Background
Shrimp-shell waste (SSW) is generated in huge quantities from the shrimp processing industries throughout the world, and it is primarily disposed of into the sea, causing intense environmental pollution (Suresh 2012). Since this chitinous waste is considered as a valuable renewable resource, related studies have been carried out to convert it into useful compounds. Recently, environmentally friendly reutilization of SSW using microorganisms has attracted our interest. Thereupon, fermentation productions of chitin, mono-, di-, and oligo-saccharides have been reported from SSW by chitin-degrading bacterial strains (Halder et al. 2013; Sorokulova et al. 2009; Wang et al. 2012). The chitosaccharides have exhibited diverse functional properties, such as antitumor activity (Liang et al. 2007; Wang et al. 2008b), antimicrobial activity (Tsai et al. 2000; Wang et al. 2008a; Wang and Yeh 2008), and antioxidant activity (Annamalai et al. 2011; Azam et al. 2014; Wang et al. 2010). In particular, N-Acetylglucosamine (GlcNAc) as a unit of chitin has been reported to have a great prospect for the treatment of several diseases, such as osteoarthritis (Talent and Gracy 1996), gastritis, and inflammatory bowel disease (Chen et al. 2011).
Among studies on microbial reclamation of shrimp processing waste, Bacillus cereus has been known as an efficient microorganism for shrimp-waste degradation (Azam et al. 2014; Banik and Prakash 2004; Ghorbel-Bellaaj et al. 2012; Sorokulova et al. 2009; Wang et al. 2009; Wang et al. 2012). The strain B. cereus EW5 was reported to produce chitinolytic (Azam et al. 2014), proteolytic, and lipolytic enzymes (Kim et al. 2010). Bioactive chitosaccharides, chitobiose, and GlcNAc were also recovered by SSW biodegradation using the B. cereus EW5 (Azam et al. 2014; Banik and Prakash 2004; Chen et al. 2011; Ghorbel-Bellaaj et al. 2012; Kim et al. 2010; Talent and Gracy 1996; Wang et al. 2009). Since competent production of the chitosaccharides from SSW has not yet reported, it is necessary to study the enhanced SSW biodegradation in a bioreactor level for commercial application.
Fed-batch is a commonly used means for the production of microbial biomass, ethanol, organic acids, antibiotics, vitamins, enzymes, and other compounds in which the culture medium is added continuously or in pulses to reach the maximum volume (Hadiyanto et al. 2013). The advantages of fed-batch over the conventional batch operation include higher biodegradation rate, higher productivity, higher dissolved oxygen (DO) in the medium, and decrease in fermentation time and toxic effect of the medium components (Abou-taleb 2015; Cheng et al. 2009). In the fed-batch operation, the design of the feeding strategy and feed control is of great importance, as both overfeeding and underfeeding of the nutrient affect the cell growth and the formation of desired products (Bretz and Kabasci 2012). The feeding strategies in the fed-batch operation include a constant feeding rate, a pulse feeding rate, and an exponential feeding rate. Since actively growing cells fed at a constant rate in fed-batch operation are overfed at early reaction stages and underfed at later stages, feeding based on the specific growth rate of the main microbe has been known to yield a better bioreaction (Salehmin et al. 2014). In this study, therefore, the production of reducing sugar, antioxidant, and DNA protective compounds was investigated in the fed-batch SSW biodegradation using a pulse feeding strategy for high production. The changes in kinetic parameters and the production of the bioactive compounds were compared with those obtained from the batch biodegradation to demonstrate the advantage of fed-batch biodegradation.
Methods
The microorganism used in this study was a chitin-degrading strain B. cereus EW5 (GenBank accession no. DQ923487) previously isolated from the earthworm viscera (Azam et al. 2014) and ordinary maintained in the deep freezer at − 70 °C. It has potentially proteolytic and lipolytic activities with fairly good salt endurance. As a competent microorganism, the strain B. cereus EW5 was first cultivated in the liquid nutrient broth after thawing, incubated for 14 h at 47 °C, and then maintained at 4 °C for further use.
The culture medium was composed of (w/v): 1% shrimp-shell powder (SSP); 0.5% NH4Cl; 0.1%, K2HPO4; and 0.05% MgSO4.7H2O (pH 7.0). The SSP was prepared from the shell parts of the frozen whiteleg shrimp (Litopenaeus vannamei) purchased from the local market. The shell parts were initially washed with tap water, boiled for 15 min, and then dried in an oven at 120 °C for 12 h. The dried shells were ground to powder form, sieved with a particle size of less than 38 μm, and stored at 4 °C until used. To activate the biodegradation, the prepared SSP was pretreated: it was sonicated for 1 h, treated with NaOH at pH 12.5 ± 0.1 on a hot plate at 80 ± 5 °C with mild stirring for 5 h for deproteinization, and then treated with HCl at pH 4.0 ± 0.1 at room temperature, followed by continuous stirring overnight for demineralization to increase its solubility. The other constituents of the culture medium excluding SSP were separately autoclaved at 121 °C for 15 min. After then, the other constituents’ solution was mixed with the pretreated SSP solution. Finally, the SSP culture medium for biodegradation experiments was prepared after pH of the mixed solution was adjusted to 7.
