Introduction
Fast development of aquaculture and increasing demand for fish have led to intensification of fish farming, magnifying stressors for fish and thus increasing the risk of disease. To date, chemotherapy has been widely used to prevent and treat disease outbreaks (Reverter et al., 2021). However, frequent use of these antibacterial agents causes serious drawbacks, such as environmental contamination, toxicity to the hosts, and even contamination of fish products with drug residues (Kemper, 2008; Koch et al., 2021; Lim et al., 2019), which prompted an urgent need for alternative therapy, including natural products, such as medicinal plants.
In our previous study, we found that Angelica gigas Nakai and Artemisia iwayomogi Kitamura showed the potent anti-bacterial, anti-parasite, anti-fungal and anti-viral activities against various fish pathogens (Jeon et al., 2020). In addition, an ethanolic extract of 1:1 (w/w) ratio of A. gigas and Ar. iwayomogi (thereafter referred to as CE) showed strong in vitro and in vivo antibacterial activities against various fish pathogenic bacteria, such as Edwardsiella tarda, Vibrio spp., Photobacterium damselae, Streptococcus spp., and Lactococcus spp. (Seo et al., 2017). A. gigas, commonly known by the Korean name as ‘Cham-dang-gui’, belongs to the Umbelliferae family and is abundantly distributed throughout northern Asia, including Korea (Joo et al., 2010). The roots of this plant have been reported to have various biological activities, such as antifungal (Yoon et al., 2011), antitumor (Lee et al., 2003b), anti-inflammatory (Shin et al., 2009), and neuroprotective (Kang et al., 2005) activities. Coumarins, such as decursinol angelate and decursin, are major components of this plant (Lee et al., 2003a) and they have been reported to have significant antibacterial activities (Lee et al., 2003c; Park et al., 2021).
Ar. iwayomogi, locally known as ‘Haninjin’ or ‘Dowijigi’, belongs to the Compositae family and is a perennial herb easily found in Korea (Lee et al., 1993). The aerial part of Ar. iwayomogi has been reported to have antibacterial (Seo et al., 2010), antifungal (Jung et al., 2005), anti-inflammatory (Kim et al., 2005), antitumor (Taleghani et al., 2020), and antioxidative (Sin et al., 2020) activities. Especially, some terpenoids (mono- and sesqui-) in the essential oil (Yu et al., 2003) and some phenolics (Seo et al., 2010) of Ar. iwayomogi are known to have inhibitory effects on bacterial growth.
Botanicals or medicinal plants are known to contain one or many chemical components that may have therapeutic purposes (Heng et al., 2013). Therefore, it is conventional to combine different medicinal plants to achieve various treatment purposes simultaneously or to enhance a single effect without causing severe side effects (Nishiyama et al., 1995). Similarly, combined extract showed extended antimicrobial spectrum, stabilities against heat and pH treatments (Hsieh et al., 2001), and synergistic antimicrobial effects against oral microorganisms (Rhim et al., 2002). However, since these plant extracts contain various components, standardization through analyses of active marker compounds is required for practical use and will play a central role in the development and modernization of these preparations (Ong, 2004). The chemical standardization of active compounds is of special significance because it directly affects the activity of natural products (Nikam et al., 2012). In addition, plant extracts of consistent quality that contain well-defined components are required for reliable clinical trials and for providing consistent, beneficial therapeutic effects (Mosihuzzaman & Choudhary, 2008). However, plants contain various compounds and analysis of plant mixtures is not simple owing to their complicated chemical compositions. The analysis procedure includes avoidance of peak interference, selection of maker compounds, modulation for a stable baseline, and specification of a single compound peak (Zhao et al., 2017).
In the present study, considering those requirements, this study aimed to isolate the active markers in CE by antibacterial activity-guided fractionations and to standardize CE using the active markers through high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS). Furthermore, the compounds were investigated for antibacterial activities against S. iniae, V. anguillarum, and E. tarda. To the best of our knowledge, this is the first report on antibacterial activities of these compounds against fish pathogenic bacteria.
