Background
Osteological development in teleost fishes involves a sequence of remarkable morphological and functional changes, occurring in different developmental stages (Löffler et al. 2008; Kang et al. 2012; Ott et al. 2012). These ontogenetic changes strongly influence the feeding, breathing, and swimming behaviors of both larvae and juveniles, and are therefore useful in functional and ecological analyses and as a basis for phylogenetic inferences about relationships among teleost taxa (Omori et al. 1996; Faustino and Power 1999; Koumoundouros et al. 2000, 2001a, b; Liu 2001; Lima et al. 2013; Voskoboinikova and Kudryavtseva 2014). Practically speaking, an accurate knowledge of skeletal development is essential for the detection and elimination of skeletal deformities appearing during artificial seedling production and to promote effective aquacultural and resource management (Koumoundouros et al. 1997a, b).
Sebastes koreanus Kim and Lee 1994, in the family Scorpaenidae (or Sebastidae sensu Nakabo and Kai 2013), is smaller than its congeneric species and is regarded as endemic to the Yellow Sea (Kim and Lee 1994; Kim et al. 2005; Choi and Yang 2008). The species may be a good model fish with which to understand the phylogenetic relationships within the suborder Scorpaenoidei, because the species is specifically adapted to the unique marine environment of the Yellow Sea. Comparisons of S. koreanus with other Sebastes species have shown that S. koreanus collected in the wild exhibit wide ontogenetic variations in their pigmentation patterns and in their head–spine development (Yu et al. 2015). In addition, the restricted distribution of the species makes populations susceptible to collapse as a consequence of environmental pollution and/or the influence of climate change on the Yellow Sea. In this respect, artificial seedling production presents a viable approach to the conservation of susceptible species. However, no studies have yet been conducted on the details of skeletal development in S. koreanus, except for studies of morphological development and parturition season (Yu et al. 2015) and a small study of osteological development of reared larvae and juveniles in the Korea Strait (Park et al. 2015). Also, we confirmed some differences in osteological development of S. koreanus when compared with reared larvae (Park et al. 2015) and wild-captured larvae from the Yellow Sea. Therefore, in this study, we describe in detail the early skeletal development of S. koreanus in the context of functional changes, based on wild-captured larvae and juvenile. We also compare the osteological development of S. koreanus with that of congeneric species.
Methods
All individuals were collected from the eastern margin of the Yellow Sea. Larvae of S. koreanus [6.11–11.10 mm body length (BL), n = 32] were collected off the Taean Peninsula in May 2011 using a bongo net (0.6 m mouth opening, with 330 and 500 μm mesh size; bottom depth 15–24 m). The juvenile of S. koreanus (18.60 mm BL, n = 1) was collected off Gang-hwa-do in July 2012 using a stow net. The individuals were preserved in 5 % formalin immediately after collection. The specimens fixed in formalin were washed with distilled water and then preserved in 99 % ethanol. Before staining, each sample was identified according to the morphological characteristics of Yu et al. (2015), which were measured to the nearest 0.01 mm with the stereomicroscope (Olympus SZX16, Japan). The methods of measurement followed Leis and Carson-Ewart (2000). The measured body parts included BL and total length (TL). The anatomical terminology relating to skeletal structures follows Russell (1976), and the terminology of developmental stages follows Kim et al. (2011). The skeletal staining technique was derived from the double staining protocol of Darias et al. (2010). After staining, the specimens were examined on their right and dorsal sides with a stereomicroscope and photographs taken with a camera lucida (Olympus SZX-DA, Japan) attached to the microscope. Drawings of the different skeletal parts were prepared from the photographs. We compared the skeletal structures of the larvae and the juvenile with those of adult S. koreanus specimens, to observe the precise locations and shapes of the skeletal elements. We also compared stained specimens with a stained Sebastes inermis complex juvenile (17.06 mm BL, n = 1) collected in the wild. The stained specimens were preserved in 100 % glycerin in glass bottles and were deposited at Pukyong National University (PKU).
Results
The osteological development of S. koreanus at various developmental stages was described in the following skeletal regions: neurocranium, jaw bones, palate series, opercular series, hyoid arch, pectoral girdle, infraorbital bone, caudal skeleton, and vertebrae. The development results are summarized in Table 1.
