Background
Matrix metalloproteinases are hailed as crucial enzymes that have important roles in cancer progression and several tumor-related complications (Jones and Walker 1997). Due to their nature, matrix metalloproteinases (MMPs) are pivotal in cell proliferation and migration which are closely linked with invasive tumor cells and onset of malignant tumor growth (Moss et al. 2012). In addition, MMPs which are zinc-dependent endopeptidases responsible for extracellular matrix degradation are known to be involved heavily in various disorders including inflammatory response, cardiovascular diseases, arthritis, and most types of cancer (Bauvois 2012; Egeblad and Werb 2002; Overall and López-Otín 2002). Different classifications of MMPs occur depending on the tissues they are found, their functions, and their expression patterns. There are several types of extracellular matrix-related enzymes defined in human cellular mechanisms. Among them, MMP-2 (72 kDa) and MMP-9 (92 kDa) are found to regulate tumor invasion and metastasis. Researches in malignant tumors reported the overexpression and enhanced activity of both of these MMPs (Ibañez and Cifuentes 2013). Enhanced metastasis is evidently linked with enhanced MMP-2 and MMP-9 expressions. Therefore, studies on hindering or inhibiting the expression or activity of MMPs gained interest and put several MMP types including MMP-2 and MMP-9 into spotlight as therapeutic targets. MMPs are regulated by tissue inhibitor of MMP (TIMP) through a negative feedback mechanism acted on activation of MMP enzymes. TIMP expression inhibits all types of MMPs other than gelatinases. Normal regulation mechanism of TIMPs ought to be deteriorated and further facilitate the enhancement of MMP expression in malignant tumors (Yu and Gu 2015).
Brown macroalgae have been of much interest due to their known ability to withstand different marine environments by producing various secondary metabolites. Some brown algae species already have been reported to possess numerous health beneficial effects (Holdt and Kraan 2011; Matanjun et al. 2009; Patarra et al. 2011). Low content in harmful lipids and high content in polysaccharides, unsaturated fatty acids, vitamins, and minerals enable marine algae to be promoted as a nutritious source for healthy diets (Plaza et al. 2010). Sargassum horneri is a common species of brown macroalgae that grows on the coastal sea of Korea and Japan. Although it has been a part of a diet in limited areas of Japan, it could not enter the market as a processed product until recently. Literature contains little information about its chemical composition and nutritional aspects (de la Mare et al. 2012; Thomas and Kim 2011). Extracts from S. horneri were observed to stimulate the formation of bone tissue and to prevent bone loss (Jiao et al. 2009). In addition, chromene isolated from S. horneri was reported to possess a protective effect against UV-A-induced damage in skin dermal fibroblasts (Reuter et al. 2010). In this context, as a part of ongoing research to develop antitumor compounds, especially MMP inhibitors from natural origin, current study aims to present understanding on the potential of S. horneri as a source for natural products that can act on MMP activity. In this manner, solvent-partitioned fractions of S. horneri extract were screened for their effects on MMP-2 and MMP-9 activity and expression.
Methods
S. horneri was purchased from Parajeju (Jeju, Korea) in 2013. The sample (1000 g) was air-dried outdoors under shade, ground to powder using a blender (NFM-3561SN, NUC Co. Ltd., Seoul, Korea) at high-speed grinding setting, and extracted in a 5-l Erlenmeyer flask with EtOH (3 l) for three times. The collected extracts were concentrated under reduced pressure with a rotary evaporator (80 mbar, 50 °C) (RV 10 Series, IKA, Wilmington, NC, USA).
The crude extract (128 g) was suspended in between CH2Cl2 and water. Later, the CH2Cl2 layer was separated by 85% aqueous MeOH and n-hexane. Next, the water layer was partitioned with n-BuOH and H2O, respectively. Overall, the solvent partition yielded the n-hexane (0.73 g), 85% aq. MeOH (4.05 g), n-BuOH (1.08 g), and H2O (71.32 g) fractions.
HT1080 human fibrosarcoma cells were cultured in T-75 culture flasks (Nunc, Roskilde, Denmark) in an incubator with 37 °C and 5% CO2 atmosphere with Dulbecco’s modified Eagle’s medium (DMEM; Gibco-BRL, Gaithersburg, MD, USA) containing 10% fetal bovine serum (FBS), 2 mM glutamine, and 100 μg/ml penicillin-streptomycin (Gibco-BRL, Gaithersburg, MD, USA). The medium was changed every 3 days.
