Introduction
Metals naturally exist in aquatic ecosystems, but side effects from industrialization have resulted in excessive concentrations. Exposure to high metal levels may negatively affect fish and other aquatic organisms, hampering physiological functions, growth rate, and reproduction, or even increasing mortality (Reddy and Reddy 2013; Öz 2018; Öz et al. 2018). Cadmium is a particularly widespread and toxic example that is documented to accumulate in exposed organisms; it is used primarily in alloys, pigments, electroplating, and batteries (Bryan 1976; Farag et al. 1995; Adriano 2001; Javed 2003).
In fish, Cd disrupts Ca metabolism through competition for transport sites on the basolateral calcium pumps of gills (Verbost et al. 1987, 1988, 1989; Pinot et al. 2000). Cd redox activity affects antioxidants, thus reducing protection against oxidative stress, increasing lipid peroxidation, and decreasing DNA synthesis (Okorie et al. 2014). In addition, Cd lowers plasma Na, Cl, and K, leading to hyperglycemia and hypermagnesemia (Larsson et al. 1981; Haux and Larsson 1984; Sjöbeck et al. 1984). Even at low concentrations, Cd deforms tissues and vertebrae, causing respiration abnormalities and death in fish (De Smet and Blust 2001). Cd and other toxic heavy metals can also accumulate through direct absorption or biomagnification; the resultant inhibition of major organ function (i.e., liver, kidney, and gills) is strongly linked to toxicity. Thus, the degree of accumulation in each organ is used frequently as a biomonitor for metal contamination (Handy 1992).
In fish exposed to Cd, reactive oxygen species (ROS), such as hydrogen peroxide (H2O2), hydroxyl, and oxygen radical, occur and induce oxidative stress. As a result, the biological system induces antioxidant enzymes, e.g., superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione S-transferase (GST), to mitigate the attack of ROS. These enzymes are used as stress biomarkers in fish by exposure or contamination of heavy metals and generation of ROS. SOD is catalyzing the transformation of superoxide anion radicals to H2O2 and oxygen (O2). Catalase (CAT) decomposes toxic H2O2 to O2 and H2O. Glutathione peroxidase (GPx) decomposes H2O2 or organic hydroperoxide to H2O or corresponding alcohols using reduced glutathione (GSH) into oxidized glutathione (GSSG). Glutathione S-transferase (GST) detoxifies the reactive intermediates and oxygen radicals by catalyzing the conjugation of GSH to various electrophilic metabolites, thereby enhancing water solubility and assisting excretion (Livingstone 2003).
Two standard tests of metal toxicity are acute or chronic exposure. In many organisms including fish, acute toxicity is defined as LC50 (median lethal concentration), a concentration that kills approximately 50% of a test group after exposure to increasingly higher toxicant levels for a specified, relatively short time frame (Schreck and Moyle 1990; Mason 1991). Acute toxicity data are supplemented with chronic toxicity tests for the same compound, exposing subject organisms to the same low concentration over a longer period. Such information is useful as a reference when performing environmental surveys of contaminated areas and determining the effects of toxicant efflux after industrial accidents.
Eels are commonly consumed in Asia and are mostly produced through aquaculture. Farmed eels are fed paste that contains a high ratio of fish meal. Thus, Cd accumulation can occur if the metal’s concentration in fish meal is high. Eels suffer particularly high mortality under Cd exposure, because their benthic lifestyle increases contact with heavy metals that sink to the floor. These factors indicate that we require data on Cd effects in eels to ensure food safety and assess environmental contamination. However, despite the progress made on understanding the outcome of Cd exposure in several fish species (Handy 1993; Yilmaz et al. 2004; Aldoghachi et al. 2016), little research has been conducted in eels, especially Anguilla japonica. Furthermore, many bioaccumulation studies focus on chronic exposure, despite the possibility of industrial accidents causing acute Cd exposure and accumulation. If Cd in eels is highly accumulated after acute exposure, it can affect the health of humans as food through a catch. Thus, the purpose of this study is to assess risk as food, identify the effect on fish health, and utilize baseline data for chronic toxicity test by investigating accumulation in major tissues (liver, kidney, spleen, gills, and muscle) and change of antioxidant enzymes (SOD, CAT, GPx, and GST) in the liver with the determination of LC50 for Cd in adult A. japonica.