Materials and Methods
The roots of Angelica gigas Nakai and aerial parts of Artemisia iwayomogi Kitamura were purchased in Kyungdong local market (Seoul, Korea). Both plant materials were identified in our own lab through comparison with reference medicinal plant materials provided by the Ministry of Food and Drug Safety of Korea. The plant materials have been verified by verification method of NHMI (National Herbal Medicine Information, https://nifds.go.kr), and detailed methods and results are described in Supplementary Material, 1. Verification of two medicinal plants.
First grade solvents (Daejung Chemicals & Metals, Siheung, Korea) were used for extraction, fractionation, and isolation. HPLC-grade solvents were purchased from Honeywell B & J (Morristown, NJ, USA). Sephadex® LH-20 was purchased from GE Healthcare (Chicago, IL, USA). Silica gel column (Si 60) was purchased from Biotage® (Uppsala, Sweden) and commercially prepared TLC plates (Silica gel 60, F254, Merck, NJ, USA) were used. Brain Heart Infusion Agar (BHIA) and Broth (BHIB) were bought from Difco (Becton, NJ, USA).
In order to antibacterial-guided fractionation, dried roots of A. gigas and aerial parts of Ar. iwayomogi (670 g as a 1:1 mixture) were finely ground and extracted three times with 50% ethanol (13.4 L) at 40°C for 3 h using ultrasonic apparatus (Powersonic 420, Hwashin Tech., Gwangju-si, Gyeonggi-do, Korea). The extract was then filtered through a Whatman No. 4 filter paper (Maidstone, UK). The 50% ethanolic extract (CE) was evaporated to dryness under vacuum, yielding 225 g extract. This extract was then suspended in water and partitioned successively with n-hexane (Hex, 29.7 g), methylene chloride (MC, 12.9 g), ethyl acetate (EA, 7.7 g), water saturated n-butanol (W-S Bu, 40.3 g) and aqueous (126 g). These fractions were then tested for their antibacterial activity. Since the MC fraction presented the highest antibacterial activities among the five fractions (Table 1), active compounds were isolated from the MC fraction through various chromatographic techniques.
Silica gel chromatography of the MC fraction of CE (12.5 g) with Hex-acetone mixture (84:16 to 0:100) as an eluent afforded six fractions (MC-I to MC-VI). Among the six fractions, MC-II (662.9 mg) was subjected to silica gel chromatography with Hex-acetone mixture (84:16 to 0:100) and yielded four sub-fractions (MC-II_fr.1 to 4). MC-II_fr.2 (115.8 mg) was subjected to medium pressure liquid chromatography (MPLC) using silica gel chromatography with chloroform-methanol mixture (99:1 to 0:100), yielding 67 fractions. Among 67 fractions, 36 to 40 fractions were combined to give compound 3 (7.5 mg). Fractions 52 to 59 (88.4 mg) were further subjected to Sephadex® LH-20 column chromatography eluted with methanol, yielding 130 fractions. Among 130 fractions, fractions 69 to 77 (15 mg) afforded compound 4 (9.2 mg) through semi-preparative high-performance liquid chromatography (S-4 µm, 8 nm, 250 × 10 mm; J’sphere ODS-H80, YMC, Kyoto, Japan). MC-II_fr.3 and 4 were combined, and the combined fraction (431.5 mg) was subjected to Sephadex® LH-20 column chromatography using methanol-acetone mixture (4:6). Among the resulting 130 fractions, fractions 48 to 65 (176.1 mg) were subjected to Sephadex® LH-20 column chromatography eluted with methanol, yielding compound 5 (13.2 mg).
Dried roots of A. gigas (40 g) were extracted four times with methanol (0.2 L) in an ultrasonic apparatus at 40°C for 2 h. After solvent removal under reduced pressure, the methanolic extract yielded was 12.1 g. The methanolic extract was suspended in water and partitioned successively with MC. The MC fraction was evaporated to dryness under vacuum and yielded 2.8 g.