For cell viability assessment, cells were cultured in 96-well plates at a 5 × 103 cells/well density. Following a 24-h incubation, cell culture medium was removed and the cells were washed with fresh medium and prior to treatment with the medium with or without S. horneri samples. After incubation for 48 h, cells were re-washed with fresh medium and 100 μl of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (1 mg/ml) was introduced to the wells, followed by a 4-h incubation. Finally, 100 μl of dimethyl sulfoxide (DMSO) was used for each well in order to solubilize the formazan crystals prior to absorbance measurement at 540 nm using a GENios® microplate reader (Tecan Austria GmbH, Grödig, Austria). Cell viability was defined by the absorbance value as a way to indicate the amount of MTT converted into formazan crystal. Viability of cells was determined as a percentage in comparison to the untreated control wells against sample-treated wells, and dose response curves were established.
Cells were grown on a 12-well culture dish to 90% confluence followed by forming an injury line with a width of 2 mm from scraping vertically across the cell layer with a sterile scraper. Floating cell debris was washed away with phosphate-buffered saline (PBS), and cell medium was changed to serum-free medium. Cells were treated with 50 μg/ml Sargassum horneri solvent-partitioned extracts (SHEs). Cell migration was observed under an inverted microscope (Nikon Eclipse TS100, Nikon Instruments, Melville, NY, USA), and photographs were taken at incubation starting time and after 24 h of incubation.
Enzymatic activities of MMP-2 and MMP-9 from HT1080 cells that were treated with or without samples were detected by gelatin zymography. HT1080 cells were cultured in 24-well plates with a density of 2 × 105 cells/well in a serum-free medium and were introduced to different concentrations of sample for 1 h. Phorbol 12-myristate 13-acetate (PMA; 10 ng/ml) was used to enhance the MMP expression, and cells were further incubated for 24 h after PMA treatment. Total protein contents of the cells were normalized using Bradford protein determination method. Next, cell culture medium was subjected to substrate-gel electrophoresis. Conditioned cell culture medium with the same amount of protein was transferred on 10% polyacrylamide gels under non-reducing conditions containing 1.5 mg/ml gelatin. Polyacrylamide gels were then washed with 50 mM Tris–HCl (pH 7.5) containing 2.5% Triton X-100 to remove any remaining sodium dodecyl sulfate. After the washing process, gels were incubated for 48 h at 37 °C in developing buffer (10 mM CaCl2, 50 mM Tris–HCl, 150 mM NaCl) in order to facilitate gelatin digestion. Areas of gelatin hydrolyzation by MMP were observed as clear zones against blue background of Coomassie Blue staining under a CAS-400SM Davinch-Chemi imager™ (Davinch-K, Seoul, Korea).
Total cellular RNA was extracted by TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA) from sample-treated and control wells. Any changes in the concentration of mRNA for MMP-2 and MMP-9 were determined by RT-PCR. Briefly, 2 μg of total RNA from cells was converted to single-stranded cDNA using a reverse transcription system (Promega, Madison, WI, USA). The target cDNA was amplified using the following primers: forward 5′-TGA-AGG-TCG-GTG-TGA-ACG-GA-3′ and reverse 5′-CAT-GTA-GCC-ATG-AGG-TCC-ACC-AC-3′ for MMP-2; forward 5′-CAC-TGT-CCA-CCC-CTC-AGA-GC-3′ and reverse 5′-CAC-TTG-TCG-GCG-ATA-AGG-3′ for MMP-9; forward 5′-AAT-TCC-GAC-CTC-GTC-ATC-AG-3′ and reverse 5′-TGC-AGT-TTT-CCA-GCA-ATG-AG-3′ for TIMP-1; forward 5′-TGA-TCC-ACA-CAC-GTT-GGT-CT-3′ and reverse 5′-TTT-GAG-TTG-CTT-GCA-GGA-TG-3′ for TIMP-2; and forward 5′-GCC-ACC-CAG-AAG-ACT-GTG-GAT-3′ and reverse 5′-TGG-TCC-AGG-GTT-TCT-TAC-TCC-3′ for β-actin. Cycles were 95 °C for 45 s, 60 °C for 1 min, and 72 °C for 45 s for amplification. Following the completion of 30 cycles, the final products were separated by electrophoresis on 1.5% agarose gel for 30 min at 100 V. Gel staining was carried out with 1 mg/ml EtBr, and visualization by UV light using AlphaEase® gel image analysis software was finalized with the assay (Alpha Innotech, San Leandro, CA, USA).