Materials and methods
Anguilla japonica specimens were collected from the eel aquafarm of Paju city, Gyeonggi province, South Korea. Fish were acclimated to a polyvinyl (PVC) tank for 2 weeks prior to experiment and food-deprived. Also, we identified no infection of parasites in some fish before acclimation and toxicity test to prevent mortality by parasites and used visually healthy fish for the experiment.
Acute Cd toxicity test was conducted under laboratory conditions. Acclimated fish (n = 90; average weight 186.6 ± 11.9 g) were selected, divided into nine groups (10 per group), and placed into plastic aquaria (555 × 395 × 310 mm) filled with underground water. Table 1 summarizes the water quality parameters measured for the bioassay. Water temperature was maintained with a heater at 29 ± 1 °C. To make conditions similar to aquafarm, the laboratory was kept in 24-h darkness except when checking fish mortality. During the exposure period, water was not renewed and fish were not fed. Analytical-grade CdCl2 (Aldrich, Inc., USA) was dissolved in triple distilled water to prepare stock Cd solution used for exposure experiments (see the “Determination of LC50 and assay of actual Cd levels in experimental water” section).
Experimental fish were exposed to waterborne CdCl2 treatments of 0.25, 0.5, 1, 3, 5, 6, 7, and 9 mg L−1, 0.15, 0.30, 0.61, 1.83, 3.08, 3.67, 4.29, and 5.51 mg L−1 as only Cd concentrations, for 96 h. A water-only control was also used. Cd exposure concentrations were established after pre-experiment by referring to Cd chronic accumulation concentration (0.1 mg L−1) in eels, Anguilla japonica, through previous study (Yang and Chen 1996). Dead fish were counted every 12 h and removed immediately from the aquaria. Experimental water was collected to measure actual Cd concentrations at 12 and 96 h after adding the stock Cd solution. Water samples were diluted with 2% nitric acid before analysis using ICP-MS (inductively coupled plasma mass spectrometry; NexION 300X, Perkin-Elmer Inc., USA). The change rate and decrement of actual Cd were calculated as follows:
(1) Change rate (%) = 100 × (1 − Cd concentration at 96 h ÷ Cd concentration at 12 h)
(2) Decrement (mg L−1) = Cd concentration at 12 h − Cd concentration at 96 h
After a 96-h Cd exposure, gills, liver, kidney, spleen, and muscle samples of live fish were collected and kept at − 80 °C until analysis. Tissues were freeze-dried and digested with nitric acid (Suprapur grade, Merck, Germany) and hydrogen peroxide (bioassay grade, Merck, Germany) in a microwave (START D, Milestone, Italy). The resultant solutions were diluted with triple distilled water and subjected to ICP-MS (NexION, Perkin-Elmer Inc., USA).
The Collected liver was homogenized with 0.1 M phosphate-buffered saline (PBS) using tissue lyzer (TissueLyser II, QIAGEN, Germany). The homogenate was centrifuged at 10,000g for 30 min under 4 °C. The supernatants were obtained and stored at − 80 °C until analysis. SOD activity was analyzed using the SOD assay kit (Dojindo Co., Japan) measuring 50% inhibition rate for the reduction reaction of 2-(4-lodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium monosodium salt (WST-1) and was expressed as U mg protein−1. CAT activity was analyzed using the CAT assay kit (Sigma-Aldrich Inc., USA) measuring the absorbance of the chromogen versus the amount of residual H2O2 after reaction with samples and was expressed as U mg protein−1. GPx activity was analyzed using the GPx cellular activity assay kit (Sigma-Aldrich Inc., USA) measuring the change in absorbance at 340 nm by the reduction reaction of tert-butyl hydroperoxide and was expressed as U mg protein−1. GST activity was analyzed using the GST assay kit (Sigma-Aldrich Inc., USA) measuring the change in absorbance at 340 nm by reaction of sample and substrate solution including 1-chloro-2,4-dinitrobenzene (CDNB) and was expressed as μmol min−1 mg protein−1. Total protein concentrations in the liver were determined using the method of Bradford (1976), with bovine serum albumin as a standard.
Finney’s probit analysis was used to determine the LC50 of Cd in eels, along with confidence limits. Between-group differences in Cd bioaccumulation and activities of antioxidant enzymes were analyzed using two tests as a one-way ANOVA depending on Levene’s test for equal variance. Duncan’s multiple range and Games-Howell tests were used at P > 0.05 and P < 0.05 in equality of variances, respectively. Significance of post hoc test was set at P < 0.05. All statistics were performed in SPSS version 20 (IBM co., USA).