Silica gel chromatography by MPLC (Isolera One, Biotage®, Uppsala, Sweden) of the MC fraction of A. gigas (2.8 g) with a mixture of n-hexane (Hex)-acetone (95:5 to 0:100) as an eluent, and afforded four fractions (Fr-I to IV). Fr-II (81.2 mg) was subjected to Sephadex® LH-20 column chromatography with methanol and yielded pure compound 1 (44 mg, purity 99.0%). Fr-III (195.4 mg) was subjected to silica gel chromatography with Hex-MC (50:50 to 0:100) and yielded six sub-fractions (fr-I to fr-VI). Among the six sub-fractions, fr-IV (144.2 mg) was subjected to Sephadex® LH-20 column chromatography with methanol and yielded pure compound 2 (51.2 mg, purity 98.7%). The purities of standards were over 98% as determined by HPLC-UV with a wavelength of 210 nm (Supplementary Material, Fig. S1, tables and figures marked with S are found in the Supplementary Material). All compounds were identified using spectral data, such as HPLC-MSn fragment pattern (HPLC: Surveyor MS Pump; Autosampler: Surveyor autosampler; MS: ThermoFinnigan LCQ Advantage MAX ion trap mass spectrometer, MA, USA), proton nuclear magnetic resonance (1H-NMR), and carbon-13 nuclear magnetic resonance (13C-NMR) by direct comparison with published data of spectra and structures. NMR spectra (Figs. S2–S6) were recorded in chloroform-d, methanol-d4, and dimethyl sulfoxide-d6 using 400 MHz NMR spectrometer (AVANCE 400 FT-NMR, Bruker, MA, USA). All isolated compounds were screened for antibacterial activity.
Streptococcus iniae KCTC 3657, Vibrio anguillarum KCTC 2711, and Edwardsiella tarda KCTC 12267 were purchased from Korean Collection for Type Cultures (Daejeon, Korea). For antibacterial susceptibility test using microdilution method (Kang et al., 2008; Langfield et al., 2004), bacterial colonies taken directly from BHIA plates were incubated in BHIB at 28°C for 24 h. From this culture, a suspension equivalent to 0.5 McFarland standard in BHIB was prepared.
The fractions or compounds and an equal volume of bacteria at 1×106 CFU/mL were mixed in a 96-well plate and incubated at 28°C for 24 h. The antibiotics amoxicillin and oxytetracycline were used as positive controls. The lowest concentration of antibiotics that visibly inhibited bacterial growth was considered as MIC. Each assay was repeated in three times.
Stock solutions for standard compounds were prepared with HPLC-grade methanol as a solvent. Working calibration solutions were prepared by successive serial dilution of the stock solution with methanol and the final concentrations were 5, 25, 50, 100, 500, and 1,000 ng/mL for compound 1, and 5, 25, 250, 1,000, and 2,000 ng/mL for compound 2.
Dried CE was dissolved in 50% methanol to prepare 1 mg/mL stock solution. The stock solution of CE was diluted to make working solutions at concentrations of 1, 3, and 10 µg/mL.
Agilent Technologies 1260 liquid chromatograph coupled to a 6460-triple quadrupole mass spectrometer equipped with an electrospray ionization (ESI) ion source was used for quantitative and qualitative analyses. Optimized conditions were as follows: drying nitrogen gas flow, 10 L/min; nebulizer pressure, 35 psi. drying gas temperature, 350°C; capillary voltage, 5,000 volts; flow rate, 0.6 mL/min; scan range, 50–500 (m/z). The analytical column was a Capcell Pak C18 MG (250 × 4.6 mm, 5 µm; Shiseido, Tokyo, Japan) connected to a short pre-column. The column was operated at 30°C. Table S1 summarizes the retention times, specific transitions, and collision energies used for each compound and solvent condition.
For linearity study, calibration plots were constructed using diluted stock solutions of a series of at least five concentrations, by plotting mean integrated chromatographic peak area against the corresponding concentration. , limit of detection (LOD) and limit of quantitation (LOQ) were calculated in accordance with the International Conference on Harmonization (ICH) Q2B Guideline. Method precision was validated through determination of intra- and inter-day variances. Recovery was used to further evaluate the accuracy of the method. Known amounts of standard solutions were mixed with known amounts of samples. Next, the resultant samples were analyzed, and triplicate experiments were performed at each level. Average recoveries were estimated by the following formula: Recovery (%) = (amount found - original amount) / amount spiked × 100 %, and RSD (relative standard deviation, %) = (standard deviation / mean) × 100 %. RSD was taken as a measure of precision and accuracy. Specificity was achieved by analyzing MRM signals. All peaks of the target compounds in CE were identified by comparison of retention time, as well as parent and product ions with standards in MRM spectra.