Immunoblotting was performed according to common standard procedures. To explain briefly, HT1080 cells were agitated in RIPA lysis buffer (Sigma-Aldrich Corp., St. Louis, USA) at 4 °C for 30 min. Cell lysates (35 μg) were then subjected to separation using 10% SDS-polyacrylamide gel electrophoresis and transferred onto a polyvinylidene fluoride membrane (Amersham Pharmacia Biosciences., England, UK), blocking with 5% skim milk and hybridization with primary antibodies (diluted 1:1000). Membranes were then incubated with horseradish-peroxidase-conjugated secondary antibodies at room temperature. Immunoreactive proteins were detected using an electrochemiluminescence kit (Amersham Pharmacia Biosciences, England, UK) according to the manufacturer’s instructions. Protein bands were observed using a CAS-400SM Davinch-Chemi imager™ (Davinch-K, Seoul, Korea).
The data were presented as a mean of three different experiments ± SD. Differences between the calculated means of the each individual group were determined by one-way ANOVA coupled with Duncan’s multiple range tests. Any difference was considered statistically significant at p < 0.05. The statistical software SAS v9.1 (SAS Institute Inc., Cary, NC, USA) was used for analyses.
Results and discussion
Particular important pathways for metastasis, oxidative stress, and fibrosis are known to be influenced by the activities of MMPs (Holdt and Kraan 2011; Plaza et al. 2010). Therefore, MMP inhibitors steadily gain high interest from various research and development studies of pharma and nutraceutical approaches. Recently, natural sources for MMP-inhibiting substances are being intensively studied, and in this context, marine organisms hold a great deal of potential being present in a unique and challenging environment. Various organisms, especially marine plants, and metabolites have been identified as potential MMP inhibitors, and possible mechanisms of action for isolated compounds have been suggested (de la Mare et al. 2012; Thomas and Kim 2011). In order to provide valuable insights on that matter, S. horneri was studied to evaluate its MMP-inhibition efficiency. In order to help the future utilization through isolated and elucidated bioactive substances, crude extract of S. horneri was fractioned with organic solvents and solvent-partitioned extracts were tested separately.
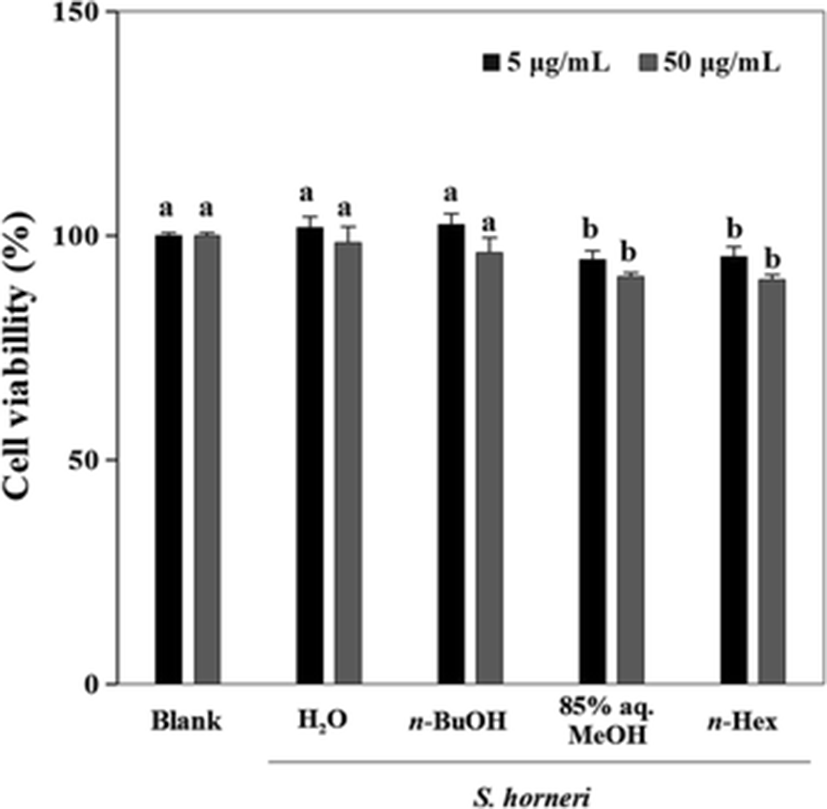
First, the solvent-partitioned extract (SHE) samples were tested for its cytotoxic presence in human fibrosarcoma cell line HT1080 for 48 h at two different concentrations (5 and 50 μg/ml) (Fig. 1). The cytotoxicity test revealed that these concentrations were not significantly toxic and any observed inhibition of MMP-2 and MMP-9 activity was not caused by any cytotoxic influence.
