Background
Processing of raw fish into food products generates large quantities of byproducts such as scales, head, viscera and roes. Byproducts utilization will improve the economic aspects of processing industry and further their nutritional beneficiation through valuable essential amino acid and fatty acid components (Narsing Rao et al. 2012). It has been estimated that the value addition of human food developed from the byproduct will increase significantly in the future (Kristinsson and Rasco 2000; Tahergorabi et al. 2012).
Yellowfin tuna Thunnus albacares is a large epipelagic species widely distributed in the tropical and subtropical waters of the major oceans (Collette and Nauen 1983; Zudaire et al. 2013). Due to its high demand, yellowfin is harvested widely, and many types of fishing gear are used. Yellowfin tuna is widely used in raw fish dishes. And yellowfin tuna roe, a byproduct generated from fish processing (1.5–3.0 % of total weight), is generally used as animal feed or pet food preparation (Chalamaiah et al. 2013; Klomklao et al. 2013; Intarasirisawat et al. 2011). In our previous study, we fractionated inhibitors from fish roes, and confirmed the distribution of protease inhibitory activity in crude extracts from fish roes (Ji et al. 2011; Kim et al. 2013a; 2013b). In the present study, yellowfin tuna roe was used as a model for fish processing byproduct, and it was the starting material for isoelctric solubilization/precipitation (ISP). ISP process is to solubilize the muscle protein at low or high pH to separate soluble proteins from bone, skin, connective tissue, cellular membranes, and neutral storage lipids through the centrifugation (Nolsøe and Undeland 2009). The solubilized proteins are recovered by isoelectric precipitation to give a highly functional and stable protein isolate (Kristinsson and Ingadottir 2006; Chanarat and Benjakul 2013). The ISP processing has been applied to beef and fish processing byproducts (Chen and Jaczynski 2007a; 2007b; Mireles DeWitt et al. 2002). Various methods of protein isolate preparation have been reported for different protein sources, including legumes (Horax et al. 2004), oilseeds (Horax et al. 2011), cereals (Agboola et al. 2005; Ju et al. 2001; Paraman et al. 2007) and fish protein (Azadian et al. 2012; Chanarat and Benjakul 2013) based on solubility behavior of their proteins. Proteins isolates are the basic functional components of various high protein processed food products and thus determine the textural and nutritional properties of the foods. These properties contribute to the quality and sensory attributes of food systems (Foh et al. 2012). The roe of marine sources are the most underutilized fish by-products, which have considerable chance for value-addition to produce food and feed. Roes are easily decomposed with short shelf-life and hence, the roes should be processed immediately or converted into value added foods to enhance their shelf-life (Narsing Rao et al. 2012).
No scientific information is available on the protein isolate preparation from yellowfin tuna roe. The aims of this study was to investigate the chemical compositions, amino acid profile, mineral profile, color from yellowfin tuna protein roe isolate and second by-products with isoelectric solubilization/precipitation using basic and acidic pH treatments.
Methods
Yellowfin tuna Thunnus albacares (YT) roe was obtained from Dongwon F&B Co., Ltd. (Changwon, Korea). Roe was stored at −70 °C in sealed polyethylene bags, and transferred to the laboratory. Frozen roe was partially thawed for 24 h at 4 °C and then cut into small pieces with an approximate thickness of 1.5–3 cm and minced with food grinder (SFM-555SP, Shinil Industrial Co., Ltd., Seoul Korea). Minced roe was frozen at −20 °C until used.
Bovine serum albumin (BSA), casein, hemoglobin, β-Mercaptoethanol (β-ME), glycerol, N,N,N′,N′-tetramethyl ethylene diamine (TEMED), sodium carbonate, sodium hydroxide, sodium L-tatarate, and potassium hydroxide were purchased from Sigma-Aldrich Co., LLC. (St. Louis, MO, USA). Copper (II) sulfate pentahydrate was purchased from Yakuri Pure Chemicals Co., Ltd. (Kyoto, Japan). Bromophenol blue and Folin-Ciocalteu’s reagent were purchased Junsei Chemical Co., Ltd. (Tokyo, Japan). All reagents used analytical grade.