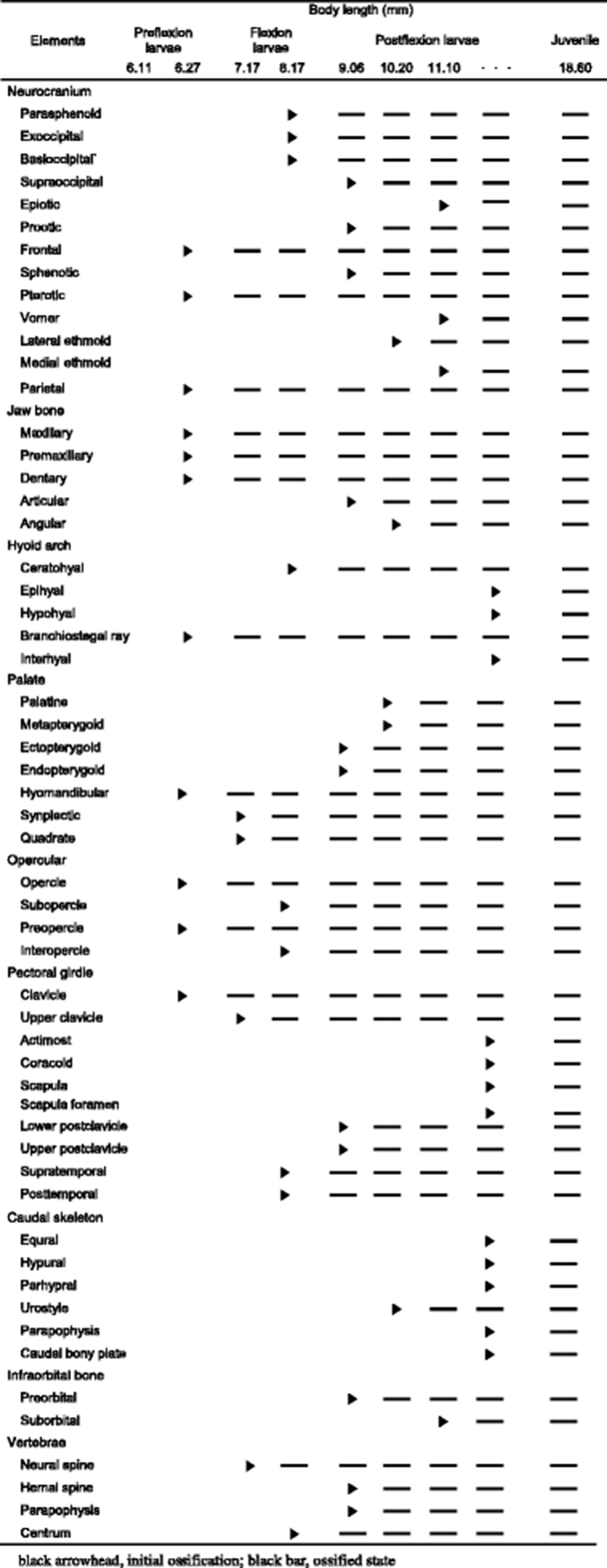
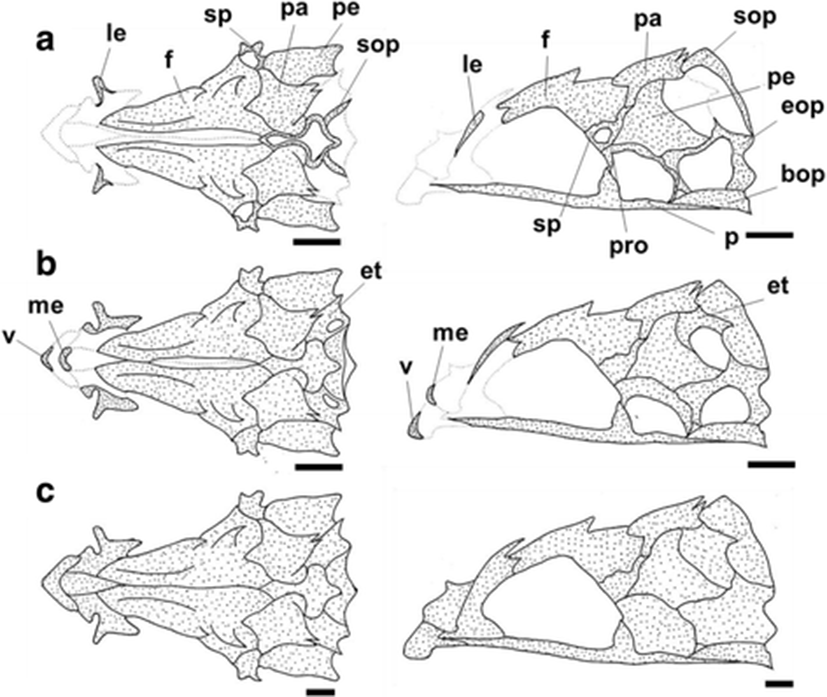
The development and ossification of the neurocranium for individuals at different developmental stages are illustrated in Figs. 1 and 2. In the smallest larvae (6.11 mm BL; preflexion stage), no skeletal structures of the neurocranium were visible. Ossification of the neurocranium started at 6.27 mm BL, with ossification of the parietal, frontal, and pterotic bones (Fig. 1a); ossification of these elements appeared to begin at the tips of the spines. In the 7.11 mm BL larva, the skeletal elements that had appeared in earlier stages continued to ossify, but no ossification of additional elements was observed (Fig. 1b). At 8.11 mm BL, the posterior of the parasphenoid and the anterior of the basioccipital had started to ossify, and the two elements were joined. At the same time, the exoccipital began to ossify along its posterior margin (Fig. 1c). At 9.06 mm BL, ossification of the frontal had extended to the dorsal area of the neurocranium, and then the frontal boundary line joined the parietal. In addition, the supraoccipital, sphenotic, and prootic elements had started to ossify along their margins at this stage (Fig. 1d). At 10.20 mm BL, the lateral ethmoid had started to ossify along its dorsal margin, and the parietal, pterotic, parasphenoid, and basioccipital were almost fully ossified (Fig. 2a). At 11.10 mm BL, ossification of the frontal had extended to most regions and ossification of the sphenotic and supraoccipital was complete. The epiotic, which appeared relatively late compared with the other neurocranial elements, had started to ossify at this stage (Fig. 2b), and the vomer and medial ethmoid appeared simultaneously along their anterior margin. At this stage, although all the elements of the neurocranium had started to ossify, some elements continued to ossify. In the juvenile stage (18.60 mm BL), the ossification of the neurocranium was fully complete (Fig. 2c).
