Introduction
The family Mormyridae belongs to the most primitive group of teleostean fishes- the Osteoglossomorpha (Nelson et al., 2016). Fishes of this family are endemic to African freshwater and include 22 genera and 230 species (Eschmeyer et al., 2020; Froese & Pauly, 2019; Moyle & Cech, 2000). In terms of genus and species diversity, the family Mormyridae exceeds all other extant Osteoglossomorph lineages (Simanovsky et al., 2020). All fish in the family produce weak electric discharges that can sense perturbations and be used as a means of communication (Carlson & Arnegard, 2011; Engelmann et al., 2009).
Mormyrids are known for a few notable structural and physiological characteristics, with the possession of a narrow caudal peduncle and a deeply forked caudal fin (Bell, 1989). They are diverse in morphology and unique in some external structures. The possession of mouths of highly variable form and often trunks like in some genera (e.g., Gnathonemus) is common. Phylogenetic trends are difficult to trace in mormyrids (Bass, 1986). Thus, morphological and molecular techniques have aided in further phylogenetic analysis (Lavoué et al., 2003). Molecular studies have provided a well-supported tree for the major mormyrid lineages (Alves-Gomes & Hopkins, 1997; Lavoué et al., 2003; Sullivan et al., 2002). The morphological works have also been employed in resolving fish taxonomical illusions (e.g., Abdurahman et al., 2016; Gelsano & Demayo, 2022; Kerschbaumer & Sturmbauer, 2011; Strauss & Bond, 1990). Both the molecular and morphological analysis in mormyridae taxonomical studies agree on: (1) mormyrid monophyly, (2) sister-group relationship between mormyridae and gymnarchidae, and (3) the basal division of the family mormyridae into two subfamilies (Mormyrinae and Petrocephalinae; Lavoué et al., 2003).
Among others, the ‘elephant-nose fish’ Gnathonemus petersii (Günther, 1862) is an African freshwater mormyrid and is native to the rivers of west and central Africa, in particular the river basins of lower Niger, Ogun and upper Chari. The species has a vast distribution and has been recorded in Mali, Benin, Niger, Nigeria, Chad, the Central African Republic, Cameroon, Democratic Republic of the Congo, and Zambia. In Lower Guinea, the species are found in the Cross, Mungo, Wouri, Lokoundjé, and Lower Sanaga Rivers (Nelson et al., 2016). The species is widely distributed throughout central Africa, from the Niger Delta to the Congo River basin. Thus, a wide distribution of the species suggests re-visionary work may uncover multiple species (Nelson et al., 2016).
G. petersii prefers slowly moving rivers and pools with muddy bottoms covered with submerged macrophytes (Engelmann et al., 2009). The species is a dark brown to black in color, laterally compressed (averaging 23–25 cm, total length [TL]), with a rear dorsal fin and anal fin of the same length with a forked caudal fin (Fig. 1). The species has two stripes on its lower pendicular and a trunk-like protrusion on the head. This protrusion is not a nose, but rather a sensitive extension of the mouth used for self-defense, communication, navigation, and finding prey. G. petersii can be recognized easily by the black brown coloration of two distinct bands in the shape of parentheses “()” running from the origins of dorsal to anal fins (Fig. 1). The species feeds on small worms and aquatic invertebrates such as mosquito larvae in the natural habitat (Nwani et al., 2011). The species has good low light vision; the eyes use a combination of photonic crystals, parabolic mirrors, and a clustered arrangement of rods and cones (Engelmann et al., 2009). As a unique feature in mormyrids, G. petersii possess a weak electric field from its electroreceptors (Engelmann et al., 2009).

The use of morphological parameters in taxonomical studies is common; this technique is very cost-effective and could be promoted as a tool for aiding in species identification, particularly for morpologically diverse fish species. Information on the external morphology of fishes is used in many taxonomical works (Hubbs & Lagler, 1958; Lagler et al., 1977; Miller & Lea, 1972; Moyle & Cech, 2000; Strauss & Bond, 1990; Trautman 1981). The morphology of fish is a result of adaptations to several forces. Environmental influences cause variations in the general structure, and thus measuring morphological variations in fish is crucial to resolving the question of taxonomical uncertainties (Strauss & Bond, 1990). The morphometric and countable traits of fish historically have been used for taxonomic and evolutionary studies (Brosse et al., 2021). Despite the value and availability of genetic, physiological, behavioral, and ecological data for such studies, systematic ichthyologists continue to depend heavily on morphology for taxonomic characters (Abdurahman et al., 2016; Gelsano & Demayo, 2022; Strauss & Bond, 1990). In addition to measurable and countable traits, species have characteristic shapes, sizes, pigmentation patterns, disposition of fins, and other external features that aid in recognition, identification, and classification (Gelsano & Demayo, 2022). Therefore, the present study aimed to characterize morphological variations in G. petersii collected from different localities in western and central Africa based on morphometric measurements, meristic counts, and observations on color pattern, positions of mouth, and fin arrangements.