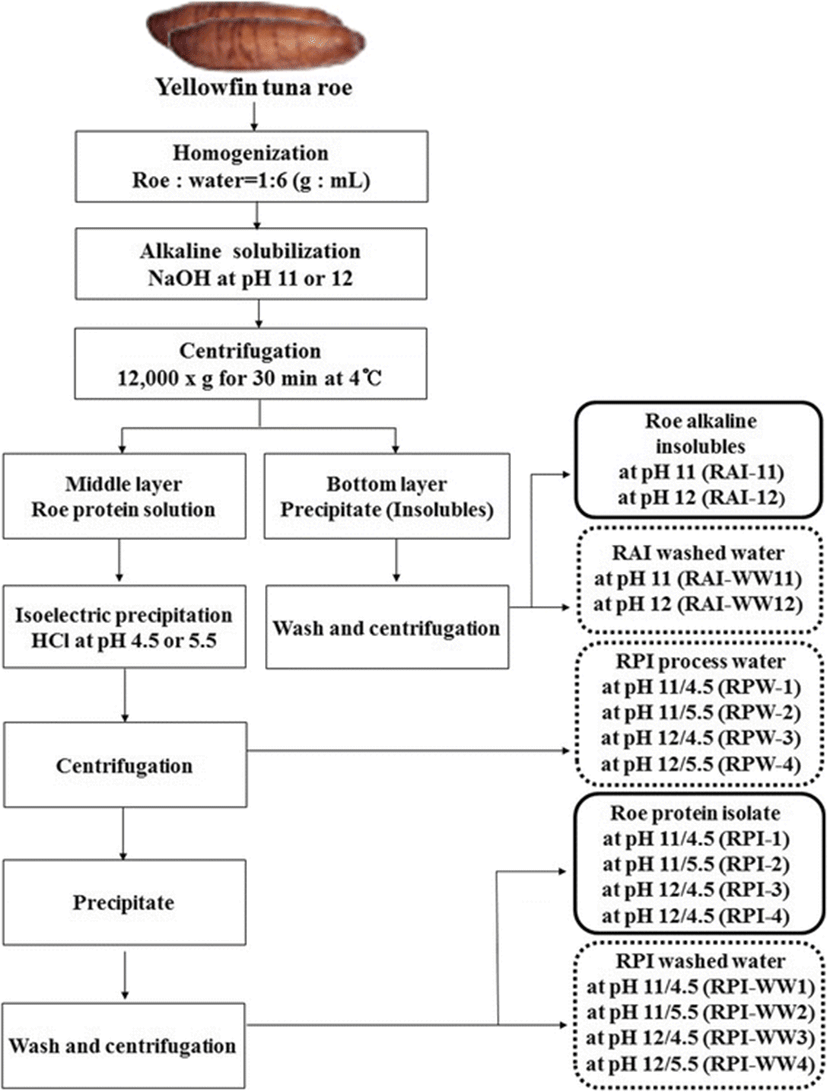
The frozen minced roe was homogenized with distilled deionized water (DDW) at a ratio of 1:6 (w/v) using a homogenizer (POLYTRON® PT 1200E, KINEMATICA AG, Luzern, Switzerland). The homogenate was adjusted to pH 11 and 12 with 2 N NaOH, respectively. Alkaline solubilization was solubilized protein and inactivated endogenous enzymes in the homogenate. Once the desired pH was reached, the solubilization reaction was allowed to take place at 4 °C for 1 h, followed by centrifugation at 12,000 g and 4 °C for 30 min using a refrigerator centrifuge (Supra 22 K, Hanil Science Industrial Co., Ltd., Incheon, Korea). After centrifugation, two alkaline solubles (pH 11 and 12) in the supernatant fraction, and two alkaline insolubles (pH 11 and 12) as processing 2’nd byproduct in the precipitate fraction were separated. First, to prepare the protein isolates from alkaline solubles through acid precipitation, those of pH were readjusted by addition of 2 N HCl to pH 4.5 and 5.5, respectively, a value near the isoelectric point of fish proteins. The suspensions were centrifuged at 12,000 g and 4 °C for 30 min. After centrifugation, the supernatants were collected as roe process waters (RPWs) and referred to as RPW-1 (pH 11/4.5), RPW-2 (pH 11/5.5), RPW-3 (pH 12/4.5) and RPW-4 (pH 12/5.5), respectively. Precipitates by alkali solubilization and acid precipitation were additionally washed with DDW by centrifugation at 12,000 g and 4 °C for 30 min to remove the NaCl. After centrifugation, the washed roe protein isolates (RPIs) were lyophilized and referred to as RPI-1 (pH 11/4.5), RPI-2 (pH 11/5.5), RPI-3 (pH 12/4.5), and RPI-4 (pH 12/5.5), respectively. The roe protein isolate washed water (RPI-WW) as the 2’nd byproduct referred to as RPI-WW1 (pH 11/4.5), RPI-WW2 (pH 11/5.5), RPI-WW3 (pH 12/4.5) and RPI-WW4 (pH 12/5.5), respectively. Alkaline insolubles were resuspended with DDW, then readjusted to pH 6.5 with 2 N HCl, and centrifuged at 12,000 g and 4 °C for 30 min. The supernatants were collected