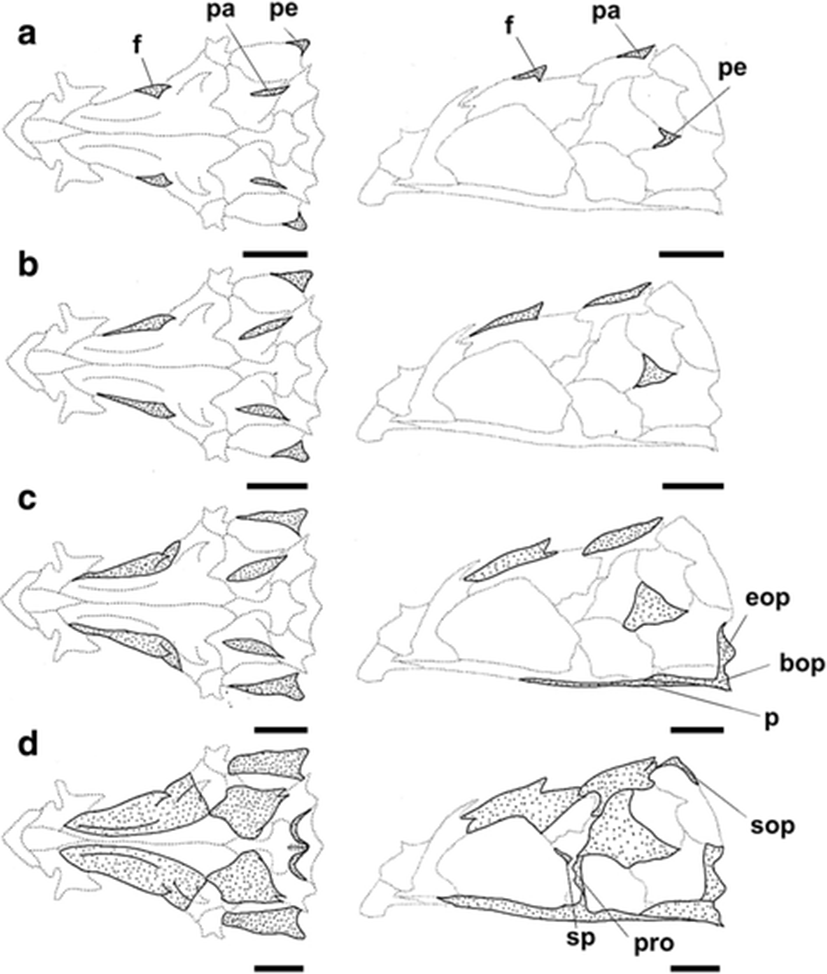
The development and ossification of the jaw bones, palatine, and opercular series for individuals at different developmental stages are illustrated in Fig. 3. No skeletal structures were visible in the smallest preflexion larva (6.11 mm BL). At 6.27 mm BL, the maxillary and premaxillary had both begun to ossify at their anterior and ventral margins, respectively (Fig. 3a). The dentary also started to ossify along its V-shaped anterior margin. The hyomandibular started to ossify at opposite medial margins (Fig. 3a). At the same time, the strongest three preopercular spines on the preopercle began to ossify, and the opercle had simultaneously ossified at its anterior margin (Fig. 3a). At 7.17 mm BL, the quadrate and the symplectic started to ossify in the region in which the two elements join (Fig. 3b). At 8.17 mm BL, the interopercle and preopercle had begun to ossify at their margins (Fig. 3c). The premaxillary, maxillary, and dentary also continued to ossify, and then the premaxillary had formed the ascending process and articular process. At 9.06 mm BL, the angular had ossified (Fig. 3d), and the endopterygoid and ectopterygoid had started to ossify along their adjacent margins. In particular, the upper part of the hyomandibular had quickly and fully ossified, and the opercle had extended to the strongest first spine. At 10.20 mm BL, the articular had started to ossify, and the maxillary and premaxillary had fully ossified and assumed their adult forms (Fig. 3e). The palatine started to ossify along its anterior margin, but the degree of ossification was small. At 11.10 mm BL, the ossification of the jaw bones was complete, and the opercular series was almost fully ossified at this stage, except for small parts of the subopercle and interopercle (Fig. 3f). At the juvenile stage (18.60 mm BL), the ossification of the jaw bones, palatine, and opercular series was complete (Fig. 3g).