To produce useful compounds from SSP, the biodegradation experiments were carried out in a 5 L bioreactor (Winpact Bench-Top Fermenter, Major Science, USA) with working volume of 3 L. This bioreactor system was equipped with three six-bladed adjustable Rushton-type impellers, four peristaltic pumps, polarographic DO sensor, pH electrode, temperature control, inlet air flow meter, baffle, condenser, and real-time recording and control system within the vessel. The stirred reactor was aerated by an air pump (LP-40A, Young Nam Yasunaga Co., Korea). The airflow rate and rotation speed were 2 LPM and 200 rpm, respectively. During the biodegradation, DO level was maintained at 50–70% saturation by adjustment of agitation speed and aeration rate. The inoculum (10%, v/v) for the biodegradation was prepared in a 250-mL conical flask containing the 100-mL SSP culture medium. After B. cereus EW5 was seeded, the flask was incubated at 47 ± 1 °C and 170 ± 5 rpm for 1 day to proliferate cells until a log-phase. To prevent severe foaming, 1% antifoam emulsion was pumped into the reactor. Since evaporative liquid loss was taken place in the reactor due to high reaction temperature and airflow, it was compensated with sterile distilled water (DW). Samples were taken periodically during the biodegradation for analyses of cell density and useful compounds produced from SSP. The culture supernatant was collected by the centrifugation at 4 °C and 10,000 rpm for 10 min.
The biodegradation was executed in both batch and fed-batch operations. The bioreactor temperature was 47 ± 1 °C, pH was not controlled, and antifoam emulsion was pumped into the bioreactor when foam occurred plentifully. In the batch operation, 2700 mL of the sterile SSP culture medium was initially filled in the bioreactor vessel. The pH and DO probes were calibrated in advance, and all process set points were entered on the control unit installed on the reactor body. The agitation speed was set at 200 rpm with airflow rate of 1.0 vvm for complete mixing. After the parameters were at their set points, 300 mL of inoculum (10%, v/v) was pumped aseptically into the bioreactor vessel using a peristaltic pump. Therefore, the batch biodegradation started with initial working volume of 3 L, and samples were taken periodically for analyses of reaction parameters.
The fed-batch biodegradation started after the characteristic data were obtained from the batch biodegradation. The fed-batch operation was initiated as a batch culture with 1080-mL SSP culture medium and 120-mL (10%, v/v) seed culture of B. cereus EW5. According to the growth characteristics of the batch culture, pulse feeding was executed at 14, 42, and 72 h of biodegradation periods when the cells reached early-, mid-, and late-exponential phases, respectively. The amounts of pulse feeding at 14, 42, and 72 h were 390, 785, and 625 mL, respectively. The final working volume was 3 L, which was equivalent to the working volume of the batch biodegradation. The SPP culture medium was injected into the bioreactor using a peristaltic pump at a flow rate of 46.80 mL/h, and the biodegradation lasted for 96 h. All the experiments were carried out in triplicate.
At each sample period, a 10-mL sample was taken from the bioreactor, and 1 mL was used for the determination of change in cell density during the biodegradation. Against control, the cell density was measured as optical density (OD) using spectrophotometer (Optizen, Mecasys Co., Korea) at 600 nm, and each measurement was carried out in triplicate. The rest of the sample was centrifuged at 4 °C and 10,000 rpm for 10 min, and then the supernatant was immediately frozen at − 20 °C for later analyses of reducing sugar, antioxidant activity, and DNA damage inhibition activity.
A slightly customized method of Imoto and Yagishita (1971) was used to determine the concentration of reducing sugar produced from the SSP. Four milliliters of the color reagent (98% dinitrosalicylic acid; Sigma-Aldrich, St. Louis, USA) was mixed with 1 mL of the culture supernatant, followed by incubation in boiling water in a glass tube for 8 min. Then, the absorbance of the mixture was measured using the spectrophotometer at 420 nm after cooled at room temperature. The concentration of reducing sugar was finally determined by means of the standard curve using GlcNAc (Sigma-Aldrich) as a reference compound.
To determine the DPPH (2,2-diphenyl-1-picrylhydrazyl) free radical scavenging activity of the culture supernatant, the slightly customized Blois (1958) method was applied. Two milliliters of 0.1 mM DPPH (dissolved in 80% ethanol) solution was added to 1 mL of the culture supernatant, and the resultant mixture was placed at room temperature in the dark for 30 min. After then, the mixture solution was measured using the spectrophotometer at 517 nm. The sample blank was prepared by replacing DPPH with 80% ethanol. DPPH radical scavenging activity was finally obtained from the following calculation:
The control sample was the mixture of 2 mL of 0.1 mM DPPH and 1 mL of 80% ethanol. As a positive control, L-ascorbic acid (0.1 mM) was used under the same measurement conditions. The assay was done in triplicate.
