Background
The genus of Grateloupia is consumed as a side dish of rice in the east coast of Korea. The price is very high as compared to the other seaweeds such as Undaria and Saccharina. Because of the economical importance of the Grateloupia spp., research on the cultivation techniques of these species should be carried out for mass cultivation in Korea. Grateloupia subpectinata, G. asiatica and G. divaricata are widely distributed along the east coast and Jeju Island (Lee and Kang, 2001; Lee, 2008; Lee et al., 2011). Grateloupia spp. have been known as invasive algae in North America and Europe possibly due to global shippings and mariculture activities (Harlin and Villalard-Bohnsack, 2001; Verlaque, 2001; Gavio and Fredericq, 2002; Verlaque et al., 2005; Wilkes et al., 2006; Mathieson et al., 2008) and supposed to have a wide range of adaptability.
Spatial and temporal variations of seaweed populations are influenced by differences in physical and biological factors (Schiel and Foster, 2006; Wichachucherd et al., 2010). Fluctuations in temperature, wind speed and upwelling index are related with annual variations in relative abundance of marine algae (Hemández-Guerrero et al., 2000). Beside the density, red algae have alternating life stages of gametophytes and sporophytes. Grateloupia is also one of them, and its life stages are isomorphic (Kawaguchi et al., 2001). However, those two life stages may respond differently to environmental factors. We investigated those differences with monthly survey throughout the year.
Studies on phenology as well as complex interactions between environmental factors and changes in abundance over seasons would be helpful for the management of this seaweed resource. These information may indicate when it is good timing for cultivation and also determine an ideal period for harvesting (Adharini and Kim, 2014; May-Lin and Ching-Lee, 2013). Therefore, the results of this research will be applied for the mariculture of Grateloupia.
Methods
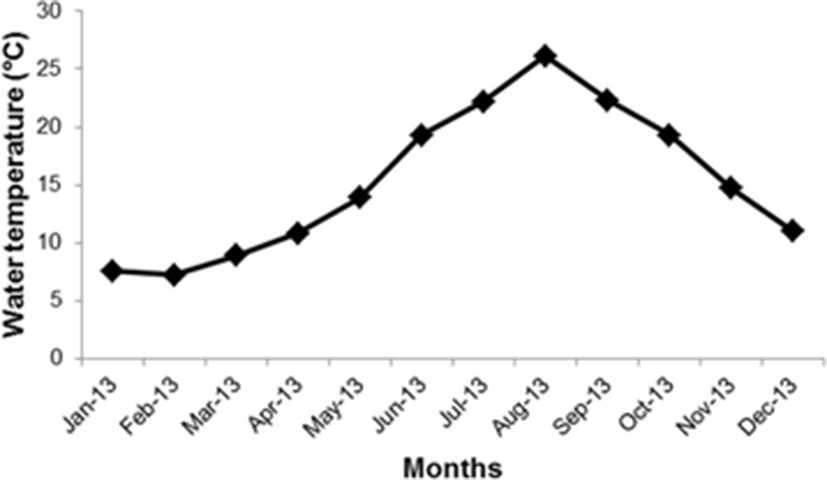
The study was carried out at Namae, Yangyang-gun in the eastern coast of Korea (38° 4′ N, 128° 37′ E). Namae coast is the intertidal zone which has rocky substrate and some parts covered by sands. Thalli of G. subpectinata were collected with holdfast from January to December 2013. Three quadrats of 50 cm x 50 cm were placed randomly to study population density. Seaweed samples were kept and stored into a cooler until carried to laboratory. Water temperature data were obtained from the database of Korea Oceanographic Data Center (KODC, 2014) in monthly averages of meteorological observations in 2013 (Fig. 1).
