyces induced skin ulcers in diseased fish (Sosa et al., 2007). In addition, fishes infected with Saprolegnia parasitica, showed epithelial desquamation with vacuolar degeneration and focal necrosis and fungal hyphae fragments appeared in dermis (Abou El Atta, 2008).
In the present work, the skin tissue of Nile tilapia fishes infected with zoospores cysts of S. parasitica showed mild mycotic dermatitis with congestion of some epithelial capillaries whilst severe damage in the skin of fishes treated with A. laevis. In this case, histopathological alterations appeared as obvious fungal hyphae, degeneration and sloughing of epithelium layer and inflammatory cell reaction in subepidermal layer associated with mycotic myositis. However, muscles of fishes infected with etiological agents of each of S. parasitica and A. laevis showed mycotic myositis, presence of inflammatory cell reaction and necrosis of the muscles fibers. In accordance, similar results were obtained by Zaki et al. (2008) in tilapia fishes infected with Saprolegnia parasitica. In addition, Aphanomyces induced deeply penetrating hyphae in the surrounding muscle tissue in diseased fish (Sosa et al., 2007). Also, degeneration in muscle bundles with aggregations of inflammatory cells between them and focal areas of necrosis were observed in Nile tilapia exposed to edifenphos pesticide (Kaoud & El-Dahshan, 2010).
Histopathological observations of the gills of fishes infected with zoospores cysts of each of S. parasitica and A. laevis showed several alterations. Gills of Nile tilapia fishes directly exposed to suspension of zoospores cysts of etiological agents of S. parasitica showed epithelial hyperplasia and increases the number of goblet cells and congestion of gill lamella capillaries. However, gills of Nile tilapia infected with A. laevis severely affected to large extent. They showed fungal hyphae in the subepithelial layer and increase in the number of goblet cells in the epithelial covering. In addition, there was a degeneration and sloughing of epithelium and congestion, separation between surface epithelium and capillary beds, telangiectasis and fusion of the secondary lamellae.
Gills of fish are vital; and sensitive organs for living and respiration of fish and they were affected negatively through direct exposure to etiological agents of pathogenic fungi which eventually causing death of fish. In this regard, Aly & El-Ashram (2000) found that when Saprolegnia infects the gills, it disrupts respiration and osmoregulation, and also leads to heamodilution. Severe gill infections can result in sudden respiratory collapse and ultimately death. Saprolegnia infection results in histopathological alterations in gills such as loss of the gill lamellae in severe cases associated with death (Ashour et al., 2017). Similarly, Gaafar et al. (2010) found that gills of Nile tilapia exposed to edifenphos pesticide showed congestion and separation between surface epithelium and capillary beds. In addition, the gills of tilapia that were exposed to heavy metals exhibited slight congestion and edema of the primary lamellae, an increase in the number of cells, fusion and focal desquamation of the epithelial lining of the secondary lamellae (Kaoud & El-Dahshan, 2010). Furthermore, Abou El Atta (2008) observed the desquamation in the majority of the secondary lamellae with few mononuclear leukocytes infiltration and the gill arch contained the fungal spores and hyphae. In addition, fungal hyphae were seen in the epitlelial lining of the secondary lamellae of Clarias gariepinus infected with yeasts (Refai et al., 2010).
When Saprolegnia invades the gills, it impairs respiration and osmoregulation as well as causing heamodilution; extensive gill infections can cause acute respiratory failure leading to death (Aly & El-Ashram, 2000). Saprolegnia infection results in histopathological alterations in gills such as loss of the gill lamellae in severe cases associated with death (Ashour et al., 2017).
Patel et al. (2018) reported that tissue samples of fish infected with Saprolegnia showed variable pathology in gills. They mentioned severe hemorrhage and edema in primary gill filaments with hyperplasia in epithelial cells and infusion in secondary gill filaments. Hyphae of Saprolegnia were seen between necrotic and edematous myofiber with inflammatory cells infiltration
In the present work, also, histopathological alterations in liver tissue of Nile tilapia treated with etiological agents of A. laevis appeared somewhat more affection when compared with S. parasitica treatment. Liver tissue of Nile tilapia treated with spores of A. laevis showed distinct necrosis of the hepatocytes and melanomacrophage center compared with vacuolar degeneration of hepatocytes at Saprolegnia parasitica treatment. In connection with our results, congestion of hepatic sinusoids, diffused vacuolar degeneration of the hepatocytes with necrotic focal areas and presence of esinophilic cells were observed in Nile tilapia exposed to edifenphos pesticide after 7 days as stated by Gaafar et al. (2010). Also, fungal hyphae were visible in hepatocytes of Clarias gariepinus infected with yeasts Refai et al. (2010). Moreover, degeneration of the hepatocytes, congestion of central vein, nuclear pyknosis were documented by Kaoud & El-Dahshan (2010) in the majority of hepatic cells of Nile tilapia exposed to heavy metals.
During this investigation, anterior kidney of fish infected with A. laevis showed multiple zoospores within the hemobiotic center and at the periarterial sheet. However, fish infected by S. parasitica showed multiple necrobiosis in the hemobiotic center with exhaustion of the lymphoid elements and chromafain cells. In agreement with our results, Gaafar et al. (2010) found that the anterior kidney of Nile tilapia exposed to edifenphos pesticide revealed severe activation of melanomacrophage centers with necrosis and depletion of the haematopoietic tissues. Moreover, the fungal hyphae were detected in the interstitial tissues of kidney of Clarias gariepinus infected by yeasts as mentioned by Refai et al. (2010).
