Background
Algae are classified into three main classes, namely, Chlorophyceae, Phaeophyceae, and Rhodophyceae, and there are approximately 6000 algal species worldwide, of which approximately 150 species are used as food (Devi et al. 2011; Meenakshi et al. 2011). Algae are known to be a rich source of bioactive substances such as carotenoids, dietary fiber, proteins, minerals, vitamins, polyphenols, and low-calorie polyunsaturated fatty acids. Research and development of health foods, cosmetics, and medicines using algae have been increasing globally (Lee et al. 2011). As aquatic algae can inhabit extreme environments, unlike terrestrial organisms, they are known to produce various bioactive substances that enable them to adapt to such environments (Jeon et al. 2012). Owing to the presence of these bioactive substances, algae have been reported to possess anticancer, antioxidant, antibacterial, antitrypanosomal, antiangiogenic, and anti-HIV activities (Synytsya et al. 2010; Veiga-Santos et al. 2010; Souza et al. 2012; Guerra Dore et al. 2013; Shanmugam et al. 2014; Thuy et al. 2015).
Several bioactive substances, including fucoidan (polysaccharide), fucoxanthin, chlorophyll, xanthophyll (carotenoid), phlorotannin (tannin), and fucosterol (sterol) have been isolated from algae (Hosokawa et al. 2004). Fucosterol is commonly found in algae and has been reported to lower cholesterol levels and possess various physiological activities, such as antidiabetic, anticancer, and antioxidant activities (Ikeda et al. 1988; Tang et al. 2002; Lee et al. 2004; Ham et al. 2010).
Currently, fucosterol is mainly isolated from Sargassum species. In this study, we isolated fucosterol from Sargassum miyabei and developed a high-performance liquid chromatography (HPLC) validation method for this compound. Furthermore, we compared the content of fucosterol in 11 algal species from Ulleungdo, Korea, using the established HPLC validation conditions.
Methods
In 2015, eleven species of algae (Agarum clathratum, Caulerpa okamurae, Codium fragile, Desmarestia tabacoides, Dictyopteris divaricata, Ecklonia cava, Eisenia bicyclis, Myagropsis myagroides, Sargassum horneri, S. serratifolium, and Sporochnus radiciformis) were collected in Ulleungdo Island, Korea, for comparison of fucosterol contents. In the same year, S. miyabei was collected in Pohang-si, Korea, for isolation of fucosterol. The specimens have been deposited in the Marine Biodiversity Institute of Korea (MABIK). All algal samples were lyophilized, pulverized, and extracted with 70% EtOH using a sonicator (WUC-N30H; DAIHAN Scientific Co., Ltd., Wonju, Korea).
Medium-pressure liquid chromatography (MPLC) and HPLC were performed using the Buchi Sepacore Flash system (Flawil, Switzerland) consisting of a UV photometer C-640 and Agilent 1260 Infinity system (Tokyo, Japan) equipped with a diode array detector (DAD). Mass spectrometry (MS) was conducted using a JEOL JMS-600-W Spectrometer (Tokyo, Japan), and nuclear magnetic resonance (NMR) spectra were recorded using a Bruker AVANCE 500 NMR spectrometer (Rheinstetten, Germany) in CDCl3 with tetramethylsilane used as the internal standard. Chemical shifts are reported in parts per million (δ) and the coupling constant (J) is expressed in Hertz (Hz). All reagents used were of analytical grade.
A dried sample of S. miyabei (1.2 kg) was powdered and extracted with 70% EtOH (12 L × 3) using a sonicator and then evaporated under vacuum. The 70% EtOH extract (91.7 g) was suspended in water and partitioned with n-hexane, chloroform, ethyl acetate, and n-butanol. A portion of the n-hexane fraction (15.6 g) was subjected to chromatographic analysis using an MPLC system and eluted in a gradient solvent system (100% n-hexane and up to 100% ethyl acetate) to yield eight subfractions (H1–H8). Subfraction H5 (8.1 g, n-hexane:ethyl acetate = 60:40) was re-chromatographed in an MPLC system and eluted in a gradient solvent system (70% n-hexane and up to 100% ethyl acetate) to yield five subfractions (H5-1 to H5-5). H5-3 was recrystallized with MeOH to obtain fucosterol (Fig. 1).
Fucosterol was obtained as a white powder; EI-MS m/z: 412 [M]+ (13.0), 314 (100), 299 (19.2), 271 (11.3), 229 (15.5); 1H-NMR (500 MHz, CDCl3, δH) 5.35 (1H, d, J = 5.5 Hz, H-6), 5.18 (1H, q, J = 6.5, 13.5 Hz, H-28), 3.53 (1H, m, H-3), 1.58 (3H, d, J = 7.5 Hz, H-29), 1.02 (3H, s, H-19), 1.00 (3H, d, J = 7.0 Hz, H-21), 0.98 (3H, d, J = 6.5 Hz, H-27), 0.97 (3H, d, J = 7.0 Hz, H-26), 0.69 (3H, s, H-18); 13C-NMR (125 MHz, CDCl3, δC) 147.2 (C-24), 141.0 (C-5), 121.9 (C-6), 115.8 (C-28), 72.0 (C-3), 56.9 (C-14), 56.0 (C-17), 50.3 (C-9), 42.5 (C-13), 42.4 (C-4), 39.9 (C-12), 37.5 (C-1), 36.7 (C-10), 36.6 (C-20), 35.4 (C-22), 35.0 (C-25), 32.1 (C-7,8), 31.9 (C-2), 28.4 (C-16), 25.9 (C-23), 24.5 (C-15), 22.4 (C-26), 22.3 (C-27), 21.3 (C-11), 19.6 (C-19), 19.0 (C-21), 13.4 (C-29), 12.1 (C-18).
