Background
Krill (Euphausia superba) oil has recently attracting more interest because of its unique compositions and health benefits including protection against cardiovascular disease (CVD) (Berge et al. 2014). The fatty acids in krill oil are composed of approximately 30–65% of phospholipid form whereas the fatty acids of other fish oil are mainly triglycerides (Tou et al. 2007). In addition, the krill oil possesses high content of unsaturated fatty acids (UFAs, 48.6%) of which contains high amount of omega-3 fatty acids such as docosahexaenoic acid (DHA, 16.6–36.5%) and eicosapentaenoic acid (EPA, 11.1–24.8%) (Virtue et al. 1995). Moreover, minor components such as astaxanthin, sterols, vitamin A, and tocopherols are also present in krill oil (Xie et al. 2017). According to the previous reports, krill oil, an extract prepared from a species of Antarctic krill, E. superba, has emerged with health benefits, including neuroprotection, anti-oxidant, anti-inflammation, anti-obesity, and improvement of metabolic disorders due to its omega-3 fatty acids, phospholipid-derived fatty acids, and the natural pigment, astaxanthin (Barros et al. 2014; Berge et al. 2013; Costanzo et al. 2016; Fasano et al. 2014; Lee et al. 2015). In addition, krill oil is known to have higher antioxidant potency compared with fish oil (Zhu et al. 2015). As like these reasons, the krill oil has been attracting interest as an attractive resource to develop novel food stuff or ingredients.
Ozone has been utilized in many fields such as being a disinfectant, an anti-inflammatory agent, and agent for improvement of regional circulation, simulation of regenerative process, and painless procedure (Deutsch 2007; Nogales et al. 2008). Ozone reacts with the double bonds of polyunsaturated fatty acids (PUFAs) to form reactive oxygen species (ROS) and bioactive products (Valacchi et al. 2013). Ozonated oils have been reported being applied in diverse diseases including burns, wound healing, inflammation, and periodontitis (Campanati et al. 2013; Guerra-Blanco et al. 2017; Shoukheba and Ali 2014; Valacchi et al. 2013). However, the above studies employed ozonated land plant oils, and there remains a lack of data regarding the biological studies of marine-derived oils by ozone treatment. Therefore, the objective of this study is to evaluate an anti-inflammatory effect of krill oil by ozone treatment on lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages and to elucidate the action mechanisms of anti-inflammatory effect.
Methods
Antarctic krill used in this study was captured in Antarctic Ocean provided in frozen state by Dongwon Industries Co. (Busan, Korea) and stored in a freezer at − 20 °C. Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), penicillin, streptomycin, and other materials needed for culturing cells were obtained from Gibco BRL Life Technologies (Grand Island, NY, USA). Griess reagent, 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and lipopolysaccharide (LPS) of Escherichia coli 026:B6 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Anti-phosphorylated JNK (anti-p-JNK; Cat. NO. 9251), anti-JNK (Cat. NO. 9252), anti-phosphorylated ERK1/2 (anti-p-ERK1/2; Cat. NO. 9101), anti-ERK1/2 (Cat. NO. 9102), anti-phosphorylated p38 (anti-p-p38; Cat. NO. 9211), anti-p38 (Cat. NO. 9212), anti-COX-2 (Cat. NO. 4842), and anti-iNOS (Cat. NO. 2982) mouse or rabbit antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). Anti-GAPDH (Cat. NO. sc-25,778) antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). The other chemical reagents were of analytical grade.
The first step in krill oil preparation was to lyophilize the whole body of krill and mix it with hexane in a 1:4 ratio. The mixture was allowed to precipitate for 4 h, and the supernatant was extracted. The supernatant was then mixed with hexane in a 1:2 ratio, followed by precipitation and filtration. Subsequently, the extracted supernatant was mixed with hexane in a 1:1 ratio, followed by precipitation and filtration. The extracted oil was treated with ozone gas at 3.006 g/h using an ozone generator for 144 h. The ozonated krill oil was dissolved in DMSO with 2% tween 80 and then used for experiments, adjusting the final concentration of DMOS with 2% tween 80 in culture medium to < 0.1%. Also, untreated group or LPS-stimulated group was treated with DMSO with 2% tween 80 of the same volume instead of ozonated krill oil.
The murine macrophage cell line RAW 264.7 was purchased from the Korean Cell Line Bank (Seoul, Korea). RAW 264.7 macrophages were cultured in DMEM supplemented with 10% heat-inactivated FBS, 100 μg/mL streptomycin, and 100 U/mL penicillin. The cells were then incubated in an atmosphere of 5% CO2 at 37 °C.