Results and Discussion
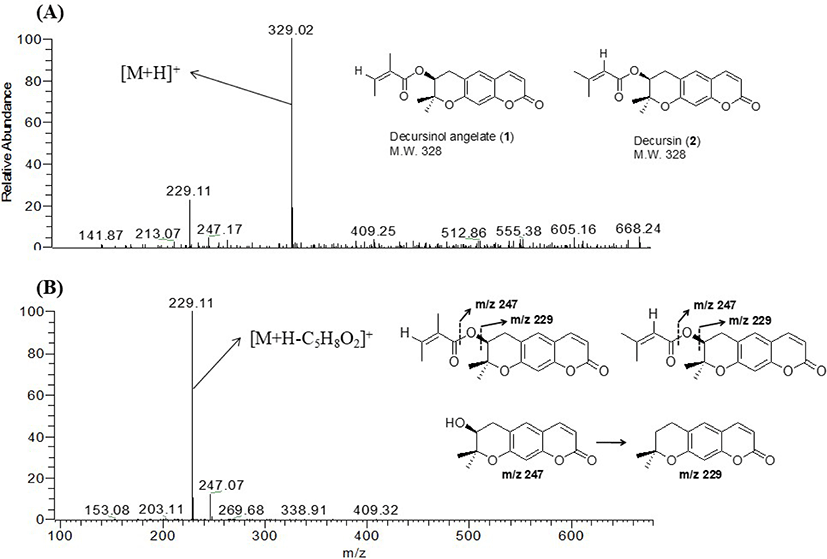
To isolate the compounds responsible for the antibacterial activity of CE as active markers, an activity-guided fractionation strategy was performed throughout the separation procedure. The MC fraction of CE showed the strongest antibacterial activity against S. iniae and V. anguillarum (Table 1). Activity-guided fractionation of the active MC fraction yielded the sub-fractions MC-I and MC-II, which showed strong antibacterial activity against S. iniae and V. anguillarum with MIC values of 156.25 µg/mL and 78.13 to 312.5 µg/mL, respectively (Table 1). The major compounds of the most active sub-fractions (MC-I and II) were identified through LC-ion trap MS (Analytical conditions are in Supplementary Material, 2. Analytical conditions of LC-ion trap MS). LC-MSn analysis suggested that major compounds with the same characteristics as those of decursinol angelate (1) and decursin (2) were present in MC-I (Fig. 1 and Figs. S7–S9), and 1, 2, xanthotoxin (3), and 2,4-dihydroxy-6-methoxyacetophenone (5) were present in MC-II (Figs. S10–S13). Because 1 and 2 were identified as major compounds of the active fractions MC-I and II, they were separated from roots of A. gigas for massive and efficient isolation of two compounds. After isolation, 1 and 2 were identified as decursinol angelate and decursin, respectively, by analyses of the spectral data using 1H-NMR (Yoo et al., 2008) and ESI-MS ([M+H]+ at m/z 329). In addition, three compounds were isolated from MC-II. Compounds 3, 4, and 5 were identified as xanthotoxin (Muller et al., 2004), demethylsuberosin (Masuda et al., 1998), and 2,4-dihydroxy-6-methoxyacetophenone (Brown, 1992), respectively, based on 1H-NMR and ESI-MS data ([M+H]+ at m/z 217 of 3, [M+H]+ at m/z 231 of 4 and [M-H]- at m/z 181 of 5). Of the five compounds, 1–4 are representative compounds reported in Angelica species, and 5 is reported to be present in Artemisia species. In the present study, only one of the compounds derived from Ar. iwayomogi was isolated in the active fractions. Essential oils of Ar. iwayomogi are known to have inhibitory effects on bacterial growth (Cha, 2007), and these essential oils are obtained by extraction with a highly polar solvent or distillation (Cha, 2007; Choi et al., 2017). Therefore, one of possible explanations for failure to isolate these essential oils with antibacterial activity is that essential oils are highly volatile, whereas CE was extracted with low-polarity solvents. This phenomenon coincided with a previous report that recovery of essential oils from plants is low at ethanol concentrations below 55% (Durling et al., 2007).