Materials and Methods
A total of 119 fish specimens used in this study were obtained from museum preserved G. petersii deposited in the Royal Museum for Central Africa (RMCA; Ichthyology section). The specimens were collected from river basins of western Africa (Cameroon, DR Congo, Nigeria) and the Central Africa Republic in 2015. Each specimen was identified to species level and soaked in alcohol solutions. Damaged specimens due to preservation procedures were excluded during the analysis.
Museum collections of undamaged G. petersii voucher specimens from the RMCA were used for this study. Regardless of the method used to acquire morphometric measurements, care was taken to ensure that specimens were not abnormal or otherwise deformed in shape or posture because of preservation. Linear morphometric measurements were carried out from the left lateral side of the fish. Over all, 119 unbroken specimens previously identified as G. petersii were measured and analyzed for morphological variations (Table 1). About 29 important morphometric, eight meristic, and four descriptive characters were analyzed during the study (Table 1). Character definitions, methods for making counts, and measurements were based on Boden et al. (1997). The morphometric measurements were taken related to general body features, length, mouth, chin lobe, and head related regions (Strauss & Bond, 1990), whereas meristic parameters include fin ray and scale counts (Abdurahman et al., 2016). A dial calliper diameter with a precision level of 0.01 mm was used for morphometric measurements. Meristic counts were taken with the help of hand lenses and microscopes. The morphometric characters measured and their respective definitions are presented in Table 1.
Descriptive statistics were employed to ascertain the measured morphometric variables into a proportion of percentage in standard length (%SL) and head length (%HL). The principal component analysis (PCA), which is a widely used technique for the assessment of patterns of variations among a set of characters, was employed to examine variations in log transformed morphometric measurements. For the morphometric characters, a PCA analysis was used on percentage data in relation to %SL and %HL. Raw values of the meristic counts were used for the PCA analysis without conversions of proportions in to %SL and %HL. The PCA also permits the examination of size and shape differences independently and does not rely on ratios of measurements (Boden et al., 1997). Thus, the PCA was used to analyze and explore the data set in PAST software version 3.40 for this study.
Results and Discussion
During the present study, eight meristic characters were enumerated (Table 2). The variation among the counts in each parameters were rarely observed. The counts of pelvic and pectoral fins for all specimens were the same (6 and 10, respectively) and thus excluded for the analysis as recommended by Abdurahman et al. (2016). Therefore, this study was intended to trace the differences among the geographical locations where the specimens were collected. This is because meristic characters can be influenced substantially by environmental factors, especially temperature during early development. In the present study, however, as it is presented in Fig. 2, there was no clear separation on counts from different localities. However, some specimens collected from the Wouri and Sanaga Rivers showed some groupings being overlapped with some Nigerian and Congo specimens and tending to be aligned on the right side (Fig. 2). This might be attributed to the higher number of lateral line scales (LLS) in Wouri and Sanaga specimens over others, as reflected on first axis (PCA1) and second axis (PCA2) as a loading factor (Table 2). The proportion of the number of LLS was relatively higher in Sanaga specimens, followed by Wouri and Congo. By considering LLS, the study tried to encounter a pairwise comparison in each locality by categorizing specimens with less than 63 LLS as one group and more on the other group. However, clear separation was not observed on the number of LLS. The number of scales around the caudal peduncle was the same (eight) except for four specimens from Congo that had 10. Concerning the scales around the caudal peduncle, this study has tried to sort out a difference that had eight and 10 scales. However, there was no clear mark of isolation in between those having 8 and 10 (Fig. 2).