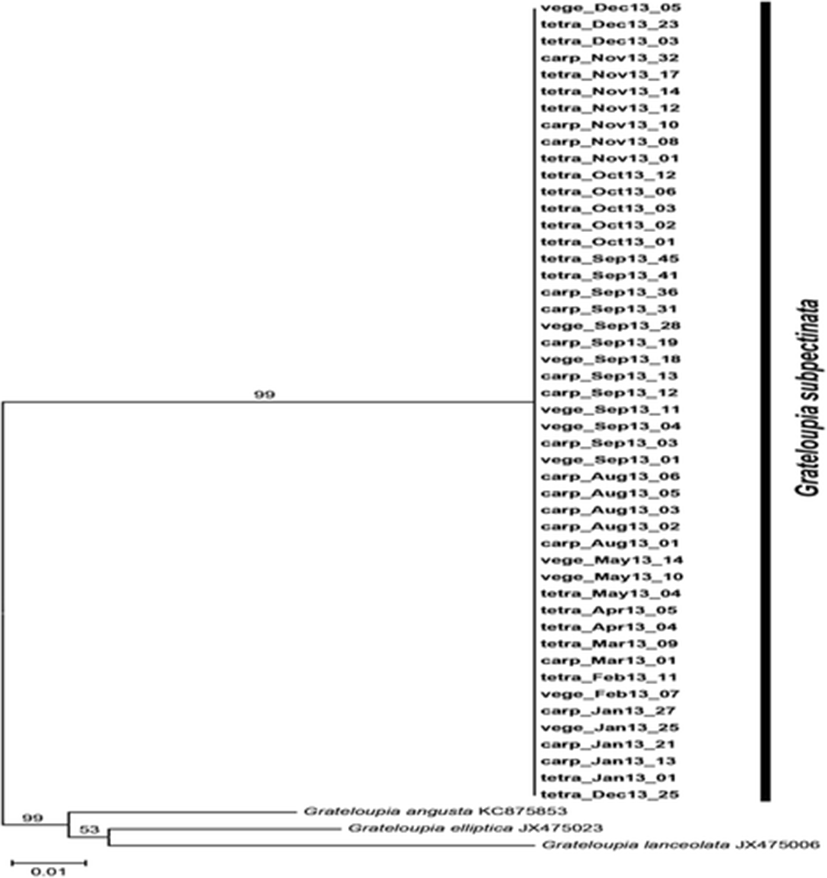
We analyzed cox1 gene from our monthly collections of G. subpectinata throughout the year. Specimens were pressed onto herbarium sheets and subsamples were dehydrated in silica gel for DNA analysis. DNA extraction, PCR amplification, and sequencing were performed as described in Boo et al. (2013). The primers used for amplifying and sequencing were COXI43F and COXI1549R for cox1 (Geraldino et al., 2006). Phylogeny of cox1 was reconstructed using neighbor joining method, using Grateloupia angustata, G. elliptica and G. lanceolata as outgroups (Fig. 2).
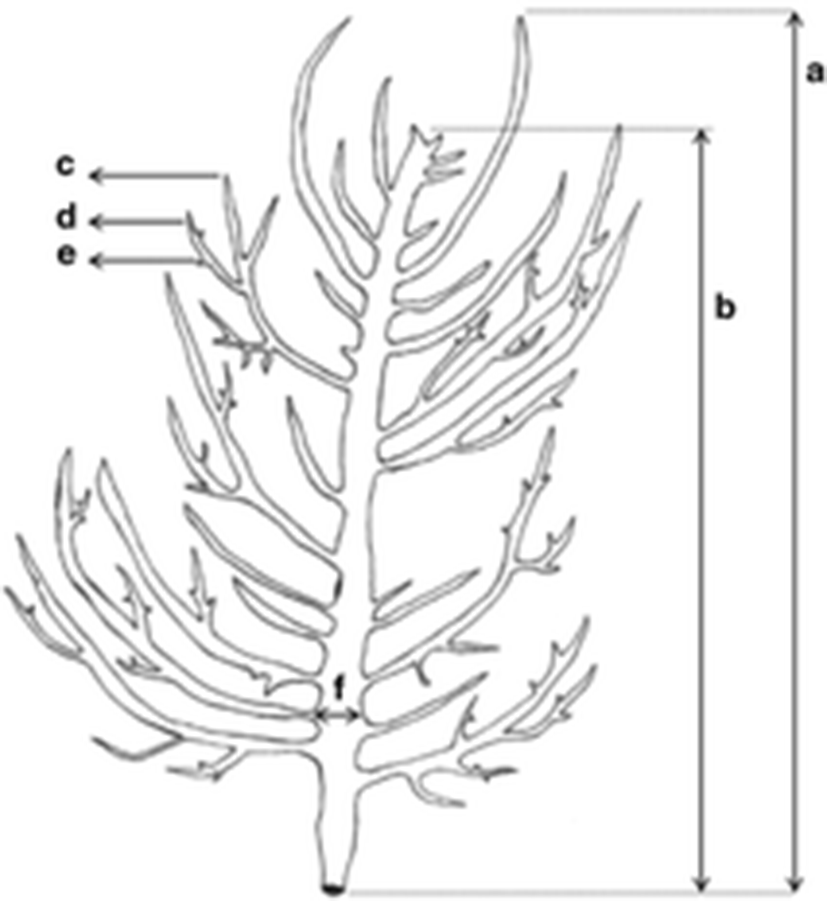
Fresh weight and total length of thalli and the width of main axis were measured. Samples were then checked for the reproductive status. Tetraposorophytes and carposporophytes were grouped and measured lengths of main axis, first branch, second branch and third branch of each thallus (Fig. 3).