The cell viability was conducted by the MTT assay as described by Mosmann (1983). RAW 264.7 macrophages were seeded in a 96-well plate and incubated aliquots of the ozonated krill oil at 37 °C for 24 h. The MTT stock solution was subsequently added to each well and incubated for 4 h. The formazan crystals in each well were dissolved in 100 μL of DMSO, and the absorbance was measured using microplate reader (BioTek Instruments, Inc., USA) at 540 nm.
Nitric oxide (NO) production in the culture medium was measured via the Griess reaction (Weissman and Gross 2001). After a 24 h pre-incubation of RAW 264.7 macrophages with various concentrations (10, 50, and 100 μg/mL) of ozonated krill oil and stimulation with LPS (1 μg/mL), the quantity of nitrite accumulated in the culture medium was measured as an indicator of NO production. Specifically, 100 μL of supernatant from each well was mixed with 100 μL of Griess reagent (1% sulfanilamide and 0.1% naphthylethylenediamine dihydrochloride in 2.5% phosphoric acid), and the absorbance at 540 nm was measured in a microplate reader. Fresh culture medium was employed as a blank in every experiment.
Western blot analysis was conducted to protein expression as described by Kim et al. (2016). RAW 264.7 macrophages were seeded in a 96-well plate and incubated with LPS (1 μg/mL) coupled with aliquots of the ozonated krill oil at 37 °C for 24 h. Then, the cells were collected and washed twice with PBS. The cells were lysed in lysis buffer for 60 min and then centrifuged at 12,000 rpm and 4 °C for 15 min. The protein concentrations were determined using the BCA protein assay kit (Thermo Fisher Scientific, IL, USA). The lysate containing 20 μg of protein was subjected to electrophoresis on a sodium dodecyl sulfate (SDS)-polyacrylamide gel, and the gel was transferred onto a nitrocellulose membrane. The membrane was blocked with 5% non-fat dry milk in Tris-buffered saline with 0.1% Tween 20 (TBS-T) for 1 h. The primary antibodies were used at a 1:1000 dilution. The membrane was shaken with the primary antibodies at 4 °C for overnight, washed with TBS-T, and then contacted with the secondary antibodies at 1:3000 dilutions. The signals were confirmed using an enhanced chemiluminescence (ECL) western blotting detection kit (Amersham Pharmacia Biotech, Little Chalfont, UK) and estimated using the Multi Gauge V3.0 software (Fujifilm Life Science, Tokyo, Japan).
RT-PCR analysis was conducted to determine the mRNA expression as described by Kim et al. (2016). The total ribonucleic acid (RNA) from the RAW 264.7 macrophages treated with LPS in the presence or absence of ozonated krill oil was extracted using the TRIzol reagent. Equal amounts of RNA were used for each complementary deoxyribonucleic acid (cDNA) synthesis reaction. Adjusted oligo dT primers (10 μM) were added and then cooled with ice. The isolated messenger ribonucleic acid (mRNA) was then used to synthesize cDNA according to the manufacturer’s instruction (Promega, Madison, WI, USA). Single-stranded cDNA was amplified by PCR using specific primers. The primer sequences used to amplify the desired cDNA fragment were as follows: cyclooxygenase-2 (COX-2) forward and reverse primers: 5′- TGA AAC CCA CTC CAA ACA CA -3′ and 5′- GAG AAG GCT TCC CAG CTT TT -3′; inducible nitric oxide synthase (iNOS) forward and reverse primers: 5′- CAC CTT GGA GTT CAC CCA GT -3′ and 5′- ACC ACT CGT ACT TGG GAT GC -3′; interleukin-1β (IL-1β) forward and reverse primers: 5′- CTG TCC TGC GTG TTG AAA GA -3′ and 5′- TTC TGC TTG AGA GGT GCT GA -3′; interleukin-6 (IL-6) forward and reverse primers: 5′- AGG AGA CTT GCC TGG TGA AA -3′ and 5′- CAG GGG TGG TTA TTG CAT CT -3′; tumor necrosis factor-α (TNF-α) forward and reverse primers: 5′-AGG CCT TGT GTT GTG TTT CCA-3′ and 5′-TGG GGG ACA GCT TCC TTC TT-3′; and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) forward and reverse primers: 5′-TGAAGGTCGGTGTGAACGGATTTGGC-3′ and 5′-CATGTAGGCCATGA GGTCCACCAC-3′. The following PCR conditions were applied by 30 cycles of denaturation at 95 °C for 30 s, annealing at 60 °C for 45 s, and extension at 72 °C for 1 min. The cDNA was separated by electrophoresis on a 1% agarose gel for 60 min at 100 V and visualized by ethidium bromide. The bands of specific genes were normalized using GAPDH as reference.