and referred to as roe alkaline insoluble washed water (RAI-WW11 and RAI-WW12, respectively). The precipitate was lyophilized and referred to as roe alkaline insolubles (RAI-1 and RAI-2, respectively). All samples were stored at −20 °C until further experiments. A flow chart for the preparation of roe protein isolates (RPIs) is shown in Fig. 1.
The proximate composition was determined according to the AOAC method (AOAC 1995). Moisture content was determined by oven-drying method at 105 °C until a constant weight was reached. The ash content was obtained by ashing a sample in a muffle furnace (Thermolyne 10500 furnace, a subsidiary of Sybron Co., Dubuque, IA, USA) at 550 °C until a constant final weight for ash was achieved. The total crude protein (N × 6.25) content of samples was determined using the semi-micro Kjeldahl method. Total lipid content was determined according to the Soxhlet extraction method.
Soluble protein concentration of sample was determined by the method of Lowry et al. (1951) using bovine serum albumin as a standard.
Total amino acid analysis was conducted according to AOAC method (AOAC 1995). The sample (20 mg) was hydrolyzed with 2 mL of 6 N HCl at 110 °C for 24 h in heating block (HF21; Yamoto Science Co, Tokyo, Japan) and filtered out using vacuum filtrator (ASPIRATOR A-3S, EYELA, Tokyo, Japan). Amino acids were quantified using the amino acid analyzer (Biochrom 30, Biochrom Ltd., Cambridge, United Kingdom) employing sodium citrate buffers (pH 2.2) as step gradients. The data are reported as mg of amino acid per 100 g of protein.
Analysis of iron (Fe), copper (Cu), manganese (Mn), cadmium (Cd), nickel (Ni), lead (Pb), zinc (Zn), chromium (Cr), magnesium (Mg), sodium (Na), phosphorus (P), potassium (K), calcium (Ca), and sulfur (S) contents in sample was carried out using the inductively coupled plasma optical emission spectrophotometry (OPTIMA 4300 DV, Perkin Elmer, Shelton, Conn., USA). Briefly, teflon digestion vessel was washed overnight in a solution of 2 % nitric acid (v/v) prior to use.
