Background
Biofouling is a natural marine ecosystem process caused by the surface colonization and development of micro- and macrofoulers on submerged natural or artificial marine structures, and can lead to economic and environmental losses worldwide. The fouling of ship hulls and fishing nets results in major costs for the marine industry through increased maintenance and fuel requirements due to greater levels of hull drag, lost productivity due to an increased frequency of dry-docking for the removal of fouling organisms, and compliance with environmental regulations (Yebra et al. 2004). Previously utilized antifouling agents, such as the common vessel tributyltin biocidal coatings, although effective against fouling, are also toxic (Minchin et al. 1996; Atanasov et al. 2005; Sonak 2009). As a result of the negative environmental impacts associated with its toxicity, tributyltin has become the subject of a relatively recent worldwide ban by the International Maritime Organization (IMO). Due to the limitations of conventional coatings, research on biomimetic surfaces and compounds inspired by natural systems has become important (Scardino and de Nys 2011). Researchers have observed that some marine species, such as mussels, can resist fouling when in good physiological condition (Scardino and de Nys 2004; Bers et al. 2006). Mussels have a tough, yet pliable, proteinaceous shell covering secreted by the mantle, known as the periostracum (Harper and Skelton 1993; Scardino et al. 2003). Wahl et al. (1998) found that when the periostracum was physically removed in Mytilus edulis, they observed an increase in the settlement of barnacles and algae on the shell. Conversely, mussels with an intact periostracum showed a greater resistance to fouling pressure (Scardino et al. 2003). Several studies have reported the benefits of microtopography on mussel shells as a physical fouling deterrent (Bers and Wahl 2004; Scardino and de Nys 2004). However, the general antifouling role of the periostracum in deterring settlement of fouling organisms and its underlying mechanisms remain unclear. Therefore, we investigated chemical elements from periostracum extracts and the physical surface of the periostracum responsible for defense against algal spore settlement. Monospores of Porphyra suborbiculata, one of common wild seaweed and easily obtainable throughout the year in a laboratory scale, were conveniently used as an assay organism for spore attachent and germination.
Methods
Aquacultured (6.5 ± 0.4 cm) and wild (4.4 ± 0.3 cm) Mytilus edulis mussels were purchased from the Namcheon fish market and collected from the rocky intertidal area at Eegidae (35°11′97″ N, 129°12′74″ E), on the east coast of Busan, Korea. For solvent extraction, shells of aquacultured mussels were gently cleaned to remove associated detritus and epibionts prior to submersion in vinegar solution.
Whole shells were submerged in a vinegar and seawater mixture (1:2 vinegar: seawater, approximately 2 % acetic acid) to aid in removing the periostracum (Grandison et al. 2011). Shells were retained in this mixture for 1 d, after which the periostracum was peeled from the shell with forceps and stored in seawater. After rinsing with distilled water, the peeled periostraca were freeze-dried and ground to a powder by hand for 5 min using a mortar and pestle. Twenty mg of periostracum powder was extracted with each one mL solvent of dichloromethane, ethyl acetate, and methanol. Extraction with each solvent was repeated three times for 1 h using pulses of an ultrasonic water bath (low-intensity frequency of 40 kHz), and the extracts were then dried with nitrogen. A stock solution of each extraction was prepared by adding 1 mL dimethyl sulfoxide (DMSO) to each 40 mg of dried extract. The prepared stocks were filtered through a 0.45-μm syringe filter before use.
The dichloromethane extracts of non-treated (i.e., no vinegar) periostraca were analyzed by gas chromatography–mass spectrometry (GC-MS) using a QP5050A instrument (Shimadzu, Kyoto, Japan) equipped with a flame-ionization detector and compared with spectral data from the database. Analysis was performed on an HP-5 column (30 m × 0.25 mm, 0.25 μm; Agilent Technologies, Santa Clara, CA, USA). The temperature was initially held at 50 °C for 2 min and raised to 150 °C at 4 °C/min and to 250 °C at 7 °C/min. Helium carrier gas was controlled at 0.6 mL/min with a split ratio of 1:50. The mass spectrometer was operated in electron-ionization mode at 70 eV.