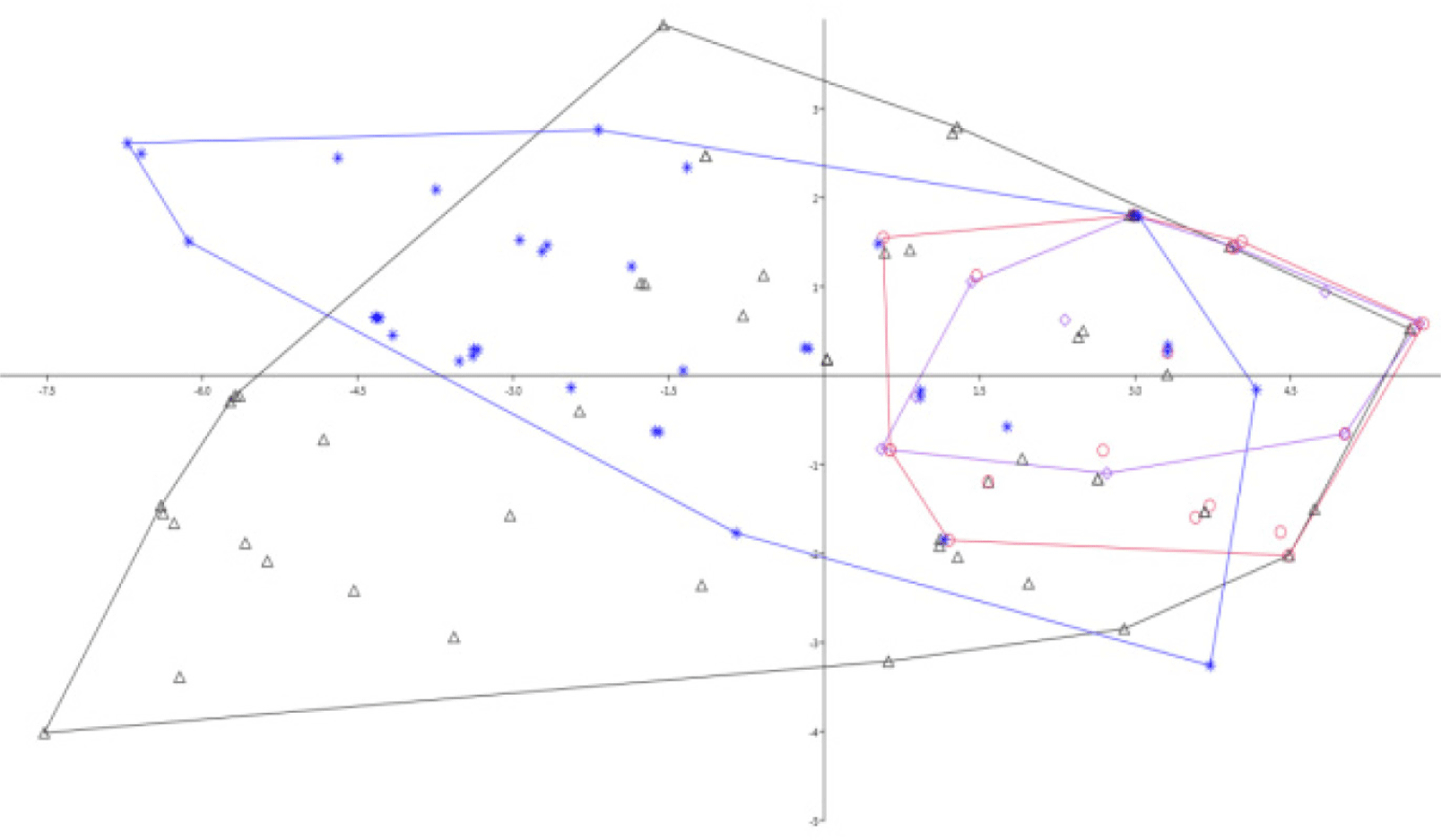
Meristic characters are the body segments and other features, primarily fin rays and scales, that once, in evolutionary history, corresponded to the body segmentation (Strauss & Bond, 1990). Meristic is a quantitative enumeration of the characteristics (body parts) of fish, for example, the number of fins. Meristic characters that are counted as many as eight characters, among others: DFR, anal fin rays (AFR), pelvic fin rays (PvFR), pectoral fin rays (PFR), scales in the lateral line (SLL), scales in the caudal peduncle (SCP), scales between dorsal fin and lateral line (SDLL), and scales between anal fin and lateral line (SALL; Tables 1 and 2). Similar counting patterns and enumeration techniques were followed by Haryono (2000). Because the evaluation of meristic and other countable characters can be subjective, published accounts should explicitly define the criteria used in making such counts accordingly Hubbs & Lagler (1958). The PCA loading factors showed that the PCA1 and PCA2 explained more of the characters counted for this study and included DFR, AFR, SLL, and SDLL (Table 2). Meristic characters can be influenced substantially by environmental factors, especially by temperature during early development (Gelasno & Demayo, 2022). As a result, countable characters vary within and among species, so they are useful in describing or identifying fishes.