For ABTS (2, 2′-Azino-bis 3-ethylbenzothiazoline-6-sulfonic acid) radical cation decolorization assay, a slightly customized method of Re et al. (1999) was applied. To prepare the ABTS radical cation (ABTS reagent), 5 mL of 7 mM ABTS was mixed with 5 mL of 4.9 mM Potassium persulfate (K2S2O8) in DW. The mixture was placed in a dark at room temperature for 16 h. The absorbance of the ABTS reagent was then modulated to 0.720 ± 0.02 at 734 nm with 80% ethanol. Finally, 1.8 mL of the ABTS reagents was added to 0.2 mL of the culture supernatant, followed by measurement of the absorbance at 734 nm. As a positive control, L-ascorbic acid (0.3 mM) was used under the same measurement conditions. The percentage of inhibition was obtained from the following calculation:
DW was used as the control, and the sample blank was prepared with 80% ethanol by replacing the ABTS reagent. The assay was done in triplicate.
A slightly customized method of Wu et al. (2010) was used for reducing power assay. One milliliter of the culture supernatant was mixed with 1.0 mL of 0.2 M phosphate buffer (pH 6.6) and 1.0 mL of 1% potassium ferricyanide, followed by incubation at 50 °C for 20 min. After the incubation, the reaction was stopped by adding 1.0 mL of 10% (w/v) trichloroacetic acid, and then the reaction mixture was centrifuged at 3,000 rpm for 10 min. Two milliliters of the centrifuged solution was taken from the upper layer and mixed with 2 mL of DW and 0.4 mL of 0.1% FeCl3, followed by incubation at room temperature for 10 min. After incubation, absorbance of the solutions was measured using the spectrophotometer at 700 nm. The control was prepared with DW by replacing the culture supernatant. The assay was conducted in triplicate.
The protective effect of the biodegraded SSP culture supernatant against hydroxyl radical-induced oxidative DNA was performed according to a slightly customized method of Lee et al. (2002). For the determination of DNA damage inhibition, 2 and 4 μL of the 48-h culture supernatants were exposed to 2 μL of 300 ng/μL λ DNA (Takara Bio Inc., Japan) with 1/4 concentration of freshly prepared Fenton’s reagents (mixture of 20 mM FeCL3, 12.5 mM ascorbic acid and 7.5 mM hydrogen peroxide). In each determination, two different amounts (2.5 and 5 μL) of Fenton’s reagents were used. Positive control was prepared by mixing DW with the 2-μL λ DNA in the absence of the culture supernatant and Fenton’s reagents, while negative control was prepared by mixing 2-μL λ DNA, 2-μL Fenton’s reagents, and 16-μL DW excluding the culture supernatant. Final volume of each mixed solution was kept at 20 μL. Each mixture was then incubated at 37 °C for 30 min, and the DNA was analyzed on 1.5% agarose gel, followed by ethidium bromide staining and visualization under UV-transilluminator using Gel Documentation system (Vilber Loumat, France).
Results and discussion
A batch biodegradation was first executed, and then a fed-batch biodegradation based on the growth characteristics of the batch biodegradation was followed to enhance the production of useful compounds from SSP. The enhancement in the fed-batch operation over the batch operation was evaluated by the comparison between their values of reaction parameters.
A batch biodegradation of SSP using B. cereus EW5 was carried out for 96 h, and changes in cell concentration and pH are shown in Fig. 1a. The profile of cell density displayed a typical batch growth pattern. For the first 12 h, a lag phase of the cell growth was revealed, followed by early-, mid-, and late-exponential phases. After then, cell growth slowly reached a stationary phase. The highest optical cell density was 2.5 at 96 h. During the biodegradation, the specific growth rate (μ) gradually slowed down, and those values at early-, mid-, and late-exponential phases were approximately 0.04, 0.03, and 0.02 h−1, respectively. This tendency in the growth rate was also shown in the previous study of SSP biodegradation (Azam et al. 2014). Meanwhile, pH dropped to 6.43 for the first 12 h, and thereafter, it started to increase to 7.09 for the next 12 h of biodegradation (Fig. 1b). The drop in pH occurred when SSP was deproteinized and demineralized by B. cereus EW5 (Rao et al. 2000), and accumulation of chitosaccharides containing an amino group caused an increase in pH (Halder et al. 2013). Similar findings were found in other studies of shellfish-waste biodegradations (Rajdeep and Krishna 2012; Wang et al. 2009). After 24 h, the pH decreased gradually and reached at the lowest value of 4.57 at 96 h. This result was supported by the study of Ghorbel-Bellaaj et al. (2011) in which pH dropped from 7.0 to 4.4 over 7 days of SSP fermentation using Pseudomonas aeruginosa. In that study, the demineralization rate was the maximum (92%) when the pH reached at 4.4. Similar result was also found from the chitin fermentation using Chitinbacter tainanesis (Chen et al. 2010). In this case, pH decreased gradually from 7.4 to 5.3 for 14 h.