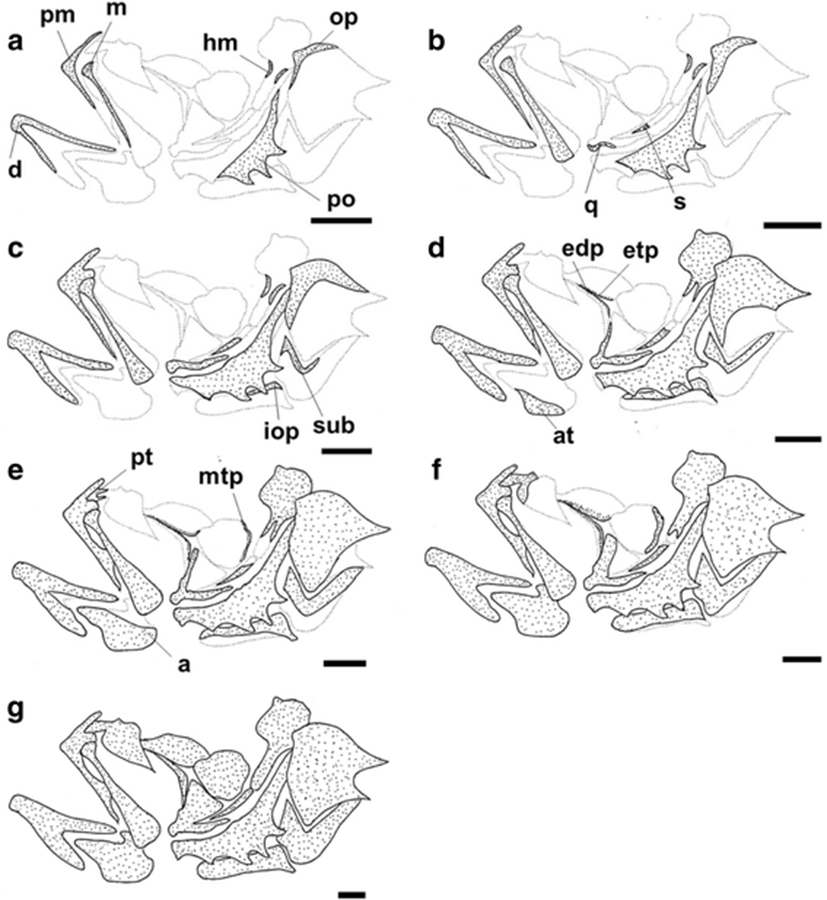
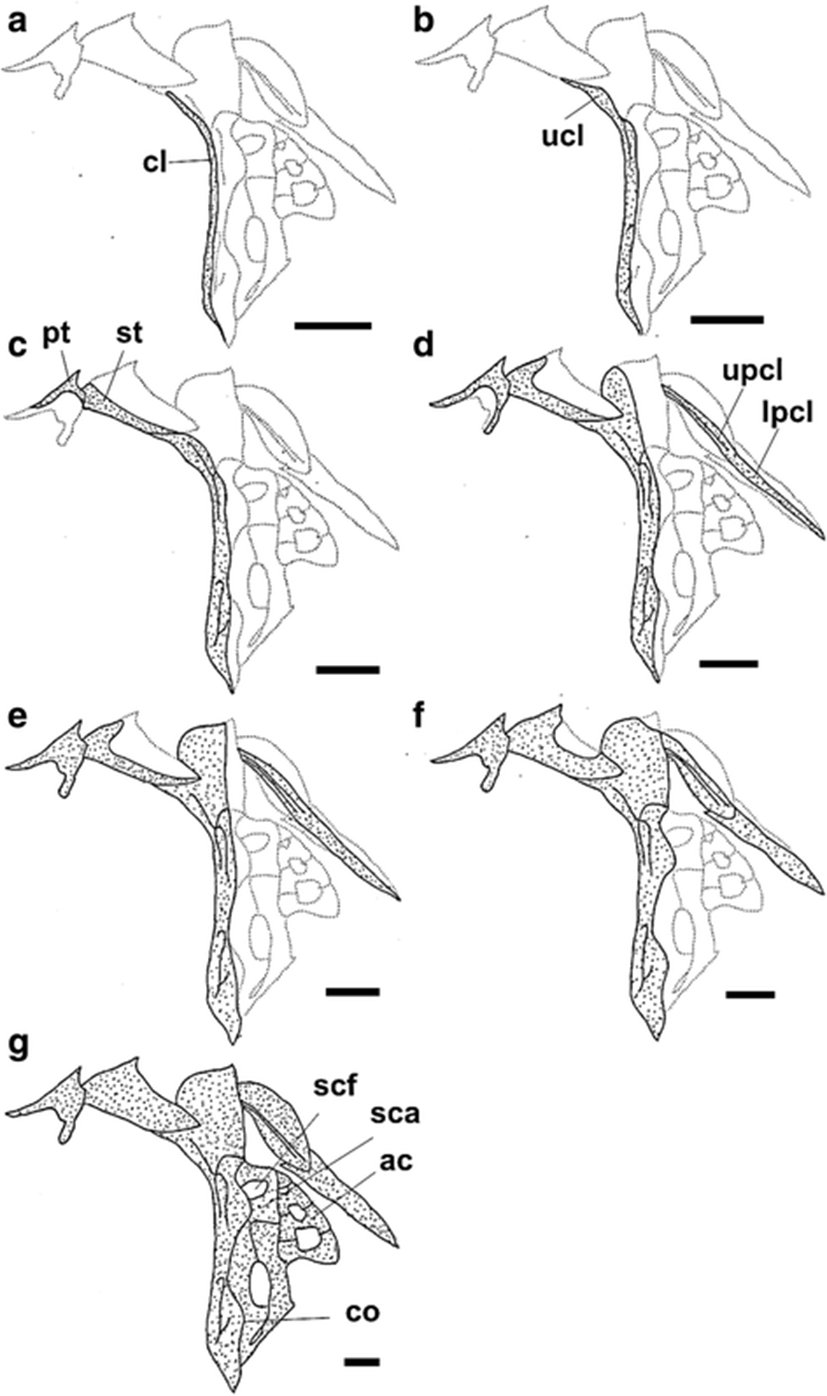
The development and ossification of the hyoid arch and pectoral girdle in individuals at all stages of development are illustrated in Figs. 4 and 5. At 6.27 mm BL, development of the hyoid arch and pectoral girdle had begun, with the ossification of the branchiostegal ray and clavicle, respectively (Figs. 4a and 5a). The fifth branchiostegal ray, which was the first branchiostegal ray to begin ossification, started to ossify in its middle region, and the clavicle was fully ossified as a long needle-shape. At 7.17 mm BL, the fourth branchiostegal ray and the upper clavicle had started to ossify (Figs. 4b and 5b). At 8.17 mm BL, all of the branchiostegal rays, except the first ray, had started to ossify, and the ceratohyal had started to ossify along its dorsal and ventral margins (Fig. 4c). The posttemporal and supratemporal had also started to ossify and were connected to the upper clavicle (Fig. 5c). At 9.06 mm BL, the first branchiostegal ray had started to ossify, and the other branchiostegal rays were fully ossified (Fig. 4d). The upper postclavicle and lower postclavicle of the pectoral girdle had also started to ossify and were connected to one other (Fig. 5d). At 10.20 mm BL, the ceratohyal had enlarged anteriorly and the posttemporal was fully ossified (Figs. 4e and 5e). At 11.10 mm BL, the anterior parts of the ceratohyal and clavicle were fully ossified, and the seven pairs of branchiostegal rays had approached their adult number and shape (Figs. 4f and 5f). In the juvenile stage (18.60 mm BL), the hyoid arch and pectoral girdle were fully ossified (Figs. 4f and 5f). However, although the scapula and uppermost radial of the pectoral girdle were nearly joined, they had not fused (Fig. 5f).