(1) Duncan’s multiple range test: kidney, SOD, CAT, GPx, and GST
(2) Games-Howell test: liver, spleen, gill, muscle
Results
Mortality was first measured at a Cd concentration of ≥ 3.08 mg L−1, and the mortality rate reached 100% at 5.51 mg L−1. The number of dead fish increased with increasing Cd concentration. Based on mortality data, LC50 of Cd in A. japonica after 24, 48, 72, and 96 h was 5.10, 4.04, 3.67, and 3.61 mg L−1, respectively (Table 2).
Period (h) | Concentration (Cd mg L−1) | 95% confidence limits | |
|---|---|---|---|
Lower | Upper | ||
24 | 5.10 | 4.37 | 6.84 |
48 | 4.04 | 3.55 | 4.66 |
72 | 3.67 | 3.25 | 4.10 |
96 | 3.61 | 3.19 | 3.99 |
Variation in the actual Cd concentration of experimental water was measured to investigate correlations between Cd accumulation in A. japonica and changes to Cd levels during the acute toxicity test. We found that actual Cd concentrations generally decreased after the 96-h exposure (Table 3). Based on measurements from 12-h post-exposure, the lowest and highest rate change in concentration were 5.1% (at exposure to 5.51 mg L−1 Cd) and 16.8% (at 1.83 mg L−1), respectively. In contrast, the lowest and highest absolute decrement of actual Cd concentration (again measured 12-h post-exposure) were 0.015 mg L−1 (at 0.15 mg L−1) and 0.664 mg L−1 (at 3.67 mg L−1), respectively.
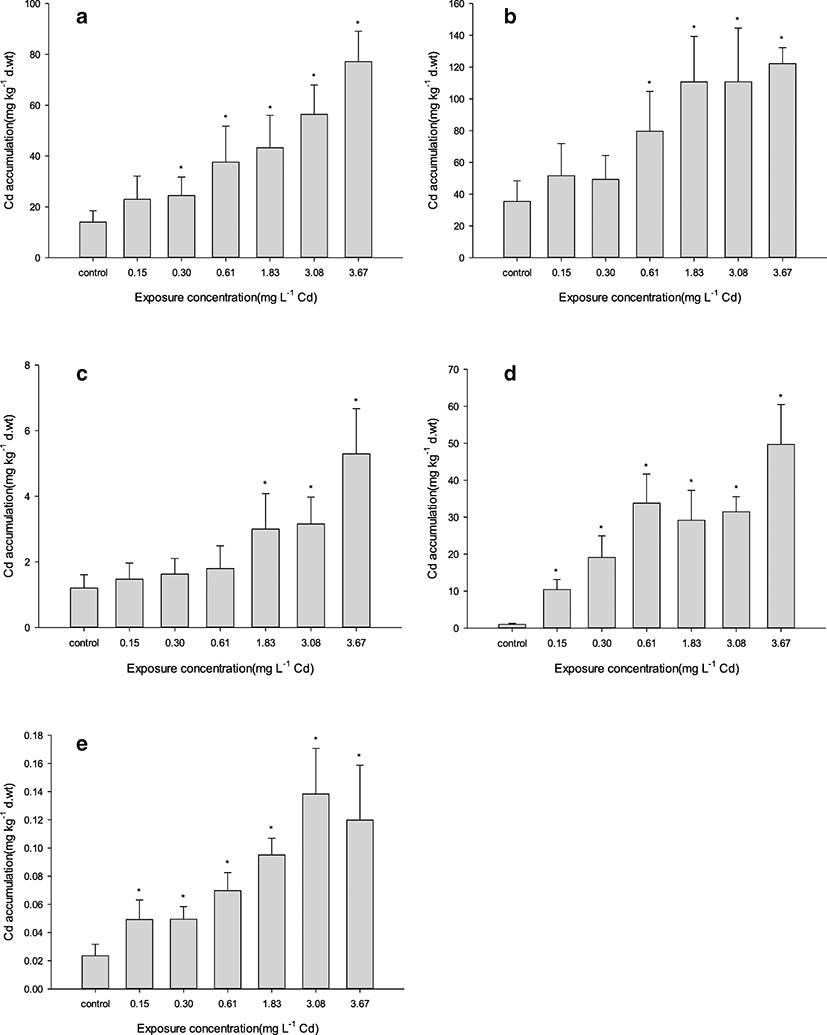
Cd exposure caused a net increase of Cd content in all tested A. japonica tissues compared with the control (Fig. 1). The order of Cd accumulation in tissues (including control) was as follows: kidney > liver > gills > spleen > muscle, with the highest and lowest concentrations being 122.190 mg kg−1 in the kidney (at 3.67 mg L−1) and 0.049 mg kg−1 in the muscle (at 0.15 mg L−1) of exposed groups, respectively. As expected, accumulation rose with increasing exposure concentration. However, significant differences as compared with control in Cd accumulation across all tissues were observable at ≥ 1.83 mg L−1 Cd exposure. Individually, significant differences were apparent at ≥ 0.15 mg L−1 in the gill and muscle, ≥ 0.30 mg L−1 in the liver, ≥ 0.61 mg L−1 in the kidney, and ≥ 1.83 mg L−1 in the spleen.