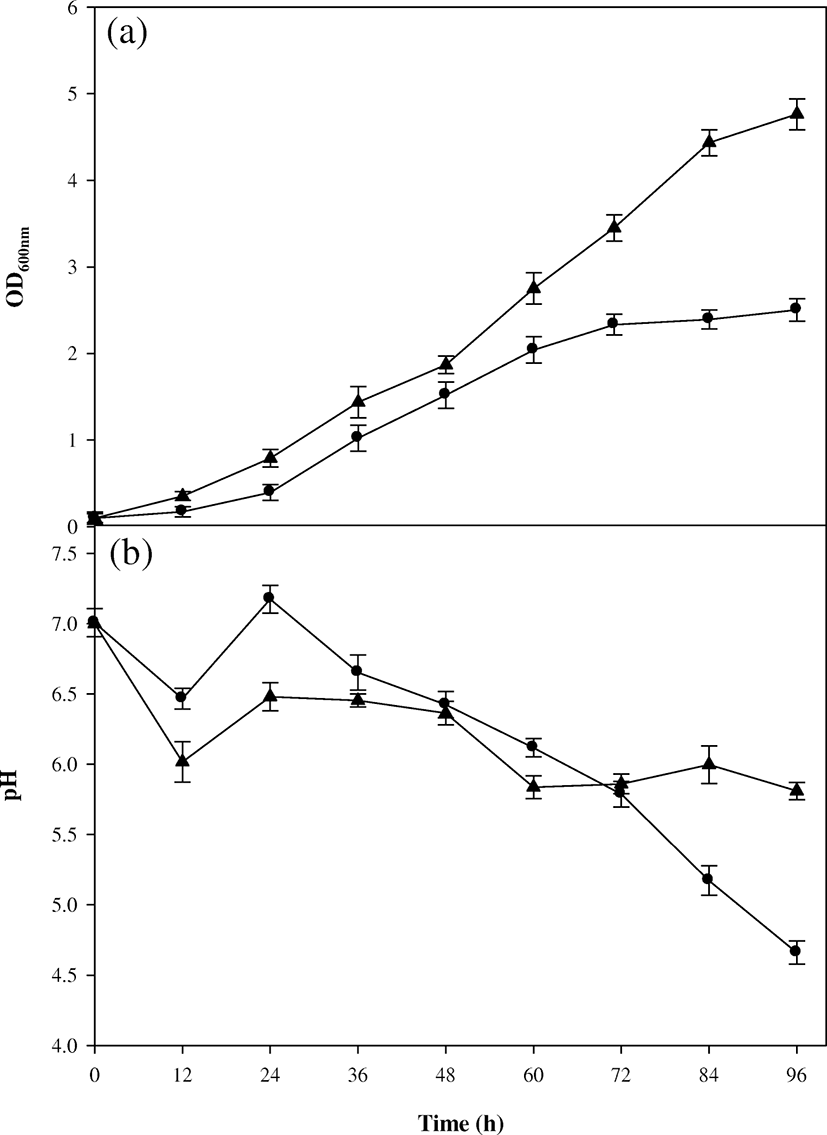
After the batch biodegradation, a fed-batch biodegradation was executed using the pulse feeding strategy. The amounts of feeding substrates were 390, 785, and 625 mL at 14, 42, and 72 h, respectively, to adjust the dilution rate to μ at each growth phase characterized from the batch biodegradation. The change in the cell density in the fed-batch operation is shown in Fig. 1a. During the first batch-type operation with working volume of 1.2 L for 14 h, a relatively high value of optical cell density was displayed, compared with that of the batch operation with working volume of 3 L. This was probably because the environmental conditions of aeration and mixing were more favorable to a smaller quantity of working volume (Gwon and Kim 2012). Cell proliferation increased after the first pulse feeding for 14 h, and this trend was more intense after the second and third pulse feedings. The optical cell density (4.76 at OD600) in the fed-batch biodegradation at 96 h was approximately 1.9 times as much as that (2.5) of the batch biodegradation. This result indicates that the fed-batch operation was more favorable for cell proliferation than the batch operation. It was because the pulse feeding was based on μ of B. cereus EW5, providing a better environment for cell growth (Abou-taleb 2015). Meanwhile, the change in pH showed a little different pattern in the fed-batch biodegradation (Fig. 1b). For the first 24 h, the pattern of pH was similar to that of the batch biodegradation. After then, pH slightly fluctuated between 5.74 and 6.50. In the batch biodegradation, on the other hand, pH gradually dropped to 4.57 until the end. Therefore, additional substrate feeding in the fed-batch operation might contribute better cell growth and consequently lead the production of various metabolites (Abou-taleb 2015). It was also reported that change in alkalinity was caused due to breakage of peptide linkages of chitin by proteolysis (Bajaj et al. 2016), and high demineralization rate was found at pH 5.86 during the batch degradation of chitin (Ghorbel-Bellaaj et al. 2011). GlcNAc was reported to result from the chitin degradation optimally at pH 5.3 (Chen et al. 2010). From all the above information, it was concluded that a different pH pattern shown in the fed-batch biodegradation was due to the enhancement in cell density with higher production of metabolites.