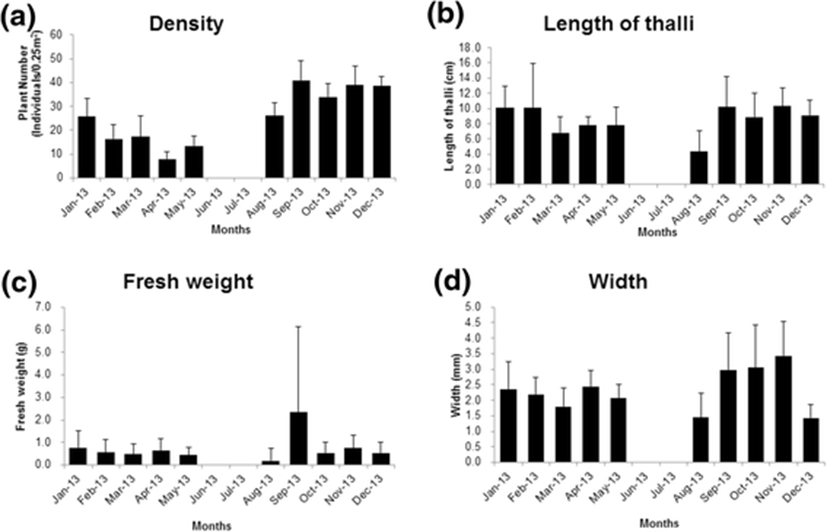
An estimation of the density, length of thalli, fresh weight, width and reproductive status in every month were presented graphically (Fig. 4). Comparison between tetrasporophytes and carposporophytes on total length, fresh weight and width of thalli were analyzed by t-test (Mann-Whitney Rank Sum Test) with significance level of 0.05. Statistical analysis was performed using the Sigma Plot 11 program.
Results
We generated 48 cox1 sequences from sample monthly collections except June and July when erect thalli disappeared. All cox1 sequences were identical with each other. Our cox1 sequences from cystocarpic and tetrasporangial fronds were exactly the same with those from vegetative fronds (Fig. 2).
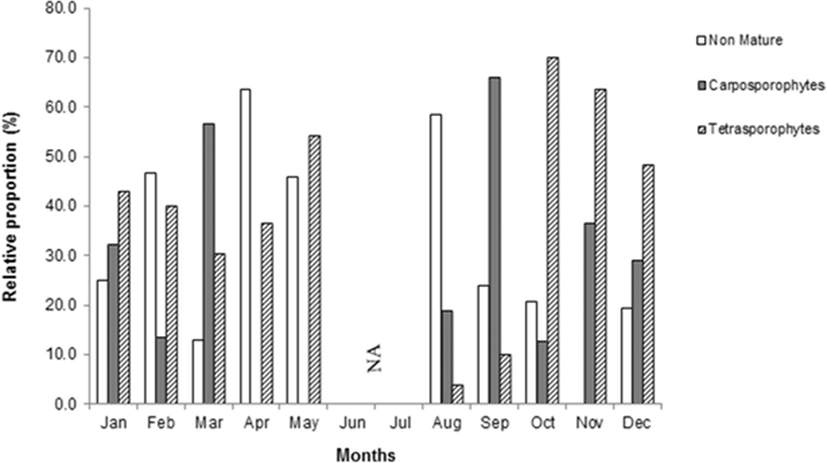
There were distinctive monthly changes in density, total length, and width of fronds (Table 1). Frond density was the greatest in September (41 individuals/0.25 m2) and it was high between autumn and winter. However, it was decreased from February to July when upright fronds were necrotic (Fig. 4a). Fronds grew well between September and February and they reached up to 10 cm in length. In March, main axis of many fronds were broken off, showing decrease in average frond lengths. After upright thalli were disappeared in June and July, they started forming juvenile thalli again in August and also became abundant (Fig. 4a, b). The highest fresh weight per individual frond was observed in September (2.34 g/ind, Fig. 4c). The widest thalli was observed in November (3.43 mm, Fig. 4d) and the spore maturation also was on peak in that month (Fig. 5).