The periostracum, peeled from the shell, was rinsed with distilled water and freeze-dried under vacuum before scanning electron microscopy (SEM) analysis. For SEM images, periostracum was mounted on conductive carbon tabs of a SEM post (Ted Pella, Inc., Redding, CA), sputter-coated using a Desk-II coater equipped with a gold target (Alfa Aesar, Ward Hill, MA), and imaged in a scanning-electron microscope (JSM-6700F; JEOL, Tokyo, Japan).
Juvenile blades of P. suborbiculata were collected from the rocky intertidal area at the mussel collection site. The fresh blades were rinsed, sonicated (40 kHz) twice for 1 min in autoclaved seawater, and immersed in 1 % Betadine solution with 2 % Triton X-100 for 1 min to eliminate epiphytes (Choi et al. 2005). To liberate the monospores, blades were cultured in Provasoli-enriched seawater (PES) medium (Provasoli 1968) under a 40 μmol/m2/s light intensity (10 L:14D) at 18 °C. Monospores were then used for attachment and germination assays under the same conditions.
For assays of algal spore attachment, aliquots of 100 μL seawater were first distributed into a 96-well plate. We added 1 μL of periostracum extract (40 mg/ml), 4 μL PES stock, and approximately 100–200 spores to each, with the final volume being 200 μL. The resulting spore suspensions were placed in the dark for 1 d at 18 °C to allow for even settlement on the bottom. At the end of this period, nonattached spores were removed from the bottom by centrifugation in an inverted position at 1500 × g for 15 min. The number of attached spores was counted under a microscope after replacing the PES solution. Relative attachment (%) was expressed as a percentage of the attached spores against total spores added. The reference for each test was prepared using the same procedure but with no extract. The minimum detectable inhibition of spore attachment by DMSO occurred at 0.5 %. Thus, the solvent extracts and reference were always added to the assay medium to provide a DMSO concentration of less than 0.5 %. For spore germination assays, approximately 100–200 spores were added to a 200-μL aliquot of PES in a 96-well plate and placed in the dark for 1 d at 18 °C to allow spores to settle on the bottom. After removing the nonattached spores by centrifugation in an inverted position, a fresh 200 μL of PES was added. Then, 1 μL of each extract (40 mg/ml) was immediately added to each 200 μL culture. The resulting germination cultures were placed at 18 °C and 80 μmol/m2/s light intensity on a 12 L:12D cycle for 1 week to permit development. The number of germlings was counted under a microscope and expressed as relative germination (%), i.e., the percentage of germinated spores to the total number of spores attached. The minimum DMSO concentration leading to detectable inhibition was 0.5 %. Thus the final concentration of DMSO was kept below 0.5 % in all assays.
Results and discussion
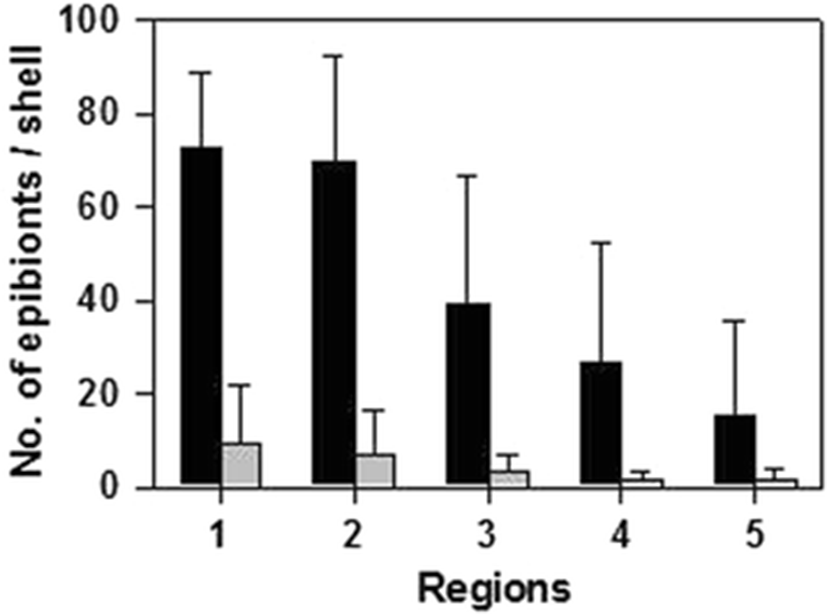
The shells of epibenthic bivalves offer substantial space for settlement of larvae and algal spores; however, the shells of mytilids often appear less fouled than adjacent biological and nonbiological substrata, where newer margin parts generally have fewer epibionts in nature. When we divided the external surface of wild mussel shells into five sections, approximately 70 epibionts of epizoons and epiphytes were observed on the oldest umbo parts, and fewer were detected on the margin parts (Fig. 1); the new margin parts have intact surfaces to defend against fouling settlement. Aquaculture mussels generally had the same trend, even with fewer numbers of epibionts on shell surfaces. This reduced settlement may be related to the intact texture and/or fresh components of the non-damaged periostracum. When looking at fouling organisms on mussel shells, we observed that the shells of aquaculture mussels had intact periostraca while those of wild mussels were damaged. Thus, the shells of aquaculture mussels were largely unfouled compared to the shells of wild mussels. In various molluscs, a range of fouling organisms, including endolithic algae, sponges, and other invertebrates, have shown a preference for areas of the shell where the periostracum is abraded or absent, such as on older shells (Kaehler 1999). Wahl et al. (1998) found that fouling by algae and barnacles on M. edulis was significantly greater on areas of the shell where the periostracum had been physically removed. Overall, a strong correlation exists between the presence of an intact periostracum and reduced fouling, which is consistent with the idea that the periostracum is used as an antifouling structure.