During the biodegradation of SSP, reducing sugar as one of useful compounds was produced by B. cereus EW5. The reducing sugar was produced from the beginning of the batch biodegradation, and its concentration increased until 60 h, with the maximum value of 0.265 mg/mL (Fig. 2). After then, it decreased slightly to 0.222 mg/mL at 96 h. This trend could be generally found in the biodegradation using polymer substrates (Azam et al. 2014). Up to the middle of exponential growth phase, the strain EW5 continuously produced the reducing sugar by its extracellular chitinolytic enzyme. Meanwhile, decrease in the reducing sugar concentration at a later biodegradation period was probably because the strain EW5 utilized the reducing sugar as an easier uptake substrate, instead of SSP. In the previous study (Azam et al. 2014), 0.24 mg/mL of reducing sugar was produced from SSP after 4 days of incubation in a shake-flask level. Therefore, batch culture in a bioreactor level clearly enhanced the production of reducing sugar and simultaneously shortened the biodegradation time.
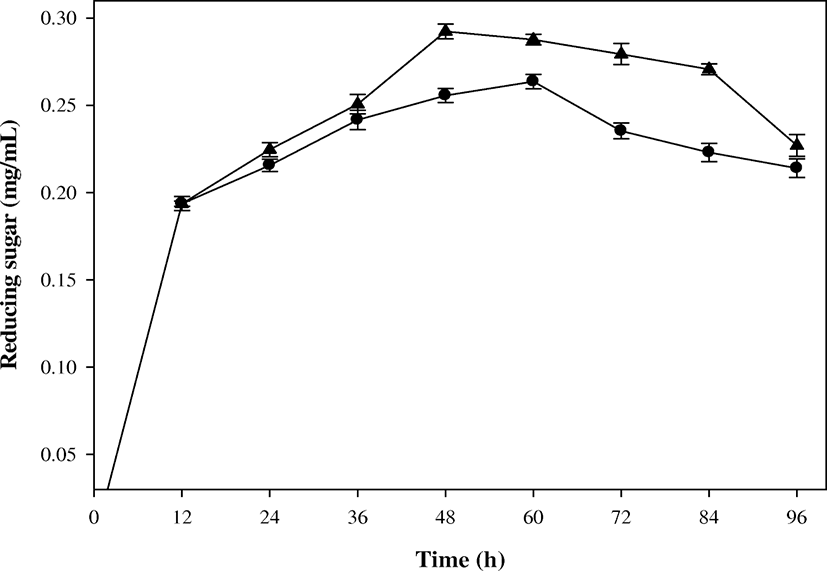
In the fed-batch biodegradation prior to the pulse feeding, the pattern of reducing-sugar production followed that of the batch biodegradation (Fig. 2). After the pulse feeding, the production rate of reducing sugar in the fed-batch biodegradation exceeded that of the batch biodegradation. The maximum value of 0.297 mg/mL was achieved at 48 h, and it maintained more or less up to 84 h. This value was approximately 12.1% higher than that of the batch biodegradation with shorter biodegradation time. As a result, the substrate feeding adjusted to μ of the strain EW5 could provide a better environment for production of reducing sugar. The concentration of reducing sugar also decreased in the later fed-batch biodegradation, as shown in the batch biodegradation.
As a bioactive compound, an antioxidant has been known to be produced from the SSP biodegradation (Azam et al. 2014). For this reason, three different types of antioxidant activities were measured for the biodegraded products produced from the batch and fed-batch biodegradations. First, DPPH radical was applied to measure the free-radical scavenging capacity of the culture supernatant. In this measurement, reduction in absorbance occurred by scavenging the free radicals when DPPH radicals encountered a proton-donating substance (Bersuder et al. 1998). As shown in Fig. 3, DPPH radical scavenging activity in the batch operation increased as SSP was biodegraded up to 60 h with a value of 89.33%, and then it decreased gradually to 66.57% at the end. The maximum activity was superior to the positive control of 0.1 mM L-Ascorbic acid (81.35%). The DPPH radical scavenging activities reported in the previous studies ranged over a wide variety of values: 56% DPPH radical scavenging activity from SSP fermentation using B. cereus (Wang et al. 2009); 64.86 and 79.84% activities from cooked and raw shells of shrimp (Litopenaeus schmitti), respectively (Lira et al. 2017); 68.5–83.4% activities during 8 days of biodegradation (Azam et al. 2014); 82% activity from shrimp waste by treatment with crude protease isolated from Bacillus cereus SV1 (Manni et al. 2010); 82.5% activity after 64 h of SSP biodegradation using Aeromonas hydrophila SBK1 (Halder et al. 2013); and 90% maximum activity after 3 days of SSP fermentation using Pseudomonas aeruginosa (Ghorbel-Bellaaj et al. 2011). Although the DPPH radical scavenging activity could become different with the reaction conditions, a relatively high activity was obtained through this study.