Values are mean ± standard error (NA Not available)
Seasonal temperature was varied from 7.8–8.8 °C in winter (Dec-Feb), 9.3–18 °C in spring (Mar-May), 20–30 °C in summer (June-Aug) and 22.5 °C to 15.7 °C in fall (Sep-Nov) (Fig. 1).
Tetrasporophytes bearing tetrasporangia were abundant in October, November and May although they were observed throughout the year except in June and July. Meanwhile, carposporophytes were abundant in September and March. Juvenile thalli grew throughout the year, particularly abundant in August, February and April (Fig. 5).
Carposporophytes showed a high variation of length due to seasonality. Carposporophytes in January had long and tufty type because they had long main axes and first branches, and they were wide. In February they grew longer and tufty because they also had third branches. However, in March many thalli were fragmented in main axis and just remained long in the first branches. In April to July carposporophytes disappeared, they were shown again in August with long and tufty type. In September, carposporophytes were abundant which had two types of thalli. They were long, big, tufty carposporophyte type and short simple thalli carposporophyte type. These types were still abundant and available until November. However, in December, long and tufty thalli were fragmented on main axis which caused shorter in length (Table 2).
Values are mean ± standard error
Tetrasporophytes were collected throughout the year. Seasonal variation gave a significant effect on their phenology. In January, tetrasporophytes had long but simple thalli. However, in February and March, some thalli were fragmented in main axis and some were still as juvenile stage. In April and May, juvenile thalli grew longer which have long main axis with, and short thalli but have tufty branches. In June and July, thalli were disappeared, subsequently in August thalli had long and simple branches. Tetrasporophytes also grew in two types in September to November, which were long and tufty branches type and short simple thalli type. However, many thalli were fragmented in main axis in December which caused shorter main axis, but they still have tufty branches in basal portion (Table 3).
Values are mean ± standard error
The length of sporophytes main axis is longer than that of carposporophytes (Table 4). However, carposporophytes had longer first and second branches than tetrasporophytes. The longest main axis was observed in January, because thalli grew simple without third branches. The longest first branch was occurred in December, but main axis were shorter. Second branches were longest in February, while third branches were longest in April. On the other hand, carposporophytes were grown more axially and shown longer first and second branches than tetrasporophytes.
There was a significant difference on total length between carposporophytes and tetrasporopytes (p = 0.04). Fresh weight and width of thalli shown no significant difference between tetrasporophytes and carposporophytes.
Discussion
We successfully analyzed cox1 (1000 bp) from 48 specimens collected during our study despite some difficulties in DNA extraction and amplification from Grateloupia. As expected from previous rbcL study of G. subpectinata (Lee et al., 2009), our cox1 tree confirmed that all collected samples belonged to G. subpectinata. Genetic uniformity in all monthly collections suggests that Namae population of G. subpectinata may be derived from a single genetic source.
Higher density of Grateloupia was occurred from September to November and persisted until the beginning of winter season (22.5 to 7.8 °C). It was recorded that the growth of thalli were maximum at high temperature during summer season. Moreover, from September until November, thalli appeared to have different morphological types. Thalli grew abundant and looked healthy when temperature decreased. In the contrary, Perfeto (1998) reported that G. doryphora grew slowly in winter season and then began to grow in spring and showed maximal growth rate in summer. Growing season is different from species to species although they belongs to the same genus Grateloupia. Density began to decrease in February to May before thalli disappeared in June and July when summer season begins. It is suggested that in summer time they changed into crust form as a survival strategy to overcome high water temperature (20–25.3 °C). This phenomenon was also suggested by Harlin and Villalard-Bohnsack (2001) that G. doryphora changed to crustose form during unfavourable conditions as a strategy for survival. While, Ilma et al. (1995) suggested that crust form is an adaptation for survival when grazing pressure is high. High temperature also caused disappearance of gametophytes in Bangiales (Sheath and Cole 1984).
Density of G. subpectinata was decreased with high temperature while the abundance of Sargassum thunbergii was increased. It may be due to the competition for nutrients and sunlight. This phenomenon also occurred in Sarcothalia crispata which declined in density and biomass associated with unusual increase in density of G. doryphora (Otaiza et al., 2001). In June and July, Yangyang had more frequency of raining that affected low salinity and sedimentation on the rocky substrate. While, Eriksson and Johansson (2003) suggested that sedimentation affected attachment and survival of spores in Fucus vesiculosus. This phenomenon also occurred in Padina boryana which caused no recruitment in rainy season (Wichachucherd et al., 2010). In August, when water temperature reached the highest (30 °C), juvenile upright thalli of G. subpectinata were grown abundantly. It is suggested that water temperature of 25–30 °C is the optimum to trigger crusts for generating juvenile upright thalli. Grazers may act to disturb the density of sporeling (Griffin et al., 1999), however, grazing was not shown for G. subpectinata in this study.