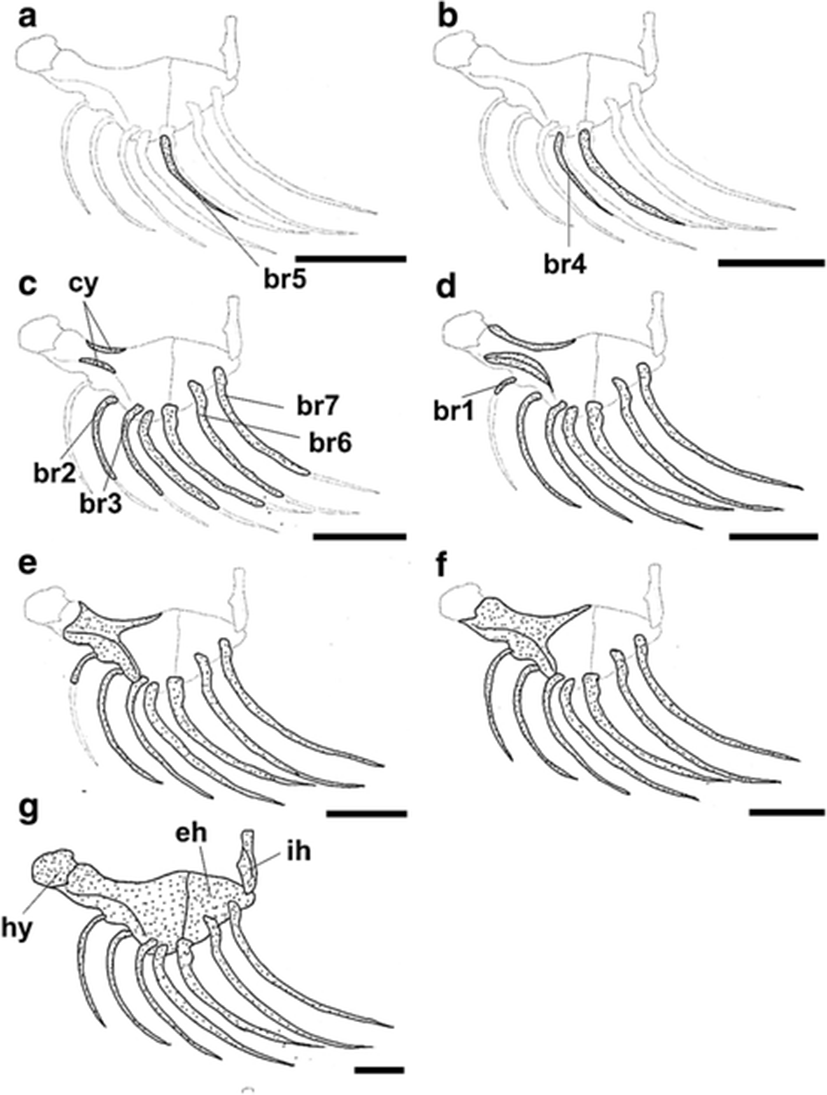
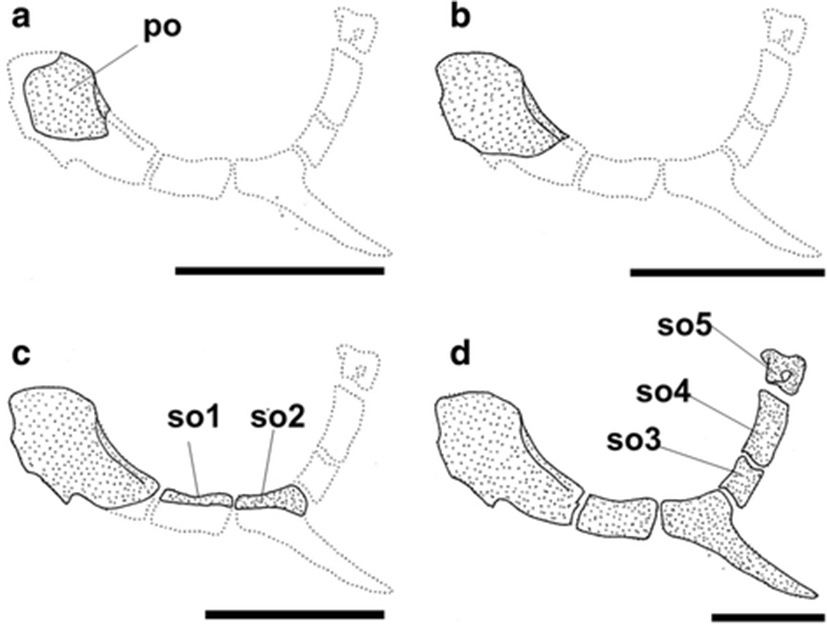
The development and ossification of the infraorbital bone in individuals at all stages of development are illustrated in Fig. 6. At 9.06 mm BL, the infraorbital bone elements on the preorbital had started to ossify (Fig. 6a). At 10.20 mm BL, the area of ossification of the preorbital had increased, but no additional elements were visible (Fig. 6b). At 11.10 mm BL, the first and second suborbital bones had started to ossify along their dorsal margins (Fig. 6c). In the juvenile stage (18.60 mm BL), the infraorbital bone was fully ossified (Fig. 6d).