A stock solution of fucosterol (1 mg) was dissolved in 1 mL of acetonitrile and was diluted to give the desired concentrations (3.91, 7.82, 15.63, 31.25, 62.50, and 125.00 μg mL−1). To analyze fucosterol content, samples of the 11 aforementioned algal species were extracted with 70% EtOH for 1 h by sonication (three times) and evaporated under vacuum. The residues were dissolved in 1 mL of 50% MeOH and filtered using a 0.45-μm syringe filter. The resulting solution was subjected to HPLC analysis, using a Kinetex C18 (4.6 mm × 100 mm, 2.6 μm) column, with a mobile phase comprising a solution of MeOH (solvent A) and 0.1% acetic acid (solvent B). The gradient solvent system was as follows: from 50 to 0% B for 5 min, and then retained at 0% B for 10 min. The injection volume was 10 μL and the flow rate was 1 mL min−1. The column temperature was maintained at 25 °C and detection wavelength was 210 nm. All injections were performed in triplicate.
The HPLC method was validated for specificity, linearity, limit of detection (LOD), limit of quantification (LOQ), accuracy, and precision according to the International Conference on Harmonization (ICH; 1997, 2005) guidelines.
The linearity of the method was established using triplicate injections at six concentrations in the range 3.91–125.00 μg mL−1. A calibration curve for fucosterol was constructed using peak area (Y), concentration (X, μg 10 μL−1), and mean (n = 3) ± standard deviation values. Correlation coefficient values (R2) were determined from the calibration curve. The LOD and LOQ of fucosterol were calculated using the following formulae: LOD = 3.3 × σ/S and LOQ = 10 × σ/S; where, σ = the deviation and S = the slope of the calibration curve. The accuracy of the method was determined based on intra- and inter-day variations. Intra-day variation was determined by analyzing triplicate samples at three different concentrations (15.63, 31.25, 62.50 μg mL−1) in a single day. Inter-day variation was determined by analyzing a single sample at three different concentrations (15.63–62.50 μg mL−1) for 3 days. Variations are expressed as percentage recoveries. Precision was assessed based on repeatability. Repeatability data were obtained from six injections of samples at a concentration of 125.00 μg mL−1 on the same day. Repeatability is expressed as the percentage relative standard deviation (%RSD).
Results and discussion
The fucosterol extracted from S. miyabei was obtained as a white powder with a molecular formula of C29H48O based on EI-MS analysis. In the 1H-NMR spectrum of fucosterol, we detected two olefinic proton signals [δH 5.35 (1H, d, J = 5.5 Hz, H-6), 5.18 (1H, q, J = 6.5, 13.5 Hz, H-28)], one oxygenated methine proton signal [δH 3.53 (1H, m, H-3)], and six methyl groups [δH 1.58 (3H, d, J = 7.5 Hz, H-29), 1.02 (3H, s, H-19), 1.00 (3H, d, J = 7.0 Hz, H-21), 0.98 (3H, d, J = 6.5 Hz, H-27), 0.97 (3H, d, J = 7.0 Hz, H-26), 0.69 (3H, s, H-18)]. In the 13C-NMR spectrum of fucosterol, we observed 29 carbon signals, including two olefin quaternary carbons [δC 147.2 (C-24), 141.0 (C-5)], two olefin methine carbons [δC 121.9 (C-6), 115.8 (C-28)], one oxygenated methine carbon [δC 72.0 (C-3)], and six methyl carbons [δC 22.4 (C-26), 22.3 (C-27), 19.6 (C-19), 19.0 (C-21), 13.4 (C-29), 12.1 (C-18)]. Fucosterol was elucidated by comparison of the EI-MS and NMR (1H-NMR, 13C-NMR, DEPT 45, 90, 135, 1H-1H COSY, HSQC, HMBC) spectral data with those previously published (Bang et al. 2011).