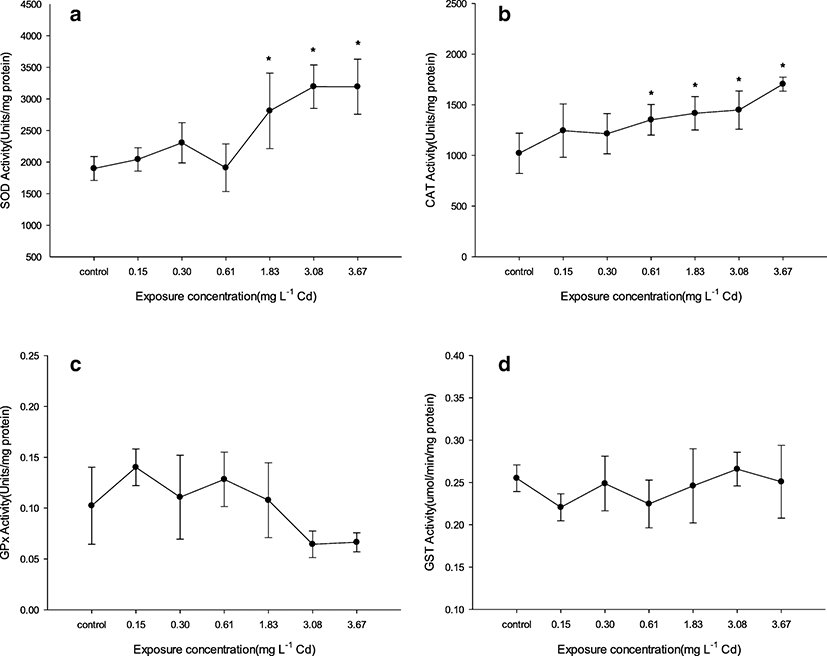
After acute exposure to Cd during 96 h, activities of antioxidant enzymes (SOD, CAT, GPx, and GST) in eel’s liver were determined (Fig. 2). Activities of SOD and CAT increased as compared with control by increasing exposure concentration. Significant increase from control (as 1898 U mg protein−1) in SOD activity was observable at ≥ 1.83 mg L−1 (as 2811 U mg protein−1) with the highest activity being 3195 U mg protein−1 at 3.08 mg L−1 Cd exposure. Significant increase from control (as 1021 U mg protein−1) in CAT activity was observable at ≥ 0.61 mg L−1 with the highest activity being 1704 U mg protein−1 at 3.67 mg L−1 Cd exposure. GPx activity from control (as 0.1024 U mg protein−1) decreased at 3.08 and 3.67 mg L−1 Cd exposure as 0.0644 and 0.0664 U mg protein−1, respectively, but it is not significant. GST activity from control (as 0.2551 μmol min−1 mg protein−1) was not changed at all groups.