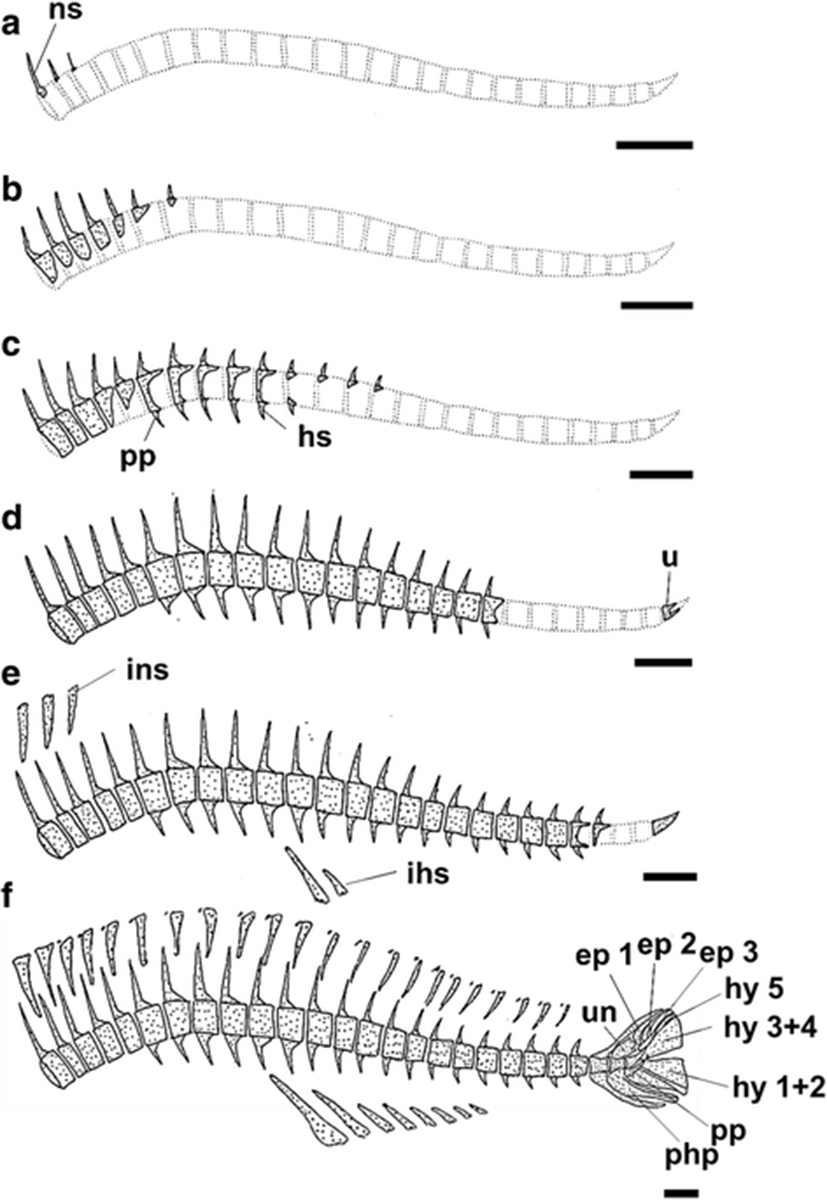
The development and ossification of the vertebrae and caudal skeleton in individuals at all stages of development are illustrated in Fig. 7. The skeletal elements of the vertebrae were first apparent at 7.17 mm BL (Fig. 7a). The first visible ossified elements of the vertebrae were the neural spines; no ossification of the centra was observed at this stage. The centra first started to ossify in the dorsal regions at 8.17 mm BL (Fig. 7b). After a centrum had formed, the neural spines appeared to elongate dorsally. The first hemal spines were observed at 9.06 mm BL, at which time ossification was visible in 10 centra, 14 neural spines, and three hemal spines (Fig. 7c). The development of the neural spines in the vertebrae occurred more rapidly than did the vertebral centra. Two to three ossified parapophyses appeared on the trunk centra at this stage. At 10.20 mm BL, the anterior centra, neural spines, and hemal spines were almost fully ossified, completely surrounding the notochord, whereas the posterior vertebrae continued to ossify consecutively towards the caudal complex (Fig. 7d). The urostyle had also started to ossify for the first time at this stage, along its anterior margin (Fig. 7d). At 11.10 mm BL, despite the progressive ossification of consecutive vertebrae, a few posterior vertebrae were still only present as cartilaginous structures (Fig. 7e). In the caudal skeleton, the urostyle had fully ossified at this stage, but no additional ossification was visible in the caudal skeleton (Fig. 7e). In the juvenile stage (18.60 mm BL), all the vertebral centra had completely surrounded the notochord, and the adjacent neural spines and hemal spines were also ossified (Fig. 7f). In addition, the hypurals, equals, parahypurals, parapophyses, and uroneural in the caudal skeleton were fully ossified in the juvenile (Fig. 7f). At the juvenile stage, the first and second hypurals and the third and fourth hypurals had also fused to form, together with the fifth hypural, three hypural segments (hy 1 + 2, hy 3 + 4, and hy 5).
Discussion
This study is the first to examine and describe in detail the sequence of osteological development in S. koreanus collected in the wild and to provide data with which to infer the phylogenetic relationships of species within the suborder Scorpaenoidei. In S. koreanus, ossification of the skeletal elements is first observed in the neurocranium, jaw bones, palatine, opercular, hyoid arch, and pectoral girdle of the preflexion larva with a length of 6.27 mm BL [6.45 mm total length (TL)], and then the only one juvenile (18.60 mm BL) has fully completed the skeletal development of all elements (Table 1). In a previous study of early skeletal development in the genus Sebastes, ossification was first observed in S. inermis complex at 7 days (7.0 mm mean TL) (Kim et al. 1993), in S. schlegelii at 6–8 days (6.85 mm TL) (Kim and Han 1991), and in S. oblongus at 3 days after release (8.0 mm TL) (Byun et al. 2012). In a study of early skeletal development in S. macdonaldi from southern California, ossification was first observed in the smallest larva (6.11 mm BL) (Moser 1972). Like this, the ossification of wild-captured S. koreanus larvae was first observed in larvae smaller than reared larvae of S. inermis, S. schlegelii, and S. oblongus (but not in S. macdonaldi). These differences in the size at the onset of ossification are probably related to the size at which the larvae are released from the adult, which is smaller than 6.11 mm BL in S. koreanus (this study), 6.12 mm TL in S. inermis (Kim et al. 1993), 5.52 mm TL in S. schlegelii (Kim and Han 1991), 7.2 mm TL in S. oblongus (Byun et al. 2012), and 4.5 mm BL in S. macdonaldi (Moser 1972). These differences may also be affected by external environmental factors, such as temperature and salinity (Fuiman 2002; Ložys 2004; Löffler et al. 2008; Ott et al. 2012), which may cause corresponding osteological differences (Matsuoka 1987; Wimberger 1993; Koumoundouros et al. 1997a) and meristic variations (Fowler 1970; Lau and Shafland 1982) between reared larvae and wild-captured larvae (Boglione et al. 2001). Therefore, despite the similar size of the released larvae of S. koreanus, S. inermis, and S. schlegelii, the first ossification size may also differ from each other as larvae of S. koreanus were collected in the wild while the other species were cultured in captivity. In addition, ossification was first observed in the reared larvae of Sebastiscus marmoratus and Sebastiscus tertius at 3.35 and 4.4 mm TL, respectively, even smaller than the larvae of species of Sebastes (Kim et al. 1997; Han et al. 2001). These results are also probably related to the size at parturition; at parturition, size is smaller than the larvae of Sebastes (Kim et al. 1997; Han et al. 2001).