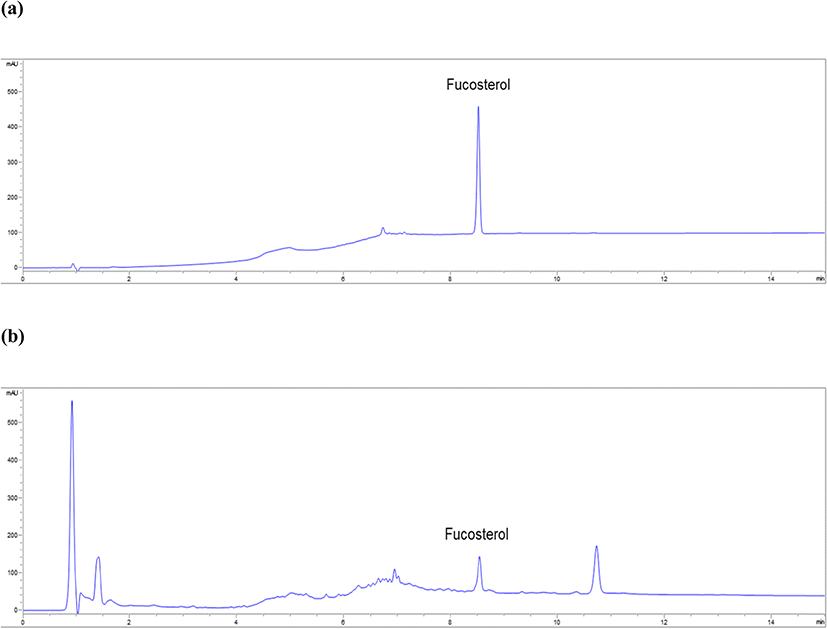
In the HPLC data, fucosterol showed a single peak unaffected by solvent and other components at 8.5 min (Fig. 2a). Good resolution of fucosterol and specificity of the method were confirmed. In the calibration curve at six different concentrations (3.91–125.00 μg mL−1) of fucosterol for determining linearity, y was 5.70x−23.90 and R2 was 0.9998. The R2 of fucosterol was greater than 0.9995, thereby verifying the linearity of fucosterol. The LOD and LOQ of fucosterol were 3.20 and 9.77 μg mL−1, respectively (Table 1). The intra- and inter-day accuracy ranges were 95.76–103.21% and 96.49–101.46%, respectively. The intra- and inter-day variations were within the range of 90–110%, which confirmed the accuracy of the method (Table 2). The precision of the HPLC method, calculated as %RSD, was evaluated for repeatability (125.00 μg mL−1). The RSD was less than 2.0% (1.07%), which confirmed the precision of the method (Table 3).
Linear range (μg mL−1) | Regression equationa | Correlation coefficient (R2) | LOD (μg mL−1)b | LOQ (μg mL−1)c |
|---|---|---|---|---|
3.91–125.00 | y = 5.7x + 23.9 | 0.9998 | 3.20 | 9.77 |
Triplicate injections at six concentrations
aRegression equation is y = Ax + B [y, the peak area; x, concentration (μg 10 μL−1)]
bLimit of detection
cLimit of quantification
Concentration (μg mL−1) | Intra-day variationa | Inter-day variationb | ||
|---|---|---|---|---|
Recovery (%) | SD (%) | Recovery (%) | SD (%) | |
62.50 | 101.05 | 0.71 | 101.46 | 0.59 |
31.25 | 103.22 | 0.13 | 99.55 | 0.28 |
15.63 | 95.76 | 2.25 | 96.49 | 0.97 |
aTriplicate injections at three different concentrations in a single day
bOne injection at three different concentration for 3 days
Concentration (μg mL−1) | Number of measurements | Area | Mean | RSD (%) |
|---|---|---|---|---|
125.00 | 1 | 682.8 | 685.4 | 1.05 |
2 | 674.5 | |||
3 | 681.7 | |||
4 | 688.0 | |||
5 | 692.0 | |||
6 | 693.6 |
The fucosterol contents in the 11 assessed algal species ranged from 0.22 to 81.67 mg g−1 (Table 4). Specifically, the content of fucosterol in A. clathratum, D. tabacoides, E. cava, and S. radiciformis was approximately four times higher than that in Sargassum species. The highest content of fucosterol was detected in the 70% EtOH extract of D. tabacoides (81.67 mg g−1, Fig. 2b), followed by that of A. clathratum (78.70 mg g−1).
aData are expressed as the mean ± SD (n = 3) in μg mL−1
bNot detected
Fucosterol has been reported to have various effect such as antioxidant, antidepressant, anticonvulsant, antipredator, and antiplasmodial activities, and it has been isolated from the Sargassum species S. micracanthum, S. tenerrimum, S. fusiforme, S. horneri, and S. linearifolium (Ham et al. 2010; Terasaki et al. 2009; Zhen et al. 2015; Majik et al. 2015; Perumal et al. 2018). Although Sargassum species are a good source of fucosterol, our present study results confirmed that 70% EtOH extracts of D. tabacoides and A. clathratum could replace Sargassum species as a potential source of fucosterol. Therefore, we anticipate that D. tabacoides and A. clathratum, which have high fucosterol contents with the various aforementioned properties, will have a high industrial value.
Conclusions
We successfully isolated fucosterol from S. miyabei and developed an HPLC validation method for this compound for comparison of the fucosterol contents of 11 selected algal species. The recorded fucosterol contents in 70% EtOH extracts of D. tabacoides and A. clathratum were 81.67 and 78.70 mg g−−1, respectively. These results indicate that 70% EtOH extracts of D. tabacoides and A. clathratum containing high contents of fucosterol with various beneficial properties can be potential alternative sources of fucosterol.