The aquacultured mussel shells were dipped in vinegar seawater, and the periostracum was peeled. When monospores of P. suborbiculata were added to the periostracum peels in PES, 36.8 % of spores attached successfully, and among attached spores, only 3.3 % germinated (Table 1). Thus, the periostracum showed potent anti-settlement effects on algal spores compared to the reference polystyrene material of the 24-well plate. The intact periostracum is able to resist fouling pressures via chemical and physical antifouling defense components. To investigate the potential antifouling effects of the periostracum, solvent extracts were first prepared and tested for efficacy in preventing the attachment and germination of algal spores. Three solvents, dichloromethane, ethyl acetate, and methanol, with different polarities were used to extract the soluble compounds from fresh periostraca and vinegar-treated periostricum peels. When 200 μg/ml of each extract was placed in the attachment and germination assay mixtures, dichloromethane extracts of both non-treated and vinegar-treated periostraca showed an attachment reduction of 31–35 % and a germination reduction of 3–5 % (Table 2). Extracts produced by the more nonpolar solvents were more effective in preventing attachment and germination, suggesting that the responsible compound also has a nonpolar lipophilic property. The chemical composition of the potent dichloromethane extract of non-treated periostraca was analyzed by GC-MS (Table 3). The major components by relative mass percentage were oleamide (19 %), an amide of the fatty acid oleic acid, and 1-tetracosanol (9 %), a fatty alcohol derived from the fatty acid lignoceric acid. One of the major compounds in the dichloromethane extract of mussel periostraca was oleamide (C18H35NO; CAS number 301-02-0), an endogenous amide form of oleic acid. This compound has shown antifouling effects on Ulva pertusa spore settlement and germination; i.e., it inhibited 100 % settlement with 10 μg/ml (Cho 2012). Oleamide is also known to induce sleep in animals by interacting with multiple neurotransmitter systems (Huitron-Resendiz et al. 2001; Mendelson and Basile 2001). In an open-field test of locomotion, the ED50 (the dose causing half of the observed effect) of locomotion reduction was an injection of 17 ± 1.5 mg/kg (Fedorova et al. 2001). Oleamide injections cause dose-dependent reductions in the time required to fall asleep, and reductions in locomotion in research animals, both with high reliability. Synthetically produced oleamide has a variety of industrial uses, including its application as a slip agent, lubricant, and corrosion inhibitor. Slip agents are used in polyethylene to bloom to the surface once the film has been produced and to reduce friction coefficients in post-processing operations (Garrido-López et al. 2006). Briscoe et al. (1972) found that adding surface films of oleamide to a substrate reduced the friction coefficient to as low as 0.03, compared to 0.4 for unlubricated surfaces. An organic corrosion inhibitor such as disodium oleamide sulfosuccinate can be used to control either corrosion attack at anodic sites or depolarizing reactions at cathodic sites (Brooman 2002). A second major compound found in the periostracum extract was 1-tetracosanol (C24H50O; CAS number 506-51-4), a fatty alcohol derived from the fatty acid lignoceric acid. Long-chain, primary alcohols are of high industrial value, mostly serving as surfactants in detergents and other cleaning products (Houston 1984). The excellent lubrication properties and stabilities of fatty alcohols make them valuable to the lubricant industry, particularly as high-performance factory machine lubricants and automobile transmission fluids. Thus, these major compounds are directly or indirectly related to the antifouling effects of mussel periostraca.