Discussion
Like the findings of a general aquatic toxicity study (Rand et al. 1995), this study demonstrated that A. japonica mortality increased with increasing Cd concentration and exposure period. Although previous studies had attempted to examine acute Cd toxicity in freshwater fish (e.g., tilapia, common carp, rasbora) (Rehwoldt et al. 1972; Shuhaimi-Othman et al. 2015), these were generally not enough for the overall assessment of environmental pollution. Nonetheless, we compared our results with several previous studies to evaluate Cd acute toxicity (Table 4). In contrast with 3.61 mg L−1 (after 96-h Cd exposure) in the LC50 of this study, the LC50 in tilapia sac fry (Oreochromis niloticus) and juvenile (Oreochromis sp.) were 1.6 mg L−1 (after 24-h Cd exposure) and 0.7 mg L−1 (after 96-h Cd exposure), respectively (Andaya and Gotopeng 1982; Aldoghachi et al. 2016). Moreover, the 96-h LC50 of Cd in adult guppies (Poecilia reticulata) was 30.4 mg L−1 (Yilmaz et al. 2004), while it was 7.42 mg L−1 in juvenile piauçu (Luciobrama microcephalus) (Gomes et al. 2009). These data indicate that between-species differences in life history, genetic composition, and individual conditions have a greater impact on fish tolerance (or sensitivity) to Cd toxicity than size and age (Rand et al. 1995). Ideally, within-species comparisons would better indicate whether our results are typical of A. japonica. However, although some studies examining acute Cd toxicity do exist for this species, differences in experimental water conditions (e.g., hardness, pH, temperature) complicate the interpretation of any cross-study variation (Shuhaimi-Othman et al. 2015). Regardless, this study provides important basic data for any future study investigating chronic Cd toxicity in A. japonica and allows for further comparative analyses of Cd tolerance among fish.
Species | Live stage | Duration (h) | LC50 (mg L−1) | Reference |
|---|---|---|---|---|
Aguilla japonica | Adult | 96 | 3.61 | This study |
Anguilla rostrata | 96 | 0.82 | Rehwoldt et al. (1972) | |
Cyprinus carpio | 96 | 0.24 | Rehwoldt et al. (1972) | |
Oreochromis niloticus | Sac fry | 24 | 1.6 | Andaya and Gotopeng (1982) |
Oreochromis sp. | Juvenile | 96 | 0.7 | Aldoghachi et al. (2016) |
Poecilia reticulata | Adult | 96 | 30.4 | Yilmaz et al. (2004) |
Luciobrama macrocephalus | Juvenile | 96 | 7.42 | Gomes et al. (2009) |
Channa marulius | Fingerling | 96 | 75.70 | Batool et al. (2014) |
Wallago attu | Fingerling | 96 | 32.95 | Batool et al. (2014) |
Rasbora sumatrana | Adult | 96 | 0.10 | Shuhaimi-Othman et al. (2015) |
Cd accumulation in tissues may differ according to metal’s form. Inorganic Cd tends to be accumulated in the liver, while Cd-thiols are readily accumulated in the kidney, considered the organ most sensitive to Cd toxicity (Hammond and Foulkes 1986; Woo et al. 1993; Okorie et al. 2014). Here, we demonstrated that Cd accumulation was higher in the kidney and liver than other tissues, with significant differences from control at ≥ 0.61 mg L−1 Cd exposure. Furthermore, both organs had greater Cd concentrations even in the control condition. This result corroborates previous study; in A. japonica exposed to 0.05 mg L−1 of Cd, the primary tissues that accumulated Cd were the kidney and liver (Yang and Chen 1996). The previous study suggested that the two tissues could function as indicators of Cd pollution in water, because they appear to be critical sites of Cd accumulation. Field studies in aquatic ecosystems generally support experimental findings. Cd concentrations in the kidney of captured A. rostrata and A. anguilla (at two reference and contaminated sites) were higher than concentrations in the liver and muscle (Pannetier et al. 2016).
We found that Cd accumulation in the gills increased about fivefold from the control level at 3.67 mg L−1 Cd exposure, the highest rate of increase observed in the experiment. Similarly, in common carp (Cyprinus carpio) exposed to 5 mg L−1 of a combined metal solution (Cr, Ni, Cd, and Pb) for 32 days, the gills exhibited a higher rate of increase in Cd accumulation compared with the other tested tissues and also contained the highest in amount of Cd (followed by the liver, kidney, and flesh) (Vinodhini and Narayanan 2008). This high Cd level is likely explained by the fact that gills are a major point of entry for Cd, which passively diffuses through gill Ca channels (Verbost et al. 1989). Also, these results indicate that gills are the most sensitive organ to Cd absorption and accumulation in freshwater fish.