In most cases, early skeletal development occurs first in elements that are necessary for feeding and respiration and therefore affect the survival of young larvae (Vandewalle et al. 1997; Wagemans and Vandewalle 1999). For example, the total resorption of the vitellus is essential for the transition from endogenous to exogenous feeding, because the efficiency of suction feeding increases with increasing prey size and the ossification of the related skeletal elements (Gluckmann et al. 1999). In S. koreanus, the skeletal elements that first start to ossify (at 6.27 mm BL) are the premaxillary, maxillary, dentary, preopercle, opercle, hyomandibular, and the fifth branchiostegal ray (Figs. 3 and 4), and the order of ossification is initially defined by the importance of the skeletal elements to feeding, swimming, and respiration. The cleithrum in the pectoral girdle ossifies in the same developmental stage (Fig. 5), and the early ossification of the clavicle produces an attachment site for the sternohyoideus muscle, which is important for swimming in subsequent growth stages (Wagemans and Vandewalle 1999; Koumoundouros et al. 2001a; Cloutier et al. 2011). Similar patterns of early skeletal development have been observed in other species (e.g., S. inermis, S. schlegelii, S. oblongus, Sebastiscus marmoratus, and Sebastiscus tertius). However, the timing of the ossification of the hyomandibular is highly variable (Kim and Han 1991; Kim et al. 1993; Kim et al. 1997; Byun et al. 2012). In most teleostei, the parasphenoid is the first element to ossify, except in some species in which the parasphenoid ossifies simultaneously with the frontals (Pagrus major; Matsuoka 1987) or the basioccipital (Heterobranchus longifilis; Vandewalle et al. 1997), or is ossified after the ossification of the frontals (Scophthalmus maximus; Wagemans et al. 1998). In S. koreanus, the first-ossified elements in the neurocranium (at 6.27 mm BL) are the parietal, frontal, and pterotic (Fig. 1). Subsequently, the parasphenoid and basioccipital begin to ossify at 8.17 mm BL (Fig. 1); these elements may help to reinforce the cranial floor to prevent damage to the neurocranium during feeding (Vandewalle et al. 1992) and to promote the balance needed for swimming (Weisel 1967). Therefore, the ossification of the parasphenoid and basioccipital during early skeletal development is important because they significantly affect feeding and swimming behavior, as do the jaw bones and clavicle, respectively.
The order of ossification of the neurocranial elements appears similar in different Sebastes species, but variations exist, particularly in the timing of the ossification of the parasphenoid. In many species of Sebastes and Sebastiscus, such as S. macdonaldi, S. inermis, S. schlegelii, Sebastiscus marmoratus, and Sebastiscus tertius, the parasphenoid is the first element to ossify (Moser 1972; Kim and Han 1991; Kim et al. 1993; Kim et al. 1997; Han et al. 2001), whereas in S. koreanus, the parasphenoid begins to ossify simultaneously with the basioccipital and exoccipital, just after the ossification of the parietal, frontal, and pterotic (present study), or in S. oblongus, the parasphenoid begins to ossify simultaneously with the supraoccipital, just after the ossification of the parietal and frontal (Byun et al. 2012). The pterotic and parietal also begin to ossify relatively early in some species, including S. koreanus, S. macdonaldi, Sebastiscus marmoratus, and Sebastiscus tertius, but no clear differences between the species of Sebastes and Sebastiscus are apparent (Moser 1972; Kim et al. 1997; Han et al. 2001). In S. koreanus, the early ossification of the hyoid arch appears on the ceratohyal and branchiostegal rays, but there is no additional ossification of elements between 8.17 and 11.10 mm BL (Fig. 4). In contrast, the ossification of the hyoid arch is clearly different in many species of Sebastes and Sebastiscus from that observed in S. koreanus and begins to occur at the same time as the ossification of the ceratohyal and epihyal (in S. inermis, S. schlegelii, Sebastiscus marmoratus, and Sebastiscus tertius), or the epihyal begins to ossify just after the ossification of the ceratohyal (as in S. oblongus) (Kim and Han 1991; Kim et al. 1993; Kim et al. 1997; Han et al. 2001; Byun et al. 2012).