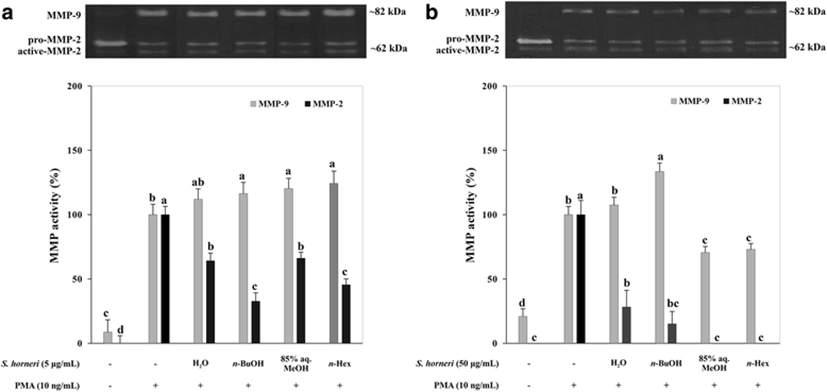
SHEs were analyzed for their possible activity to inhibit MMP-2 and MMP-9 enzymes following a PMA stimulation. Gelatinolytic activity of MMP-2 and MMP-9 secreted from fibrosarcoma cell line HT1080 was evaluated with gelatin zymography which was carried out with PMA-stimulated conditioned medium of SHE-treated cells (Fig. 2). Introduction of PMA (10 ng/ml) to cells resulted in enhanced activation of MMP-2 and MMP-9; hence, gelatinolytic activity in gelatin zymography was elevated. Among tested SHEs, 85% aq. MeOH decreased both MMP-2 and MMP-9 activity in a dose-dependent relevantly higher manner. The remaining SHEs were observed to inhibit both MMP activity in an order of n-BuOH, n-hexane, and H2O, respective to their efficiency. In Fig. 2, the MMP-2 activity was depicted as a percentage to that of activation of MMP-2 from pro-MMP-2. Data showed that the n-BuOH and H2O SHEs notably increased the MMP-9 activity while showing decreased MMP-2 activity. This can be linked to the regulatory dynamic between MMP-2 and MMP-9. In general, MMP inhibitors are usually selective; for example, an inhibitor for MMP-2 can be ineffective against MMP-9 (Benjamin and Khalil 2012). Additionally, suppressed MMP-2 production was suggested to result in increased MMP-9-mediated gelatinase activity (Kato et al. 2015) which might explain the inconsistencies among the tested samples with regard to their inhibition and/or enhancing effect towards MMPs. Inhibition of MMP activities indicated that the SHEs possess bioactive compounds that could have effects on the extracellular activity of MMP-2 and MMP-9.
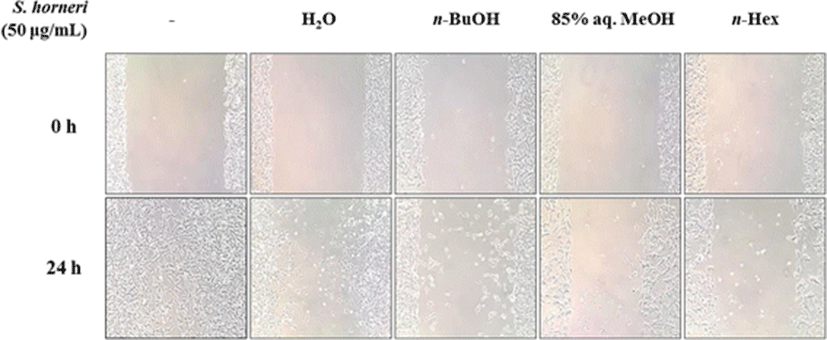
With the possible MMP inhibitory presence of SHEs, effect on cell migration was observed using cell migration assay on HT1080 human fibrosarcoma cells. Cells without any treatment showed signs of migration after a 24-h incubation while SHE-treated cells had hindered migration patterns (Fig. 3). Treatment with 50 μg/ml SHE inhibited the migration ability of tumor cells significantly, indicating a possible inhibition of MMPs which are important enzymes for the invasive nature and migration of tumor cells (Thomas and Kim 2011). SHEs were ordered as 85% aq. MeOH, n-hexane, n-BuOH, and H2O according to their level of cell migration inhibition.