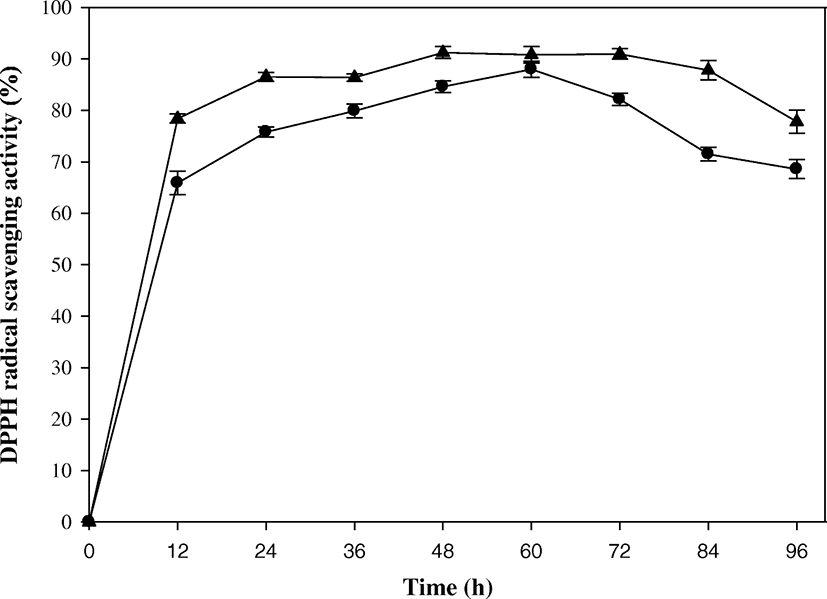
The DPPH radical scavenging activity was enhanced by the fed-batch operation. The maximum value of 92.35% was determined at 48 h, and the activity remained more or less until 84 h. Thereafter, it decreased to 75.63% at the end. The DPPH radical scavenging activity was overall enhanced, the maximum activity was achieved in a shorter biodegradation time, and the activity was maintained for a longer time, compared with the result of the batch operation. The enhancement in the DPPH radical scavenging activity could result from the maintenance of active biodegradation using the pulse feeding adjusted to μ of B. cereus EW5. Protein-astaxanthin complexes of shrimp waste were reported to produce a complex mixture of bioactive compounds (free amino acids, peptides, carotenoids, etc.) by the bacterial enzyme hydrolysis (Manni et al. 2010). Consequently, the fed-batch culture supernatant could contain more oxidant compounds that acted as electron donors, which converted the free radical to more stable products and terminated the radical chain reaction.
The ABTS radical cation decolorization assay was applied to measure the antioxidant activity of the culture supernatant. It is often used to evaluate the antioxidant activity of both lipophilic and hydrophilic antioxidant (Bersuder et al. 1998). The ABTS radical scavenging activity of the batch culture supernatant ranged from 71.42 to 93.33% during 96 h of biodegradation (Fig. 4). These activities were superior to that (73.2%) of 0.3-mM ascorbic acid as a positive control. In the same manner, Sachindra and Bhaskar (2008) reported high radical scavenging activity of 94.82% from the fermentation of SSP, and Azam et al. (2014) reported high radical scavenging activity of 93.4–99.6% during the biodegradation of SSP. On the contrary, Walke et al. (2014) reported 24% ABTS scavenging activity from shrimp shell against a positive control (0.05 mg/mL butylated hydroxyanisole), Sujeetha et al. (2015) reported 41% activity from mud crab (Scylla serrata) extracts, and Lira et al. (2017) reported 43.86 and 45.23% activities for cooked and raw shells of shrimp (Litopenaeus schmitti), respectively.
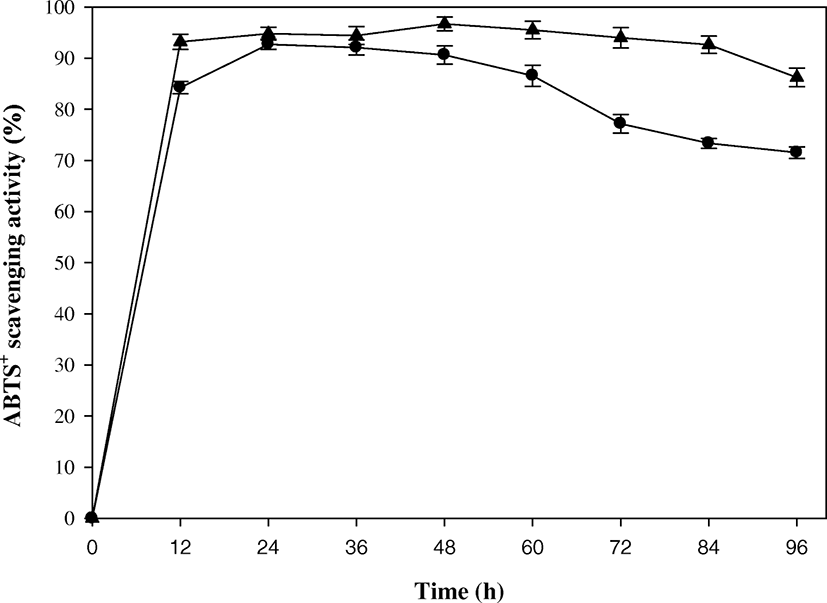
In this study, the ABTS radical scavenging activity was enhanced by the fed-batch operation (Fig. 4). The ABTS radical scavenging activity ranged 85.70–98.16% during 96-h fed-batch operation, and the highest activity was achieved after 48 h. In the fed-batch operation, there was no big drop in the ABTS radical scavenging activity until the end of biodegradation, which was not displayed in the batch operation. This indicates that the pulse feeding provided a better environment for the production of antioxidant compounds in the fed-batch operation. High ABTS radical scavenging activity was known to be caused mostly by the production of GlcNAc and chitobiose from SSP (Azam et al. 2014). This high ABTS scavenging activity was also reported in the study of SSP fermentation by Sachindra and Bhaskar (2008).