Results and discussion
The cytotoxicity of the ozonated krill oil in RAW 264.7 macrophages was estimated using the MTT assay at various concentrations (10, 50, 100, and 200 μg/mL) of ozonated krill oil. The ozonated krill oil did not show significant cytotoxicity up to the concentrations of 100 μg/mL while cell cytotoxicity was observed at the concentration of 200 μg/mL (Fig. 1a). Thus, those concentrations (10, 50, and 100 μg/mL) were used in the subsequent experiments.
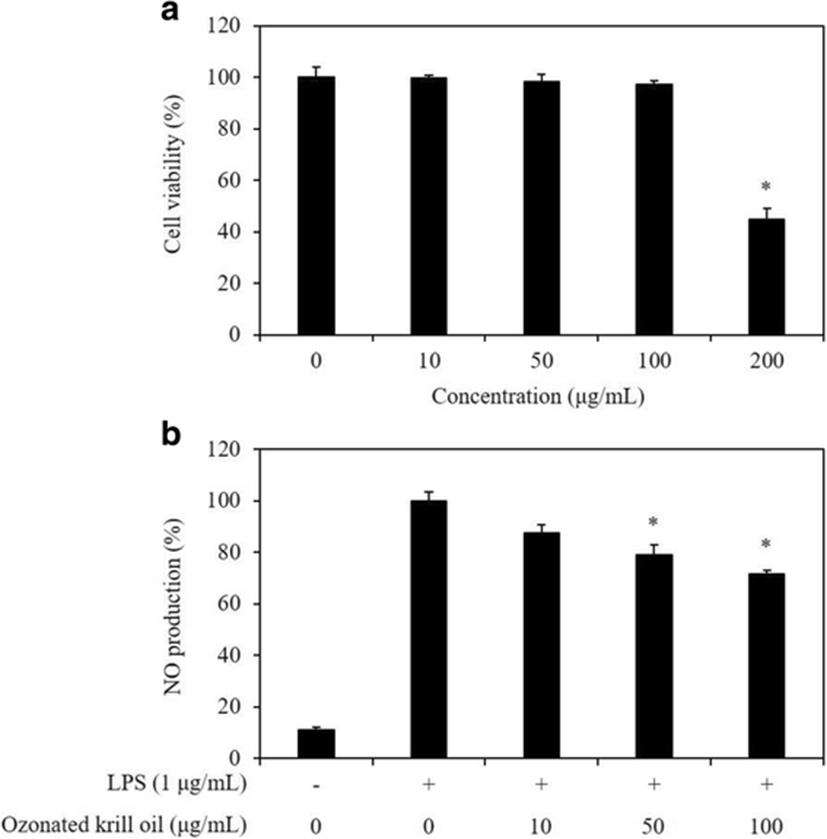
Macrophages play an important role in passive and active immunity and are involved in inflammatory response by being activated by a stimulus of LPS (Chelsky et al. 2015). In addition, activated macrophages produce enzymes, such as inducible nitric oxide synthase (iNOS) that is known to cause fatal results in a host by producing an inflammatory mediator, NO (Ulevitch and Tobias 1999; Akira et al. 2001). Macrophage-derived intercellular NO is a free radical with a short lifespan that plays an important role in the physiological and pathophysiological mechanisms in immune systems (Asamitsu et al. 2003). We evaluated the inhibitory effect of the ozonated krill oil on NO production to evaluate whether the ozonated krill oil exerts potential anti-inflammatory effect in LPS-stimulated RAW 264.7 macrophages. As shown in Fig. 1b, NO production was substantially higher in LPS-stimulated cells that that in the untreated cells. However, ozonated krill oil treatment inhibited the effects of LPS in a dose-dependent manner. The addition of 100 μg/mL ozonated krill oil caused a 29% inhibition in LPS-stimulated NO production. Krill oil is a marine-derived oil rich in phospholipids, astaxanthin, and omega-3 polyunsaturated fatty acids (Costanzo et al. 2016). According to a previous study, astaxanthin is known to have an anti-inflammatory effect (Santos et al. 2012). In addition, Ohata et al. (1997) previously reported that suppression of NO production was observed with the omega-3 polyunsaturated fatty acids such as DHA and EPA in a dose-dependent manner. Thus, these results supported the idea that the inhibitory effect of ozonated krill oil on NO production was mainly originated from its abundant n-3 high unsaturated fatty acids. According to the results of previous studies, ozone has been demonstrated to possess an anti-inflammatory effect (Delgado-Roche et al. 2017; Simonetti et al. 2017). Therefore, subsequent experiments for evaluating the anti-inflammatory effects and elucidating its action mechanisms were performed using ozonated krill oil.