Morphometric characters is a quantitative description, which have been successfully used for taxonomic inferences. Thus, documentation of morphological information is important to validate the taxonomical status and kinship relationship within or between species. A number of studies have shown that morphometric characters are suitable parameters for describing morphological variations among populations (Abdurahhman et al. 2016; Ahirwal et al., 2023; Biswal et al., 2018; Costa et al., 2003; Jacquemin & Pyron, 2016; Murta, 2000) and determining possible differences between individual unit stocks of the same species (Table 3; King, 2007). Out of the 29 morphometric variables measured for 119 samples, 18 variables showed significant differences. Without denying the role of genetic divergence, these differences might be caused by the differences in environment, as the studied specimens were sampled from different agroclimatic zones. Abdurahman et al. (2016) reported that freshwater fish display a range of morphological variations across a wide variety of physiological states and environmental conditions as a result of phenotypic plasticity. In the present study, the PCA analysis of measurements revealed that there was no clear separation among specimens collected from different localities. Morphometric variations are widely used to compare and differentiate among species and groups based on overall body type or distinctive anatomical forms in relation to one or two major body parts (Biswal et al., 2018; Jacquemin & Pyron, 2016).
SL, standard length; BD, body depth; HL, head length; HD, head depth; HW, head width; SnL, snout length; ED, eye diameter; IoW, inter orbital width; PoL, post orbital length; DNN, distance between nostrils; DNE, distance between nostril and eye; MW, mouth width; CIL, chin lobe length; PDD, predorsal distance; PAD, preanl distance; PPlD, prepelvic distance; PPcD, prepectoral distance; DFL, dorsal fin length; DFH, dorsal fin height; AFL, anal fin length; AFH, anal fin height; PlFL, pelvic fin length; PcFL, pectoral fin length; D-PlF-AF, distance between pelvic and anal fin; D-PcF-AF, distance between pectoral and anal fin; D-PcF-PlF, distance between pectoral and pelvic fin; D-DF-AF, distance between dorsal and anal fin; CPL, caudal peduncle length; CPH, caudal peduncle height; CPD, caudal peduncle depth.
Accordingly, all the measurements used for this study were in comparison with percentages of SL and HL (Table 4). The PCA2 is mainly defined by pelvic fin length, followed by chin lobe length, head width, and snout length (Table 4 and Fig. 3). On the other hand, the third axis (PCA3) is mainly defined by head width, inner orbital width, post orbital length, and distance between pectoral fin length and anal fin length. The analysis was also made separately for each locality, and it showed the same distribution on the plane (Figs. 3 and 4). A comparison was also made between Nigeria and Congo, but no clear separation was apparently observed.
PCA, principal component analysis; PCA1, first axis; PCA2, second axis; PCA3, third axis; PCA4, fourth axis; SL, standard length; BD, body depth; HL, head length; PDD, predorsal distance; PAD, preanl distance; PPID, prepelvic distance; PPcD, prepectoral distance; DFL, dorsal fin length; DFH, dorsal fin height; AFL, anal fin length; PIFL, pelvic fin length; PcFL, pectoral fin length; D-PIF-AF, distance between pelvic and anal fin; D-PcF-AF, distance between pectoral and anal fin; D-PcF-PIF, Distance between pectoral and pelvic fin; D-DF-AF, distance between dorsal and anal fin; CPL, caudal peduncle length; CPH, caudal peduncle height; CPD, caudal peduncle depth; HD, head depth; HW, head width; SnL, snout length; ED, eye diameter; IoW, inter orbital width; PoL, post orbital length; DNN, distance between nostrils; DNE, distance between nostril and eye; MW, mouth width; CIL, chin lobe length.