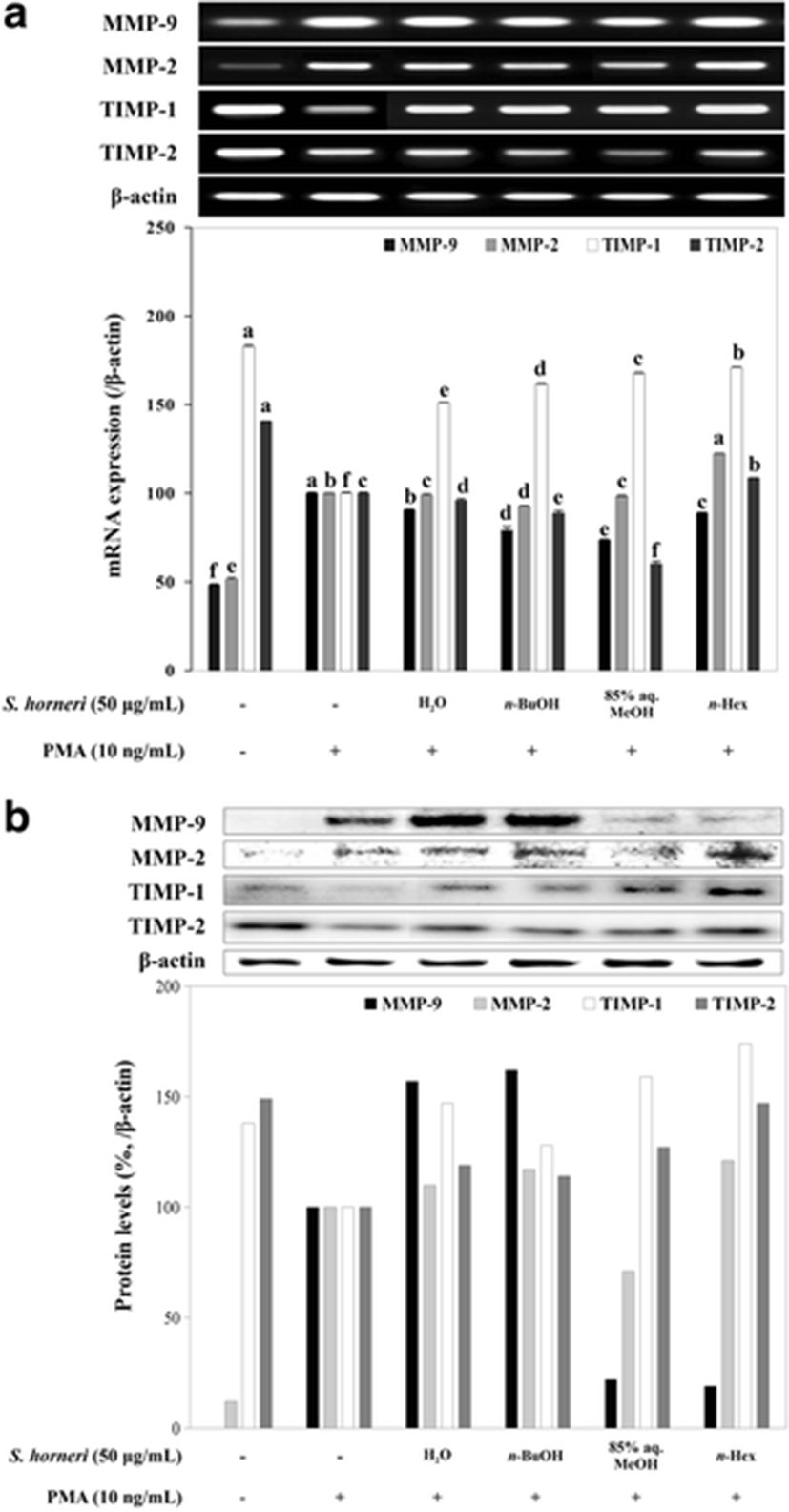
Further, RNA and total protein levels of MMP-2 and MMP-9 were determined by RT-PCR and immunoblotting with the levels of TIMP-1 and TIMP-2. TIMPs are known inhibitors of MMPs that are also reported to elevate the activity of MMP-2 in some presented situations (Jiao et al. 2009). RT-PCR and immunoblotting results suggested the treatment with SHEs was able to hinder the expression of MMP-2 and MMP-9 in terms of both mRNA (Fig. 4a) and protein levels (Fig. 4b). The presence of TIMPs is considered to imply for inhibited MMP activity as a part of cellular response for extracellular stimuli (Reuter et al. 2010). Hence, the PMA stimulation caused TIMP levels to decrease and MMP expression to increase (Fig. 4). However, treatment with SHE was observed to produce mixed results regarding the effect on the TIMP levels following the PMA stimuli. Expected results were to inhibit MMP expression while enhancing TIMP expression in order to regulate the extracellular matrix degradation. In these terms, only 85% aq. MeOH and n-hexane were able to regulate the MMP-2, MMP-9, TIMP-1, and TIMP-2 levels. On the other hand, protein levels of MMP-2 and MMP-9 were slightly elevated after H2O and n-BuOH SHE treatment with elevated TIMP-1 and TIMP-2 levels while mRNA levels did not depict any significant changes for all tested SHE samples. In addition, as mentioned earlier, elevated levels of TIMP-2 have caused an increase in MMP-2 protein levels in terms of n-hexane SHE treatment. Nonetheless, SHEs were shown to have an effect on both activity and expression of MMP pathways but with suggested different mechanisms of action. In cases of H2O and n-BuOH samples, a possible intervention for the activation of MMP-2 and MMP-9 enzymes was suggested following the elevated protein levels of MMPs which would explain the inhibited enzyme activity and elevated protein levels. In terms of the remaining SHEs, a mechanism where TIMP-linked regulation of MMP activity as well as direct bonds between substances in SHE and enzyme results in inhibition of MMP-2 and MMP-9. In other terms, certain discrepancies among the activities of samples in different assays were suggested to be the outcome of