Studies are insufficient about accumulation in the spleen by acute exposure of heavy metals. In our study, Cd accumulation in the spleen showed a significant increase at ≥ 1.83 mg L−1 Cd exposure as a higher concentration group than other tissues. It means that Cd depuration in the spleen is higher than other tissues at the exposure to low Cd concentration. For example, accumulation in the spleen of brook trout exposed to waterborne 0.001 mg L−1 Cd as sub-lethal concentration during 77 days has not increased compared with control (Sangalang and Freeman 1979). However, when referring to LC50 concentrations for Oreochromis species in Table 4, accumulation in the spleen of Oreochromis niloticus exposed to waterborne 1 mg L−1 Cd as high concentration during 15 days has increased highly from control (Cicik et al. 2004). Depuration ability of heavy metals in the spleen could be related to metallothionein (MT) expression and positive effect in specific tissues to remove non-essential metals in tissues. Fold change of MT mRNA levels in the spleen of Korean bitterling, Acheilognathus signifier (cyprinidae), exposed to waterborne 0.5 μM copper (Cu) during 48 h was the high increase following liver among 6 tissues (Lee et al. 2011). Also, as results which inject MT for detoxification in grass carp, Ctenopharyngodon idellus, on the 4 days after injection of 20 μM/kg CdCl2, the increase of Cd accumulation in the spleen suppressed highly more than head-kidney (Huang et al. 2019).
Accumulation of heavy metal in the muscle is important, because it is related to the health of a person by eating muscle as food. Cd accumulation in the muscle showed a significant increase at ≥ 0.15 mg L−1 Cd exposure. In a previous study, Cd accumulation in the muscle of Sparus aurata was higher than control by acute Cd exposure (0.5 mg L−1) for short period (2, 4, and 24 h) (Souid et al. 2013). Because of rapid accumulation in the muscle by acute Cd exposure, it is necessary to investigate food safety for fishery and a catch of fish surrounding industrial accident of Cd spill.
Significance of variance homogeneity was < 0.05 in all tissues except for the kidney about bioaccumulation between the test groups. These results were related to higher dispersion in 3.08 and 3.67 mg L−1 exposure groups than other groups, because low sample number by mortality affected the degree of dispersion statistically. The high dispersion means that ability of accumulation and depuration can differ between individuals, though the species and environment of the experiment are the same. Nevertheless, the kidney may be considered to be a better selection than other tissues as an indicator of bioaccumulation in eels, Anguilla japonica, by Cd acute exposure, because the significance of variance homogeneity was > 0.05.
We also demonstrated that the degree of bioaccumulation reflects variation in waterborne Cd concentrations. For example, all tissues differed significantly in Cd accumulation (compared with control) at ≥ 1.83 mg L−1, a point that also marked the highest change rate to Cd concentrations in experimental water. Additionally, Cd was present in all tissues (except for muscle) at the highest concentrations under 3.67 mg L−1, a point that also marked the highest Cd decrement in the water.
Some studies have suggested that Cd transference from the digestive canal to the liver (via the portal system) does not occur if the fish is exposed to heavy metals for only a short term (Handy 1993). In this study, Cd below a certain concentration (≤ 0.30 mg L−1) accumulated primarily in the gills (the main absorption route), likely because such levels are quickly removed by the liver, the most important organ for detoxification in acute exposure (Chowdhury et al. 2005). In contrast, Cd over a certain concentration (between 0.61 and 3.67 mg L−1) accumulated significantly more in the kidney and liver. Although the water in high-exposure groups (4.29 and 5.51 mg L−1) had lower rates of change and decrement in Cd concentrations than water from low-exposure groups, this was due to high eel mortality from Cd toxicity before bioaccumulation could occur. Therefore, Cd transference in fish exposed to Cd for a short term can occur depending on Cd concentrations without mortality.