Sample was dissolved in 10 mL of 70 % nitric acid. The mixture was heated on the hot plate until digestion was completed. The digested samples were added in 5 mL of 2 % nitric acid and filtered using filter paper (Advantec No. 2, Toyo Roshi Kaisha, Ltd., Tokyo, Japan). Sample was massed up to 100 mL with 2 % nitric acid in a volumetric flask
Hunter color properties of samples were equilibrated to room temperature for 2 h prior to the color measurement. Colors were determined using color meter (ZE-2000 Nippon Denshoku Inc., Japan). The colorimeter was calibrated by using a standard plate (L* = 96.82, a* = −0.35, b* = 0.59) supplied by the manufacturer. The values for the CIE (Commission Internationale d’Eclairage of France) color system using tristimulus color values, L* (lightness), a* (redness), and b* (yellowness) were determined. The whiteness was calculated by the following equation:
The molecular weight distribution of protein isolates and their 2’nd byproducts was observed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE) according to the method of Laemmli (1970). Briefly, 10 mg of sample was solubilized in 1 mL of 8 M urea solution containing 2 % β-mercaptoethanol and 2 % sodium dodecyl sulfate (SDS) solution. Protein solution was mixed at 4:1 (v/v) ratio with the SDS-PAGE sample treatment buffer (62.5 mM Tris–HCl (pH 6.8), 2 % SDS (w/v), 10 % glycerol, 2 % β-mercaptoethanol and 0.002 % bromophenol blue) and boiled at 100 °C for 3 min. The sample (20 μg protein) was loaded on the Any KD™ Mini-PROTEAN® TGX™ Precast gel (Bio-Rad Lab., Inc., Hercules, CA, USA) and subjected to electrophoresis at a constant current of 10 mA per gel using a Mini-PROTEAN® Tetra cell (Bio-Rad Lab. Inc., Hercules, CA, USA). Electrophoresed gel was stained in 0.125 % Coomassie brilliant blue R-250 and destained in 25 % methanol and 10 % acetic acid until background was clear. Molecular weight of protein bands was estimated using Precision Plus Protein™ standards (10–250 K, Bio-Rad Lab., Inc., Hercules, CA, USA).
All experiments were conducted in triplicates. The average and standard deviation were calculated. Data were analyzed using analysis of variance (ANOVA) procedure by means of the statistical software of SPSS 12.0 KO (SPSS Inc., Chicago, IL, USA). The mean comparison was made using the multiple range Duncan’s test (P < 0.05).
Results and discussions
Roe protein isolates (RPIs) from yellowfin tuna roe were prepared according to previously described ISP process. Proximate compositions of RPIs and positive controls (casein and hemoglobin) are shown in Table 1. Yields of RPIs prepared from YTR by ISP process were in range of 11.6–14.1 % with slight difference. Moisture content of the RPIs ranged 2.3 to 5.0 %. RPI-1 (87.8 %) had the highest protein content than RPI-2 (83.2 %), RPI-4 (79.6 %) and RPI-3 (79.1 %), respectively (P < 0.05). protein content of RPIs was but lower than hemoglobin (94.4 %, P < 0.05). Protein content of RPIs were higher than that reported for fish protein powders obtained from different raw materials (Sathivel et al. 2004, 2005, 2006; Sathivel and Bechtel 2006; Shaviklo et al. 2011). Protein powder prepared from fish roe or surimi (Sathivel et al. 2009; Huda et al. 2001) had similar to protein content. Lipid (5.6–7.4 %) and ash content (3.0–3.8 %) of RPIs were lower than those of FDC (P < 0.05). Pires et al. (2012) reported that mineral and fat were eliminated in the supernatant obtained after ISP process. Protein content of RPI-1 and −2 precipitated at pH 4.5 and 5.5 after alkaline solubilization at pH 11, was significantly higher than those of RPI-3 and −4 after alkaline solubilization at pH 12 (P < 0.05). Whereas, yield of RPI-1 and −2 was lower than that of RPI-3 and −4. In this result, total protein yield of all RPIs was not significantly different (P > 0.05). Final supernatant referred to roe process water (RPW) as 2’nd byproduct was generated through alkaline solubilization and acid precipitation. Their moisture and protein contents are shown in Table 2. Moisture content of the RPWs ranged from 98.9 to 99.0 % and protein concentration (mg/mL) of RPWs ranged from 2.6 to 3.0 mg/mL. RPW-1 and RPW-3 (1478.5 and 1420.9 mg, respectively) had lower total protein than RPW-2 and RPW-4 (1861.2 and 1605.3 mg, respectively) in this experiment. Lower total protein of supernatant (RPW-1 and −3) obtained by acid precipitation at pH 4.5 could be more efficient to raise the yield of RPI as precipitate, compared with protein isolate at pH 5.5. Roe alkaline insoluble-washed waters (RAI-WWs) and roe protein isolate-washed waters (RPI-WWs) were obtained through washing process to reduce remaining salt in RAI and RPI. Their moisture and protein content are shown in Table 3. Moisture content of all washed water was ranged from 99.5 to 99.7 % with no difference. Total protein of RAI-WWs in range of 315.0–340.3 mg was higher than that of RPI-WWs in range of 133.5–192.4 mg). During ISP process, it was possible to recover more than 90 % of the process water (RPWs, RAI-WWs and RPI-WWs) contained 0.50-3.4 mg/mL protein.