different chemical compositions and more than one bioactive compound presence. While most active SHEs were able to interact with the MMP enzymatic activity to show their effect, least active SHEs were suggested to interact with the intracellular pathways of expression and activation of MMPs. Accordingly, as the most active of all samples and all results considered, 85% aq. MeOH SHE was observed to show its efficiency against MMP activity tested by gelatin zymography and cell migration. On the other hand, while n-BuOH SHE was ineffective in hindering cell migration and inhibiting MMP gelatinase activity, it was able to suppress the MMP expression while enhancing the mRNA levels of TIMPs. The MeOH fractions which were suggested to be a phenol-rich extract (Seo et al. 2004; Shipeng et al. 2015), therefore, were suggested to inhibit the enzymatic activity of MMPs through direct interaction. The n-BuOH fractions which were reported to be rich in chromones and coumarins (Kim et al. 2015), however, were able to regulate the intracellular pathways of MMP expression while having little to no effect on enzymatic activity.
Conclusions
Possible chemical composition of the 85% aq. MeOH SHE, the most active sample according to current results, was suggested to be formed mostly with phenol-based compounds which are common bioactive substances of brown algae with health beneficial effects (Bhatnagar and Kim 2010) while H2O and n-BuOH SHE possibly contain more glycol-based compounds. Some MMP-inhibiting polysaccharides (Tu et al. 2008) and benzopyran derivates from different sources were already isolated and reported while similar compounds were also found in S. horneri with different bioactivities. Nevertheless, current results indicated that S. horneri is a source for MMP inhibitors that might lead to future development of anti-tumor compounds. On the other hand, detailed evaluation of S. horneri and its constituents will also provide valuable insights for its utilization as functional food, and future studies on its detail action mechanism are urged for a better understanding of its potential. In the current state, S. horneri was suggested as a potential nutraceutical due to its potential anti-MMP effect.