Relative attachmentc (%) | Relative germinationc (%) | |
|---|---|---|
Periostracum peelsa | 232/629 (36.8 %)* | 10/299 (3.3 %)** |
Referenceb | 1532/1823 (84.0 %) | 1042/1288 (80.9 %) |
aSpores were added to the periostracum peels in the 24-well plate
bPolystyrene material of the 24-well plate without peels was used as a reference
cValues are means ± SD (n ≥ 4)
*P < 0.1, **P < 0.01
aRelative activities (%) are expressed as means ± SD (n ≥ 3)
*P < 0.1, **P < 0.01
aComposition values are percentages of the relative peak areas
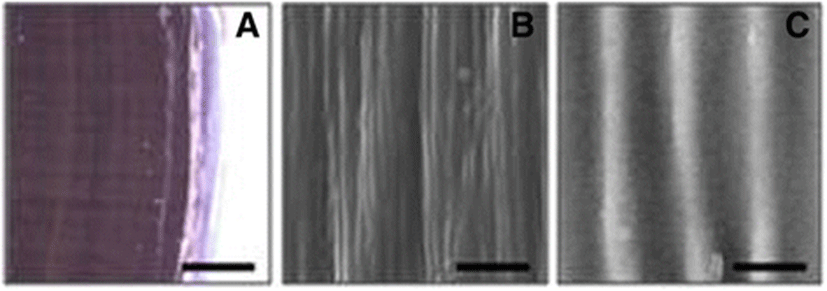
To understand physical antifouling defenses, a fresh margin part of mussel periostracum was used for scanning electron micrography (SEM). The natural surface microtopographies of the periostracum may contribute to the antifouling strategies of mussels. As shown in Fig. 2, the margin part of periostracum has a thin transparent layer. Using a scanning electron microscope at 10,000× magnification, a regular ripple structure was found on the periostracum, where regular corrugated ridges run parallel to each other and stretch across the entire shell without branching. The distance between ridges is approximately 1.4 μm. Periostracum microtopography is on a substantially smaller scale than the roughly 15 μm diameter of the typical algal Porphyra monospore. Furthermore, intact Mytilus ripple structures are also known to reduce settlement of shellfish larvae. Previous studies have shown that intact Mytilus ripple structures significantly reduce the settlement of larvae of the barnacle Balanus amphitrite (Scardino et al. 2003). Behavioral experiments show that barnacle cyprids have a higher propensity for smooth surfaces than for micro-textured surfaces. Surface textures with profile heights within a topographic range of 30–45 μm reduced settlement and recruitment by 92 % compared to smooth surfaces. These repellent effects disappeared when the microtopography was destroyed by periostracum erosion (Scardino et al. 2003). Thus, marine mussels have physical defensive structures to complement their chemical antifouling defenses (Bers et al. 2006). Biogenically derived microtopographies may represent a promising nontoxic and environmentally friendly substrate, but the surface structures of biomimetic antifouling materials must parallel natural microtopographies.
Conclusions
Our research has shown that periostracum extracts display some antifouling effects, while the periostracum also physically deters the settlement of spores. These findings strengthen mimetic application claims holding that the components and surface microtopographies of M. edulis periostraca can be used as models for antifouling materials. Our results, periostracum composition and structure results, suggest that mussel periostraca or their associated biomimetic materials may become environmentally friendly antifouling materials preventing the attachment of diverse fouling organisms.