The reducing power assay is often used to appraise the ability of an antioxidant to donate electron or hydrogen (Gao et al. 2012). It was known that there was a direct correlation between antioxidant activity and reducing power of certain bioactive compounds (Bahri-Sahloul et al. 2014). Accordingly, the action of antioxidant compounds resulted in the neutralization of free radicals, converting them into more stable non-reactive species and thus terminating the free radical-initiated chain reactions (Bersuder et al. 1998). In this study, the reducing power of the batch culture supernatant showed a linear increase with time (Fig. 5). The highest value (1.43) of reducing power was recorded at 96 h against the control (0.025). As reported in other studies (Ghorbel-Bellaaj et al. 2012; Sachindra and Bhaskar 2008; Wang et al. 2009), a positive correlation between reducing power and antioxidant activity based on the radical scavenging activity was also found in this study. The reducing power reported in previous studies of SSP were in a wide range of values: Azam et al. (2014) reported a reducing power of 0.34 after 5 days of incubation in a flask level, whereas Maruthiah (2017) reported 1.32 as the reducing power of SSP hydrolysate. Furthermore, a reducing power of 1.7 was reported after 1 day of SSP fermentation using P. aeruginosa (Ghorbel-Bellaaj et al. 2011), and a reducing power of 1.55 was reported for hydrolysate of SSP achieved by Bacillus pumilus A1 (Ghorbel-Bellaaj et al. 2012). Although the production of reducing power compounds from SSP could vary under different culture conditions, a relatively high reducing power was obtained through this study.
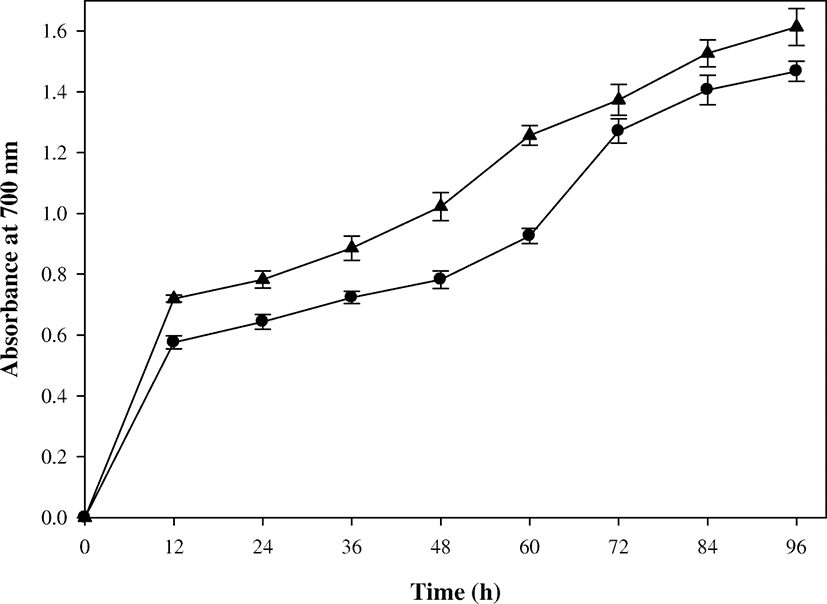
In the fed-batch operation, the reducing power of the culture supernatant also increased linearly with time (Fig. 5). The highest value of reducing power was 1.55 at 96 h, which was enhanced by 8% in comparison with that of batch operation. It was reported that antioxidant activity was closely related to the concentrations of bioactive compounds, such as phenolics, chitooligosaccharides, oligopeptides, peptides, and free amino acids, and they were most likely produced during the fermentation of shrimp waste (Ghorbel-Bellaaj et al. 2012; Sachindra and Bhaskar 2008; Wang et al. 2009). As a result, the enhancement in reducing power in the fed-batch operation could result from high production of antioxidant compounds by the pulse feeding adjusted to μ of the strain EW5.