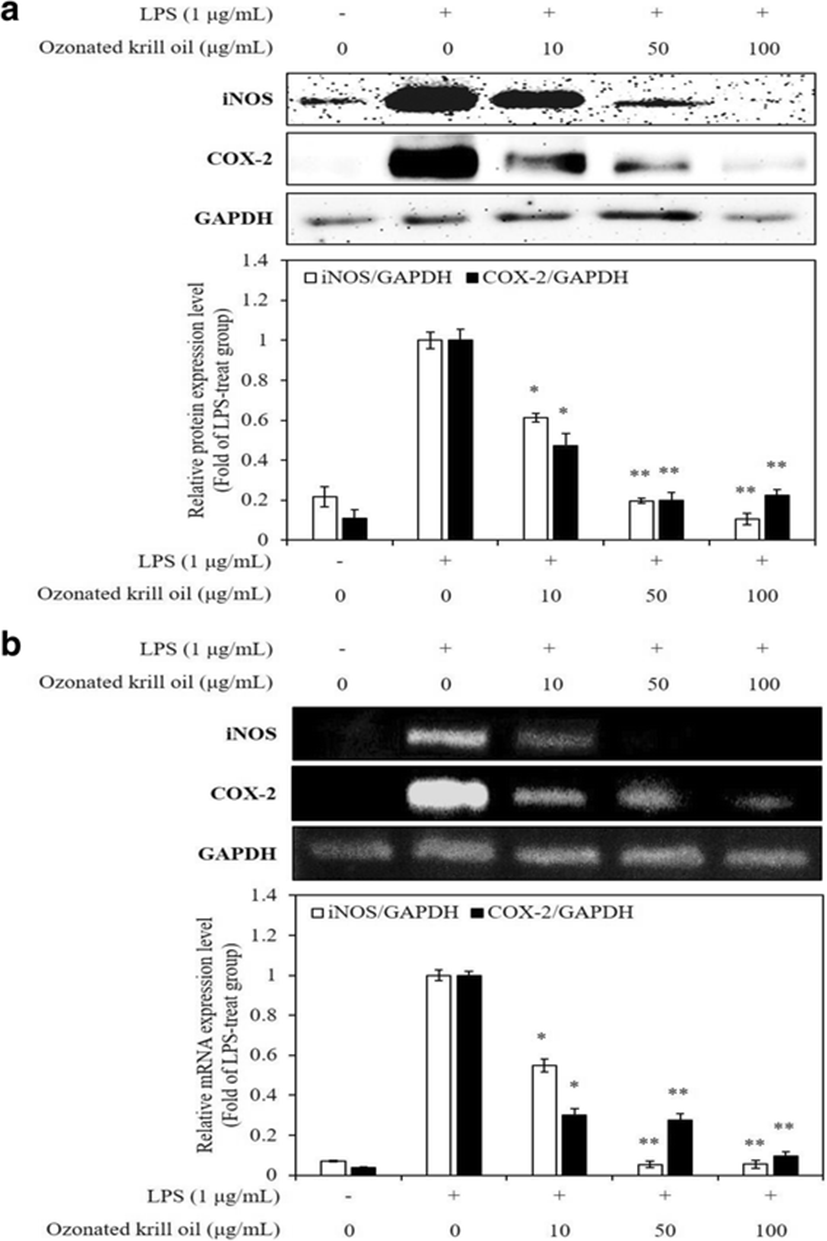
Inflammatory processes were mediated by multiple molecular mechanisms. iNOS and COX-2 play a significant role in immunity against infectious factors by producing an amount of NO and PGE2, respectively (Akira et al. 2001). These enzymes have attracted attention for their detrimental roles in inflammation-related diseases (Yun et al. 1996; Kim et al. 2009). It has been known that iNOS induces NO production, which leads to inflammation (Liu and Hotchkiss 1995), and COX-2 is a significant mediator of inflammation involved in the NO group (Kim et al. 2014). Thus, inhibition of iNOS and COX-2 expression is a pivotal goal in the treatment of inflammatory diseases. Western blotting and RT-PCR were performed to determine whether the inhibitory effects of ozonated krill oil on NO production were related to the expression of iNOS and COX-2. The ozonated krill oil significantly inhibited the LPS-stimulated increases in the protein and mRNA expression of iNOS and COX-2 in a dose-dependent manner (Fig. 2). According to the previous study, treatment of krill oil inhibited the LPS-stimulated expression of iNOS and COX-2 in mice brain (Choi et al. 2017). In addition, EPA and DHA modulate the expression of several inflammatory factors such as iNOS and COX-2, which subsequently lowers the induction of inflammation in cells (Allam-Ndoul et al. 2016; Mullen et al. 2010). These results suggest that ozonated krill oil principally acts by regulating NO production at the transcriptional level and that it could be an inhibitor of macrophage activation.