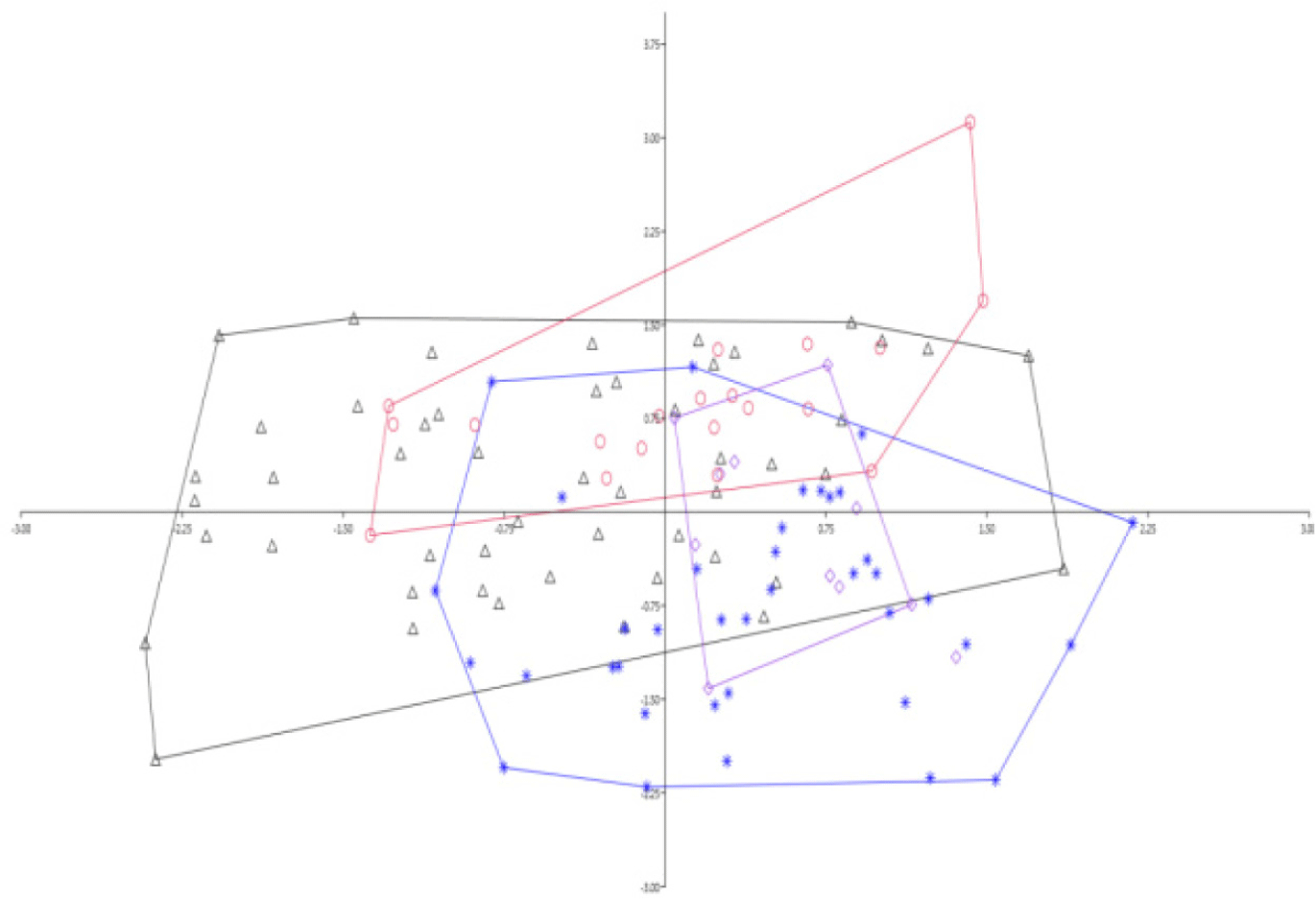
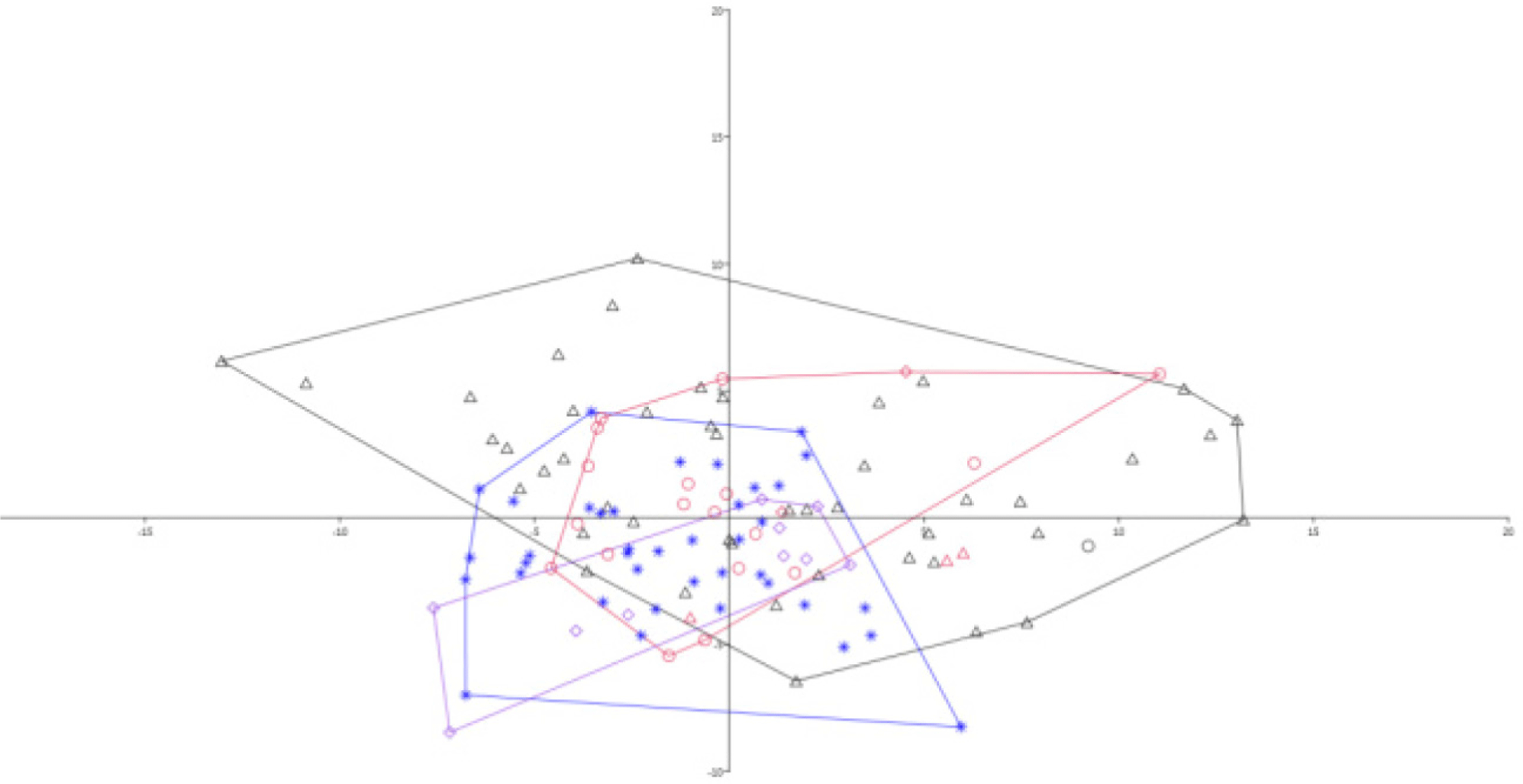
In the present study, the test for clear separation analysis applied for the G. petersii species using morphometric measurements showed somehow separations in between Congo and Sanaga specimens (Fig. 4). The taxonomic description of a species has commonly relied on the description of unique sets of morphological characters. The morphometric measurements, which represent %SL and %HL, were the most discriminate characters used in the present analysis (Table 4; Figs. 3 and 4). Specific to the long-term morphology of fishes, morphological variation that is correlated with physiological parameters such as body size or sex can be directly assessed from measurement or dissection of preserved specimens and analyzed by collection year (Jacquemin & Pyron, 2016). In this particular aspect, natural history museums like the RMCA play a significant role in fish taxonomy studies not accessible for molecular and genetic studies (Biswal et al., 2018). Morphological variation studies are also important to adopt management implications in addition to solving taxonomical uncertainties (Stange et al., 2018). This is because morphological variations triggered by habitat differences are typically manifested in freshwater ecosystems, which are vulnerable to change in global climate impacts (Biswal et al., 2018).
Conclusion
This study assessed the morphological variation of G. petersii specimens collected from west and central African rivers. The morphology of fish has been the key source for taxonomic studies and has been able to clearly showed variations among fish. However, the current study could not clearly show differences among the different voucher specimens of G. petersii collected from different water bodies. Accordingly, ichthyologists recommend the use of genetic markers for verification before drawing conclusion on relationships among morphologically assessed varieties. The present finding is important to make revisions on the diverse African mormyrids.