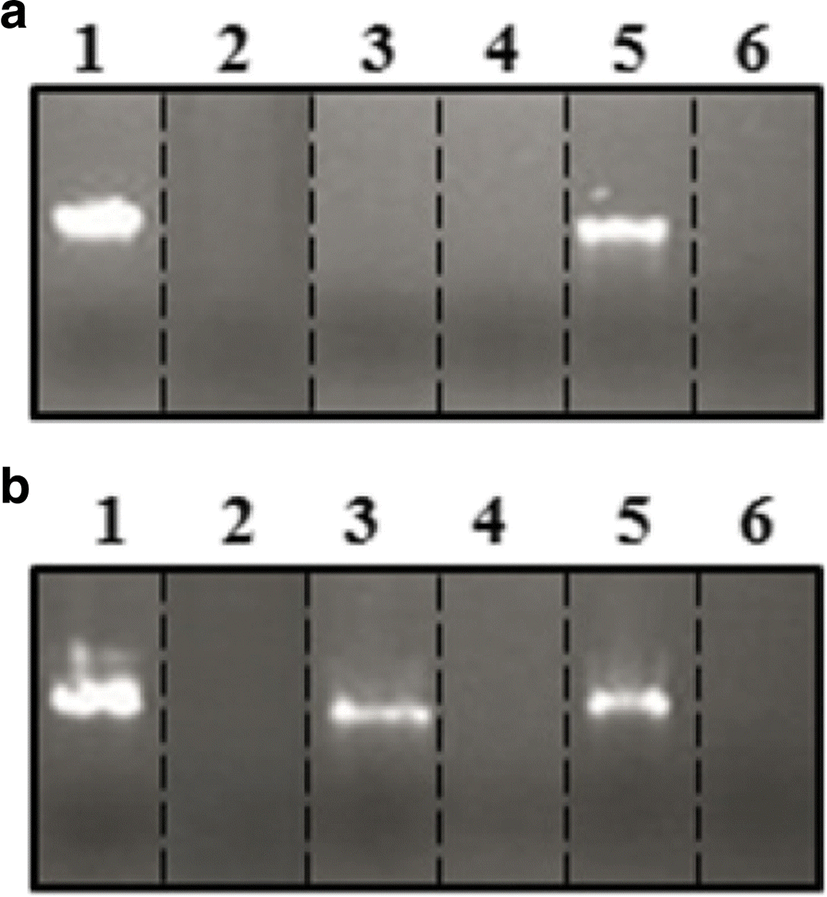
Free hydroxyl radicals were known to induce some damage to the DNA, leading to mutation (Saenjum et al. 2010) or further to cell death (Kim et al. 2012). For this reason, natural antioxidant compounds are concerned about their protective ability of cellular components. To evaluate DNA damage inhibition activity of the 48-h culture supernatant, hydroxyl radicals were exposed to λ DNA either in the presence or in the absence of the culture supernatant. As shown in Fig. 6, DNA damage inhibition activity did not appear in the absence of the culture supernatant. When 2 μL of the 48-h culture supernatant was applied to this assay, λ DNA treated with 2.5 μL of Fenton’s reagents (containing hydroxyl radicals) displayed a clear band exclusively in the presence of the fed-batch culture supernatant (lane 5 in Fig. 6a). Compounds produced from SSP were reported to have DNA protection activity against damage by hydroxyl radicals and recommended for potential use in gene therapy: astaxanthin (Sila et al. 2013) and chitosaccharidies (Halder et al. 2014) from the treatment of protease and chitinase, respectively. In this study, however, the DNA damage inhibition activity did not appear in the presence of either the batch culture supernatant or a higher free-radical concentration at 5 μL of Fenton’s reagents. When 4 μL (as a double dose) of the 48-h culture supernatant was exposed to λ DNA, the DNA damage inhibition activity of the batch culture supernatant could be clearly visible on 1.5% agarose gel (lane 3 in Fig. 6b), which was not shown in 2 μL of the batch culture supernatant. However, any DNA damage inhibition activity was still not displayed in the present of 5 μL of Fenton’s reagents, even if the dose of the culture supernatant was doubled. From the above results, it was concluded that the DNA damage inhibition activity of the fed-batch culture supernatant was superior to that of the batch culture supernatant. This was because relatively high production of DNA damage inhibitory compounds was obtained from the fed-batch operation under a better environment for SSP biodegradation (Abou-taleb 2015).
Conclusions
For commercial-scale production, production of useful compounds from SSP in a reactor level was investigated. A fed-batch biodegradation using a pulse feeding strategy demonstrated an enhancement in reducing sugar concentration, antioxidant activities, and DNA damage inhibition activity, compared with those obtained from the batch biodegradation. This enhancement was accompanied by high cell density and shortened biodegradation period. As a result, it could not only increase the reutilization value of SSW, but also provide a solution for environmental pollution problem. To our knowledge, this is the first scientific report about the enhanced production of useful compounds from SSP through the fed-batch operation.