The length and fresh weight of G. subpectinata thalli were highest in September when water temperature was 22.3 °C at Yangyang. Fresh weight were also peaked in September, thalli contained matured spores and were grown long, big and tufty. However, length of thalli became shorter after the high length period. It is suggested that after thalli get the high length and matured, those thalli were getting weak which caused fragmented in main axis.
Carposporophytes were abundant during winter season whereas the tetrasporophytes were available around the year except in June and July. Thus, thalli which were found mostly are in sporophytic stage than in gametophytic stage. The dominance of tetrasporophytes were also found in Gracilaria gracilis in Thau Lagoon in France (Marinho-Soriano et al.,1998), Gelidium pussilum in Songkhla, Thailand (Prathep et al., 2009) and G. gracilis from north-eastern Sicily, Italy (Polifrone et al., 2006). However, tetrasporophytes were abundant in October and November after carposporophytes grew abundant in September. It is suggested that carpospores had higher survivorship and then resulted higher abundance of tetrasporophytes. This phenomenon also occurred in G. gracilis which reported that diploid phase showed a higher resistance to environmental stress than haploid (Polifrone et al., 2006). Destombe et al. (1989) also mentioned that G. gracilis at juvenile stage of diploid had twice the survival rate than haploids. Meanwhile, tetraspores of Gracilaria skottsbergiiwere better adapted for growth in winter and carpospores to spring and summer, thus diploidic tetrasporophytic plants were more abundant in summer (Westermeier et al.,1999).
Seasonal variation gave significant effects on phenology of carposporophytes and tetrasporophytes of G. subpectinata at Yangyang. It is suggested that it was a kind of survivorship strategy for adapting the seasonally variating conditions like water temperature, photoperiod and wave hight. In January and February, carposporophytes grew with long main axis and tufty branches. However, in March when matured carposporophytes were grown abundantly, they were fragmented in main axis. Besides the effect of high waves, it may be also a kind of reproductive strategy to spread the carpospores into a wider space by the drifting of fragmented thalli. This phenomenon also reported in Sargassum muticum and its genus. At the end of reproductive period, fertile laterals were detached and would float to colonize distant area (Stiger and Payri 1999; Plouguerne et al., 2006). Dispersal strategy also occurred in Turbinaria ornata, when there was greater wave motion, mature plants with high number of receptacles would be driven away to increase the dispersal range (Prathep et al.,2007).
In August, carposporophytes of G. subpectinata at Yangyang grew with long main axis and tufty branches. Meanwhile in September, there were two kinds of carposporophyte types: 1) carposporophytes which have long and tufty thalli; 2) carposporophytes which have short and simple thalli. These two kinds of carposporophyte types were available from September to November. Meanwhile, tetrasporophytes grew with long and simple thalli in January. Subsequently, in February and March thalli of tetrasporophytes were decreased in the length of main axis. However, they still had long first and second branches. It may be some kind of survival strategy to respond defferent environmental conditions. The difference in phenology throughout the year also occurred in Sarcothalia crispata, that changed in abundance along season also have different types of fronds (Otaiza et al.,2001). Meanwhile, Prathep et al. (2007) suggested that shorter frond length or blade and fewer number of blades which was found in shore, can be assumed as acclimations to resist the higher energy wave force by minimizing thalli became fragmented. Instead of adaptation to the environmental changes, seaweeds sometimes use them such as the application of high waves to fragment and spread their fertile thalli in a wider place as a reproduction strategy. Carposporophytes have relationship between length and fresh weight, length and width, fresh weight and width higher than tetrasporophytes. It is suggested that tetrasporophyte have type of growth by elongating main axis, while carposporophyte have type of growth by elongating branches aside.
Conclusions
Grateloupia subpectinata showed various ratio of carposporophytes and tetrasporophytes. Tetrasporophytes were more abundant than carposporophytes in most of months except March, August and September. This is due to different growth and survivorship of haploid and diploid each month. Fresh weight of this alga showed high value in September. We can collect tetrasporophytes all around a year except June and July for seedling production, an also carposporophytes in September and March if we need diploid seeding. Preferred cultivation season of G. subpectinata will be good September to March of the next year. Base on those informations, we hope to easily develop mass culture methods of this alga in the future.