FDC freeze-dried concentrate; RAI-11 and RAI-12, roe alkaline insolubles after alkaline solubilization at pH 11 or 12 respectively. RPI-1 and RPI-2, roe protein isolate adjusting at pH 4.5 and 5.5, respectively, after alkaline solubilization at pH 11; RPI-3 and RPI-4, roe protein isolate adjusting at pH 4.5 and 5.5, respectively, after alkaline solubilization at pH 12
Data are given as mean values ± SD (n = 3)
Means with different letters within the same column are significantly different at P < 0.05 by Duncan’s multiple range test
-; not determined
aYield is weight (g) of roe protein isolate obtained from 100 g of raw YTR
bProtein yield (g) = yield x protein (%)
RPW-1 and RPW-2, roe process water adjusting pH 4.5 and 5.5, respectively, after alkaline solubilization at pH 11; RPW-3 and RPW-4, roe process water adjusting pH 4.5 and 5.5, respectively, after alkaline solubilization at pH 12
Values are means ± standard deviation of triplicate determinations
Means with different letters within the same column are significantly different at P < 0.05 by Duncan’s multiple range test
aBased on Lowry’s method (1951)
RAI-WW11 and RAI-WW12, roe alkaline insoluble washed waters of RAI-11 and RAI-12, respectively
RPI-WW1-4, roe protein isolate washed water of RAIs (1–4)
Values are means ± standard deviation of triplicate determinations
Means with same letters within the moisture (%) and protein (%) are not significantly different at P > 0.05, and means with different letters within the protein concentration are significantly different P < 0.05 by Duncan’s multiple range test
aBased on Lowry method (1951)
Total amino acid composition (g/100 g protein, %) of RPIs, RAIs, and positive controls (casein and hemoglobin) are shown in Table 4. Protein content of all samples ranged from 81.6 to 96.3 % on a dry base. From the result, RPIs had a EAAs/NEAAs acid ratio in range of 1.08 to 1.17. These were higher than that (0.92) of casein, but slightly lower than that (1.33) of hemoglobin. Leucine (8.8–9.4 %), lysine (8.5–8.9 %) and isoleucine content (5.8–6.3 %) of RPIs were significantly higher than those of RAIs (P < 0.05). It is indicated that total essential amino acid content of RPIs in range of 51.9–53.9 % were higher than that (47.1–49.0 %) of RAIs. Intarasirisawat et al. (2011) reported that leucine (8.28–8.64 %) and lysine (8.24–8.30 %) were the predominant essential amino acids in defatted tuna roe from skipjack, tongol and bonito. Lysine is often considered a first limiting amino acid for cereal food. Therefore, it needs to be emphasized that the RPIs had a higher content of lysine than egg white (8.2 %) (P < 0.05). The lysine content of RPIs was higher than that reported for Channa (6.94 %) and Lates (6.86 %) roe protein concentrates (Narsing Rao et al. 2012). The major non–essential amino acids (NEAAs) of RAIs as 2’nd byproduct by alkaline solubilization were glutamic acid (13.7–13.8 %), aspartic acid (8.8–8.9 %) and arginine (6.4–6.6 %), respectively. Amino acids namely glutamic acid (13.14 g), aspartic acid (8.08 g) and arginine (5.76 g) were reported per 100 g roe protein of Channa roe protein concentrate (Narsing Rao et al. 2012).