In this study, activities of SOD and CAT increased generally, similar to a significant increase of Cd accumulation in the liver. Significant increase in activities of SOD and CAT by Cd exposure is related to an increase of ROS in fish. As the highest activities of antioxidant enzymes (SOD, CAT), the liver is stronger for oxidative stress than other tissues (Atli et al. 2006). According to a report of Safari (2015), stress from heavy metals induces expression of genes encoding SOD and CAT to detoxify ROS (Rastgoo and Alemzadeh 2011). Similar results about SOD and/or CAT reported in various fish species, e.g., sturgeon, murrel, tilapia (Atli and Canli 2007; Dabas et al. 2012; Safari 2015). Results of this study mean that some fish in 3.08 and 3.67 mg L−1 Cd exposure maintained life by sufficiently increasing activities of SOD and CAT to eliminate ROS in the body. But some studies reported about the decrease of SOD and/or CAT activities unlike the results of ours. For example, CAT activity in the liver of Channa marulius and Wallago attu decreased with increasing exposed Cd concentrations, but SOD activity increased (Batool et al. 2014). And, in the liver of Cyprinus carpio, both CAT and SOD activities decreased after Cd exposure during 96 h (Karaytug et al. 2011). Roméo et al. (2000) reported that the decrease of CAT activity is attributed to the direct binding of Cd in CAT at Cd exposure. These differences can come by fish species, metal species, environmental factors in the experiment, etc. In the study of Saglam et al. (2014), SOD and CAT activities in the liver after Cd and Cu exposure were different depending on water hardness, respectively.
Cd induce hepatotoxicity by tightly binding to thiol groups of GSH as the first defense line of acute Cd exposure. Decrease of GSH induces oxidative stress in the liver by free radical production and disruption of the cellular GSH system (Dudley and Klaassen 1984; Liu et al. 2009). Saglam et al. (2014) reported that GPx activity can be considered complementary to CAT activity, but its capacity is smaller than CAT activity for decomposition of the peroxides (Sampaio et al. 2008). In the study of Choi et al. (2007), the expression level of GPx mRNA in the liver of goldfish decreased after Cd exposure by injection and became undetectable after 12 h exposure. GPx activity in the liver of gilthead sea bream decreased after waterborne Cd exposure at 0.1 mg L−1 concentration for 4 days (Cirillo et al. 2012). Crupkin and Menone (2013) reported that GST activity in the liver had no significant change in Australoheros facetus exposed to various Cd concentrations except for a group of lower concentration, but GST activity of other tissues (gill, brain) altered significantly more than the liver. With consideration for these facts and similar studies, we suppose that our results for decrease tendency of GPx activity in high concentrations and changeless of GST activity were affected by an increase of Cd ion and decrease of GSH in the liver.
Despite mortality over the majority, the activity change of antioxidant enzyme at 3.67 mg L−1 exposure was smaller than 3.08 mg L−1 exposure. In a previous study, the activity change of antioxidant enzyme was small against the change of mortality with Cd concentration increase by acute exposure (Batool et al. 2014). Also, the graph of antioxidative activity shows bell shape or changeless with Cd concentration increase, even though mortality does not occur (Atli et al. 2006; Crupkin and Menone 2013; Souid et al. 2013). On the basis of our and other studies, thus, the activity change of antioxidant enzyme is considered to have limits with experiment species, individuals, kinds of enzymes, etc., even if it is exposed to heavy metal of high concentration that could cause mortality.
Most studies with ours are different in the experimental condition, such as fish species, size, and temperature. They are all necessary to establish accurate environmental pollution indicators. The studies on the effect of antioxidant enzyme activity and accumulation by heavy metal exposure are mostly conducted in chronic toxicity test. So, it is important that our study results have a similar pattern to those of the chronic toxicity test. This is because the effects on chronic toxicity can be inferred through acute toxicity test. We expect that our findings are used as a direct data on food availability of polluted fish by heavy metal exposure of high level, as our study was conducted using fish of the size available as food.
Conclusions
We investigated Cd acute toxicity and bioaccumulation in adult A. japonica after 96 h of exposure. The LC50 of Cd after 96 h was 3.61 mg L−1 Cd. Cd accumulation was acute in all measured tissues (following the order kidney > liver > gill > spleen > muscle) and corresponded to Cd decrement and change rate in experimental water. Cd exposure at ≥ 1.83 mg L−1 led to significant increases as compared with control in Cd accumulation for all tissues, but the accumulation rate was highest in the gills. In activity alteration of antioxidant enzymes as biomarkers for oxidative stress, both SOD and CAT activities increased ≥ 1.83 mg L−1 significantly. GPx activity showed a decrease tendency at 3.08 and 3.67 mg L−1 Cd exposure, and GST activity was not changed. Our study results emphasize the need for additional studies on Cd chronic exposure and depuration of A. japonica. The results obtained could aid in setting standards for the influence investigation of Cd contamination in aquatic environments and processing methods (e.g., instant disuse, usage conversion, or long-term acclimation) for Cd-accumulated eel.