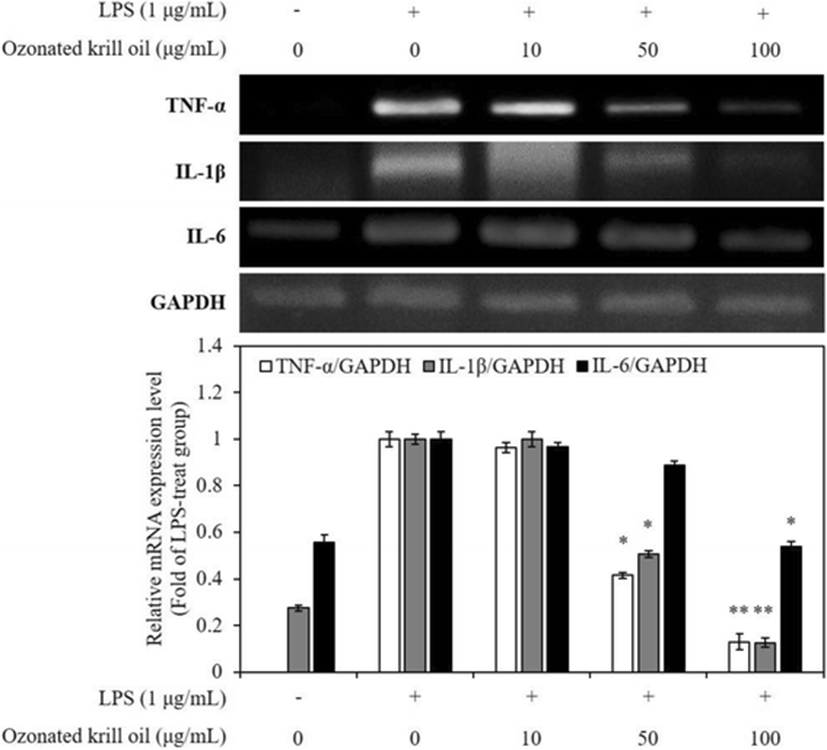
The pro-inflammatory cytokines including TNF-α, IL-1β, and IL-6 mediate and regulate immunity and inflammation during the inflammatory process (Trikha et al. 2003; Huang et al. 2006). These cytokines play key roles in the induction of inflammatory process (Kim et al. 2016). Among the several inflammatory cytokines, TNF-α is known to synthesize in the macrophages, and it stimulates the production of other inflammatory cytokines, such as IL-1β and IL-6 (Aggarwal and Natarajan 1996). IL-1β and IL-6 are well-known major pathogenic factors for many inflammatory diseases and regarded as endogenous mediators of LPS-stimulated fever (Ko and Jeon 2015). Because of their major roles in inflammatory response, reduction of several pro-inflammatory cytokines is of utmost importance during anti-inflammatory treatment. Thus, we determined the effects of ozonated krill oil on the mRNA expression of IL-1β, IL-6, and TNF-α in LPS-stimulated macrophages. RT-PCR was performed to determine whether ozonated krill oil reduces the expression of the pro-inflammatory cytokines at the mRNA expression level. All mRNA expression levels of genes related with pro-inflammatory cytokines were increased by the stimulation with LPS, and the levels were significantly decreased by ozonated krill oil treatment in a dose-dependent manner (Fig. 3). This result demonstrated that ozonated krill oil effectively inhibits the production of pro-inflammatory cytokines that are paramount in the production of an inflammatory response in activated macrophages.