TAA total amino acid, EAA essential amino acids, NEAA non essential amino acids
FDC freeze-dried concentrate; RAI-11 and RAI-12, roe alkaline insolubles after alkaline solubilization at pH 11 or 12 respectively. RPI-1 and RPI-2, roe protein isolate adjusting at pH 4.5 and 5.5, respectively, after alkaline solubilization at pH 11; RPI-3 and RPI-4, roe protein isolate adjusting at pH 4.5 and 5.5, respectively, after alkaline solubilization at pH 12; Hb hemoglobin
Values are means ± standard deviation of duplicate determinations
Means with different letters within the same row are significantly different at p < 0.05 by Duncan’s multiple range test
aBased on dry weight
bEssential amino acids for infant
cHydrophobic amino acids
NEAAs of RAIs were relatively higher than those data of FDC. Predominant essential amino acids (EAAs) of RAI-11 were leucine (8.0 %), lysine (7.6 %) and valine (6.7 %), respectively. These were similar to those of RAI-12 which had leucine (7.5 %), lysine (7.3 %) and valine (6.3 %). EAAs/NEAAs ratio of RAI-11 (0.96) was higher than that of RAI-12 (0.89). From the result, total essential amino acid (isoleucine, leucine, lysine, methionine, phenylalanine, threonine, valine) content of RAI-11 (49.0 %) was higher than that of RAI-12 (47.1 %). However, EAAs content of RAIs (47.1–49.0 %) were lower than that of FDC (51.1 %). Thus, EAAs/NEAAs ratio of RAIs (0.89–0.96) were lower than that (1.04) of FDC. RPIs were rich in glutamine, asparagine, leucine, and lysine which accounted for 12.4–12.7 %, 9.1–9.3 %, 8.8–9.4 % and 8.3–8.9 % of total amino acid, respectively. The hydrophobic amino acid content of RAIs and RPIs were similar in range of 45.4–46.3 %. However, RAI was richer in proline and glycine than RPIs (P < 0.05). In the case of proline and glycine the difference may be due to the elimination of collagenous material during the protein recovery by the alkaline solubilization. Lysine content of RAIs and RPIs was similar to that (6.1 to 9.7 %) of pollock protein samples reported by Sathivel et al. (2006). RPIs and RAIs can be used as a nutritional supplement due to the content of essential amino acids composition.
The mineral contents (mg/100 g) of FDC and RAIs and RPIs are given in Table 5. The main functions of essential minerals include skeletal structure, maintenance of colloidal system and regulation of acid–base equilibrium, and mineral also constitute important components of hormones, enzymes and enzyme activators (Belitz and Grosch 2001). The major mineral contents of RPIs and RAIs were S (591.4–715.7 mg/100 g) K (36.2–130.6 mg/100 g), Na (78.2–303.3 mg/100 g), and Ca (7.8–39.4 mg/100 g). The K content of RPIs was found to be in ranged of 36.2–68.5 mg/100 g, respectively. Among RPIs, the RPI-4 (pH 12/5.5) had the highest content of Na (244.4 mg/100 g) and K (68.5 mg/100 g) than RPI-3 (pH 12/4.5), RPI-2 (pH 11/5.5) and RPI-1 (pH 11/4.5) (P < 0.05). However, Na content of RPIs was lower than that of crab (Gokoglu and Yerlikaya 2003), rainbow trout (Gokoglu et al. 2004) and fish based dishes (Martinez-Valverde et al. 2000). P content of RPIs (215.3–254.6 mg/100 g) was lower than that (337.8 mg/100 g) of rainbow trout