Ossification of the pectoral girdle also shows a high degree of variability between different species of Sebastes and Sebastiscus. In S. koreanus, the ossification of the pectoral girdle begins with the clavicle, followed by the upper clavicle and soon thereafter by the supratemporal and posttemporal (Fig. 5). In contrast, in S. oblongus, the ossification of the clavicle first begins 3 days after release, and the ossification of the upper clavicle and posttemporal begin at 20 days, soon after the initial ossification of the supratemporal (Byun et al. 2012). In S. inermis, the first ossification on the clavicle begins 7 days after release, followed by the ossification of the postclavicle at 45 days and the upper clavicle at 50 days. The supratemporal begins to ossify at 65–69 days (Kim et al. 1993). In Sebastiscus marmoratus, the ossification of the clavicle first appears 5 days after release, and the ossification of the upper clavicle, posttemporal, scapula, and coracoid begin at 28 days (Kim et al. 1997). Therefore, it is difficult to determine a common ossification pattern for the pectoral girdle because of the observed variability between species. Also, S. koreanus ossified faster than other species of Sebastes and Sebastiscus, because this species might be to adapt to the harsh environment of the Yellow Sea such as strong current. According to Ishida (1994), adults of Sebastes, Sebastiscus, and Hozukius (suborder Scorpaenoidei) share the derived characteristic of a fusion of the scapula and uppermost radial in the pectoral girdle. However, although the fusion of the scapula and uppermost radial was observed in adults of S. koreanus, fusion was not observed in the S. koreanus juvenile (18.60 mm BL) (Fig. 5g). In some species of Sebastes (e.g., S. oblongus, Byun et al. 2012; S. schlegelii, Kim and Han 1991; Omori et al. 1996; S. macdonaldi, Moser 1972), fusion between the scapula and uppermost radial is not observed during skeletal development. Thus, it appears that the scapula and uppermost radial fuse slowly after (or starting in) the juvenile stage. Actually, the scapula and uppermost radial were closely adjoined along a thin boundary line in the S. koreanus juvenile observed in this study, presumably just prior to fusion. Since some ontogenetic studies have resolved taxonomic uncertainty that cannot be defined by adult morphology (Hubbs and Kampa 1946; White et al. 1983; Parin 1996), further ontogenetic study is needed to understand interrelationship between S. koreanus and Sebastes spp.
With respect to locomotion, the swimming of larvae immediately after their release from the adult is possible only through the antagonistic interactions of the notochord and trunk muscles (Ott et al. 2012). With growth, the notochord is gradually replaced by vertebrae, and the ossified vertebrae contribute stronger attachment sites for the powerful dorsalis trunci muscles, which are primarily responsible for swimming (Rojo 1991). In S. koreanus, after the ossification of the neural spine at 7.17 mm BL, the ossification of the vertebral centra mainly proceeds from the abdominal to the caudal vertebrae, and the urostyle is fully ossified just before the ossification of the caudal vertebrae is complete (Fig. 7). This pattern is similar to that observed in other species of Sebastes and Sebastiscus, except in S. schlegelii (Kim and Han 1991; Omori et al. 1996), e.g., in S. oblongus (Byun et al. 2012), S. inermis (Kim et al. 1993), Sebastiscus marmoratus (Kim et al. 1997), and Sebastiscus tertius (Han et al. 2001). Furthermore, in adults, the caudal skeleton in Sebastes and Sebastiscus species is formed by three hypurals (hy 1 + 2, hy 3 + 4, and hy 5), because the first and second hypurals and the third and fourth hypurals are fused (Ishida 1994). A similar trend is observed in the skeletal development of Sebastes and Sebastiscus species, including S. koreanus, S. inermis, S. schlegelii, S. koreanus, S. macdonaldi, Sebastiscus marmoratus, and Sebastiscus tertius (Fig. 7f) (Kim et al. 1993; Omori et al. 1996; Kim et al. 1997; Han et al. 2001; Byun et al. 2012). Therefore, the ontogenetic characteristics reflect the taxonomic characteristics of the adults well. In particular, the hypural cartilages fuse before ossification, unlike the fusion of the scapula and the uppermost radial (Omori et al. 1996).
Park et al. (2015) provided a brief overview of the external and osteological development of S. koreanus based on the artificial breeding of hatched larvae, using a gravid adult collected from the Korea Strait. However, compared with larvae and juvenile of S. koreanus collected from the wild in the Yellow Sea (present study; Yu et al. 2015), there are several differences, such as the pigmentation patterns and sequence of osteological development. We established three hypotheses regarding their differences. The first hypothesis is that it could be caused by the different population, as the present study, and Park et al. (2015) used different sampling sites (Yellow Sea vs. Korea Strait). In this respect, there is a possibility of existence of different population. Similarly, Kim et al. (2010) confirmed that the two populations of Ammodytes personatus larvae showed morphological differences in morphometric characters and pigmentation. The second hypothesis is that it could be caused by the difference in sea water temperature. The larvae and juvenile of S. koreanus (present study) were collected at the average sea water temperature 12.0–15.1 °C from Taean Peninsula (May and June), whereas larvae and juveniles were reared at the sea water temperature 13.5–15.5 °C. This slight water temperature difference (ca. 1.5 °C) could possibly cause the ontogenetic differences (Löffler et al. 2008; Ott et al. 2012). Finally, the third hypothesis is that it could be caused by differences of food source between reared larvae and wild-captured larvae. Particularly, calcium deficiency at the food source induces a delay in the ontogeny of skeletal development without affecting final bone mineralization (Fontagné et al. 2009). In addition, the different fixatives, formalin or alcohol, may have reduced or removed some pigmentation. Therefore, further research with microsatellite DNA and comparison of rearing is required to confirm the observed difference between reared larvae and wild-captured larvae.
Conclusions
In summary, although the sequences and periods of osteological development in Sebastes and Sebastiscus species show some variation, the early ossification of the skeleton proceeds in a sequence that prioritizes the elements required for feeding, swimming, and respiration. In this way, larvae are equipped with functional capacities that enhance the probability of their survival at this stage of their life cycle. Also, the ossification of parasphenoid and epihyal in the neurocranium appeared relatively later than congeneric species, this features indicated that S. koreanus is a unique species, which has been evolved in distinctive marine environment of the Yellow Sea.