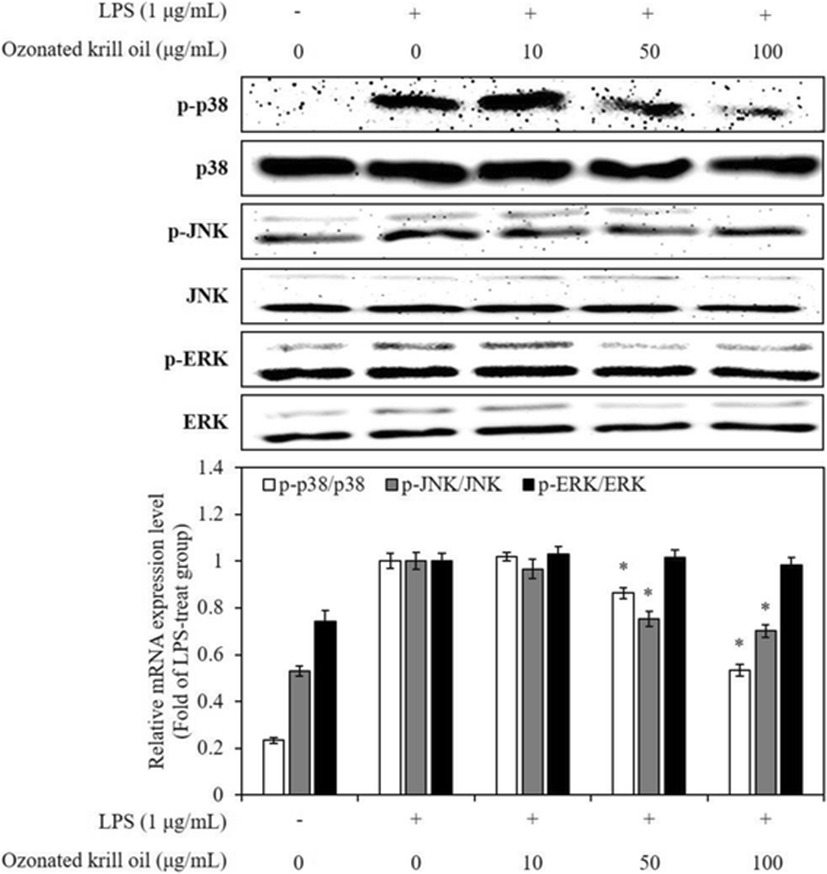
The expression of various inflammatory mediators activates phagocytosis of external factors and is involved in inflammatory response through the activation of various inflammatory signaling network including a transcription factor, mitogen-activated protein kinases (MAPKs) (Akira et al. 2001). MAPKs, including JNK, extracellular signal-regulated kinase (ERK), and p38 kinase promote expression levels of iNOS and COX-2 in LPS-stimulated macrophages (Kyriakis and Avruch 2012). Moreover, activation of MAPKs induces production of pro-inflammatory cytokines, including IL-1β, IL-6, and TNF-α in LPS-stimulated macrophages (Ajizian et al. 1999). Thus, suppression of MAPK activation or function is a major mechanism. Therefore, in order to evaluate the action mechanism of ozonated krill oil on other inflammatory pathway besides pro-inflammatory cytokines, we investigated the effect of the ozonated krill oil on the activation of MAPKs in LPS-stimulated macrophages using western blot analysis. This result indicated that ozonated krill oil inhibited LPS-stimulated p38 MAPK and JNK phosphorylation, but not that of ERK, in RAW 264.7 macrophages (Fig. 4). These results suggest that the suppression of p38 MAPK and JNK phosphorylation might be involved in the inhibition of pro-inflammatory mediators and cytokines in LPS-stimulated RAW 264.7 macrophages.
Conclusions
Ozonated krill oil was prepared by the treatment using ozone gas, and its anti-inflammatory effect was evaluated in LPS-stimulated RAW 264.7 macrophages. The ozonated krill oil exhibited the inhibitory effect on NO production. Ozonated krill oil also reduced the mRNA expression of IL-1β, IL-6, and TNF-α in LPS-stimulated RAW 264.7 macrophages. These effects are exerted by blocking phosphorylation of p38 MAPK and JNK. These findings provide a partial molecular explanation for the anti-inflammatory properties of ozonated krill oil.