reported for Gokoglu et al. (2004) and higher than that (215.0–231.0 mg/ 100 g) of European perch reported Orban et al. (2007). Mg content of RPI-2 and RPI-4 (32.3–21.4 mg/100 g) were higher than those (6.6-4.6 mg/100 g) of RPI-1 and RPI-3 (P < 0.05). S and Ca content of RPIs were no significant difference (P > 0.05). Na, K and Mg content of RAI-11 (213.0, 108.9 and 23.4 mg/100 g, respectively) were lower than those (303.3, 130.6 and 51.7 mg/100 g, respectively) of RAI-12 (P < 0.05). These values were higher than RPIs (P < 0.05). This result is indicated that Na, K and Mg content of RPIs could be eliminated during the alkaline solubilization process. Mg of RAIs was similar to that (33.0–34.0 mg/100 g) of sea bream (Orban et al. 2000) and that (25.1–33.6 mg/100 g) of Baltic herring (Tahvonen et al. 2000). But, P content of RAIs contained in range of 84.3–97.1 mg/100 g was lower than those of RPIs (P < 0.05) because of solubilization of P content for RAIs during alkaline solubilization process. Ca content of RAIs (39.4-37.2 mg/100 g, respectively) was significantly higher (P < 0.05) than those found in RPIs. During the ISP process, K, Na, Ca and S content of RPIs and RAIs were significantly lower than those (912.0, 706.3, 987.2 and 1984.1 mg/100 g, respectively) of casein (P < 0.05). In case of hemoglobin, similar results were observed in RAIs and RPIs except for Fe and S. Potentially, the RAIs as recovered insoluble may be useful in animal feeds as a mineral additive due to the relatively high concentration of minerals (Ca, Mg, and K).
FDC freeze-dried concentrate; RAI-11 and 12, roe alkaline insolubles after alkaline solubilization at pH 11 and 12, respectively; RPI-1, 2, 3 and 4, roe protein isolate adjusting at pH 4.5 and 5.5, respectively after alkaline solubilization at pH 11 and 12; Hb hemoglobin
Values are means ± standard deviation of triple determinations
Means with different letter within the same row are significantly different at P < 0.05 by Duncan’s multiple range test
-; not determined
The color properties of FDC, RAIs and RPIs are presented in Table 6. L*, a*, and b* of RAIs were higher than RPIs (P < 0.05). Among the RPIs, RPI-1 was the lightest with L* value (59.6) followed by RPI-3 (57.8) RPI-4 (57.7) and, RPI-2 (57.2), respectively (P < 0.05). RPI-1 and RPI-3 had the lower b* value of 16.4 and 16.2, respectively than those (17.3 and 17.4, respectively) of RPI-2 and RPI-4 (P < 0.05). RPI-2 and RPI-4 had higher a* value (5.6 and 5.5, respectively) than that (4.6 and 5.0, respectively) of RPI-1 and RPI-3 (P < 0.05). However, RPI-1 and RPI-3 had higher whiteness values (56.2 and 54.5, respectively) than the other RPIs (53.5 for RPI-2 and 53.9 for RPI-4). RAI-11 was lightest (P < 0.05) with L* value of 64.3 than that (63.7) of RAI-12. a*, b* and ΔE values of RAI-11 (5.7, 19.5 and 38.2 respectively) were slightly lower than those of RAI-12. Whiteness value of RAI-11 (58.9) was higher than that (58.1) of RAI-12 (P < 0.05). This different in color could be due to the separation pigment content caused by ISP process. Among the RAIs and RPIs, RAI-11 could be more useful as a food ingredient because of high value of whiteness.
FDC freeze-dried concentrate; RAI-11 and 12, roe alkaline insolubles after alkaline solubilization at pH 11 and 12, respectively; RPI-1, 2, 3 and 4, roe protein isolate adjusting at pH 4.5 and 5.5, respectively after alkaline solubilization at pH 11 and 12; Hb hemoglobin
Values are means ± standard deviation of triplicate determinations
Means with different letters within the same row are significantly different at p < 0.05 by Duncan’s multiple range test
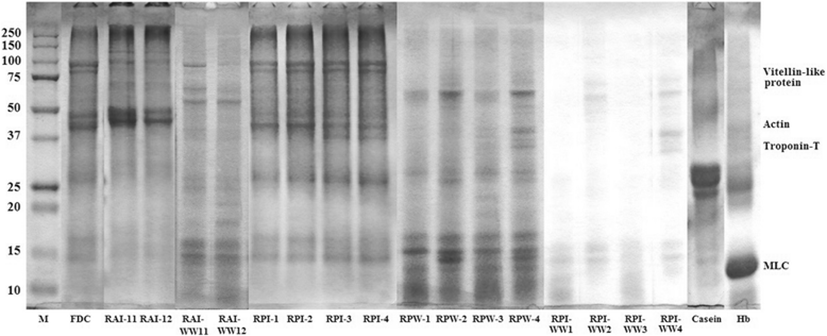
SDS-PAGE patterns of FDC, RAIs, RPIs, RPWs, RAI-WWs and RPI-WWs obtained by ISP process are shown in Fig. 2. Protein with a molecular weight (MW) ranged from 37 to 50 K was dominant in RAI-11 and RAI-12. Other protein bands in range of 100–150 K, 25–37 K and 15–20 K, respectively were also observed. There was no difference between RAIs except for protein band in range of 37–50 K where RAI-11 was clearer than that of RAI-12. The protein with a MW of 97 kDa might be a vitellin-like protein, which was found in salmon Oncorhynchus keta and sturgeon Acipenser transmontanus roes (Al-Holy and Rasco 2006). Similar proteins with MW of 32.5, 29 and 32.5 K were found in skipjack Kasuwonous pelamis, tonggol Thunnus tonggol and bonito Euthynnus affinis roe, respectively reported Intarasirisawat et al. (2011). Those proteins might be ovomucoid (Al-Holy and Rasco 2006) or phosvitin (Losso et al. 1993). Generally, different roe samples showed different electrophoretic patterns, indicating the differences in protein compositions among all samples (Intarasirisawat et al. 2011). Compared with FDC, low molecular protein bands (0–15 K) of RAIs were more faint than those of FDC that because a little protein compounds and others were transferred to RAI-WWs. Washed soluble proteins were moved on RAI-WW11 and RAI-WW12. Overall, RAI-WW11 had lower molecular protein bands than RAI-WW12 because of difference on solubilization condition during alkaline solubilization and precipitation (ISP). Prominent protein bands of RPI-1-4 were in range of 75–100 K, 37–50 K, 15–20 K, and 10 K respectively. A similar SDS-PAGE pattern of lower molecular weight protein (10 K) was observed in meriga roe hydrolysate (Chalamaiah et al. 2010). This result was also similar to hemoglobin which protein band observed in range of around 25 K and 10–15 K, respectively. Protein band of casein with molecular weight ranged 25–37 K was clearer than that of RPIs. Protein band of RPIs in range from 50 to 75 were faint because those proteins already been washed and moved to RPI-WWs. Protein band of RPWs with molecular weight of 10 K were was clearer than that of RPI. This result is indicated that acid was affected to degradation on aggregation and association of low molecular weight. Al-Holy and Rasco (2006) reported that three prominent proteins of salmon caviar had MW of 96, 20 and 10 K, which could be vitellin and possibly lysozyme or phosvitin. Protein with a MW of approximately 27 K in the soluble fraction of sturgeon caviar may possibly represent ovomucoid, a glycoprotein, which normally has a MW of 27–29 K (Al-Holy and Rasco 2006). For RAIs and RPIs, new protein bands with molecular weight over the 250 K were formed instead of FDC. Azadian et al. (2012) reported that this can be explained by the dissociation of high molecular weight myosin and association of the protein to form a high molecular weight.
Conclusion
The roe of marine sources are the most underutilized fish by-products, which have considerable chance for value-addition to produce food and feed. This study was to investigate the chemical compositions, amino acid profile, mineral profile, color from yellowfin tuna protein roe isolate and second by-products with isoelectric solubilization/precipitation using basic and acidic pH treatments. RPIs and RAIs can be used as a nutritional supplement due to the content of essential amino acids composition. The RAIs as recovered insoluble may be useful in animal feeds as a mineral additive due to the relatively high concentration of minerals (Ca, Mg, and K). Therefore, yellowfin tuna roe isolate could be a promising source of valuable nutrients for human food and animal feeds.