The five compounds were investigated for antibacterial activities against S. iniae, V. anguillarum, and E. tarda (Table 1). To the best of our knowledge, this is the first report on antibacterial activities of these five compounds against fish pathogenic bacteria. The isolated compounds showed MIC values of 31.25 to 1,000 µg/mL, and compounds 1-4 showed strong antibacterial activities against the test strains. In contrast, compound 5 was less active (500–1,000 µg/mL) than the other compounds. The positive controls amoxicillin and oxytetracycline showed MIC ranges of 0.0156 to 1 µg/mL and 0.156 to 0.625 µg/mL against the test strains, respectively. Similar susceptibilities of several terrestrial bacteria to the isolated compounds have been reported in other studies. According to previous studies (Lee et al., 2003c; Taechowisan et al., 2013), 2 exhibited antibacterial activity against Bacillus subtilis with MIC values of 12.5 and 64 µg/mL, and 1 has an MIC of 50 µg/mL. In addition, 1 and 2 possess strong antibacterial activities with MIC values of 6–200 µg/mL against Helicobater pylori (Bae et al., 1998). Compound 3 had MICs at a range of 500–2,000 µg/mL against various gram-positive and -negative strains (de Souza et al., 2005). Compound 5 at 800 µg/mL has previously been shown to exert a slight inhibitory activity against Clostridium perfringens (Ivarsen et al., 2014). This evidence suggest that major compounds of CE possess antibacterial activities against both gram-negative and -positive bacteria. In the present study, among the five compounds, 1 and 2 showed the most potent, broad-spectrum activities against S. iniae (62.5 and 250 µg/mL, respectively) and V. anguillarum (62.5 and 125 µg/mL, respectively). For this reason, 1 and 2 were selected as active marker compounds of CE and then quantified by HPLC-MS/MS.
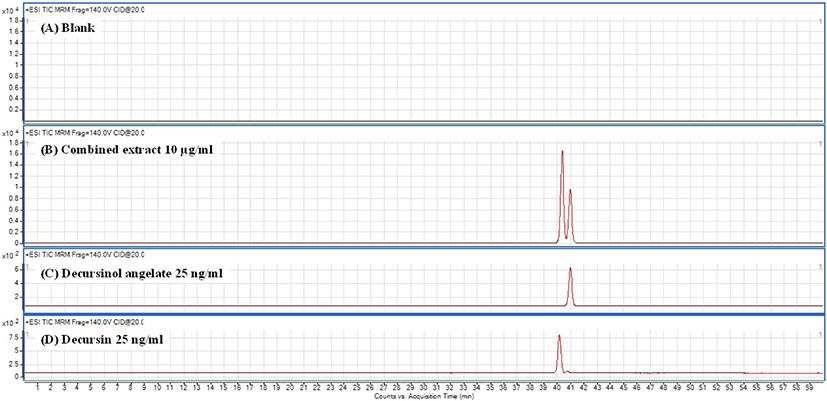
A. gigas contains dihydropyranocoumarins, 1 and 2 as major compounds, and these compounds are structural isomers having the same formula (Fig. 2). To separate, detect, and quantify these two peaks, LC-UV methods using buffer elution have been reported (Ahn et al., 2008; Kim et al., 2009; Lee et al., 2003a). However, considering the complicated chemical composition of combined plant extracts, HPLC-MS/MS method may have advantages. Although previous studies performed quantification using HPLC-MS/MS (Park et al., 2013), direct comparisons between their and our results are inappropriate because they did not separate the two peaks. Recently, one study has reported separation of 1 and 2 by LC-MS/MS using columns of various sizes, but it was not possible to separate two isomeric peaks with a single column (Kim et al., 2018). In the present study, three different C18 columns (50, 150, and 250 × 4.6 mm) were tested for the separation of 1 and 2. Among them, 250 × 4.6 mm column showed to be the most suitable and it provided good peak separation (Fig. 3). In contrast, other column showed poor peak separations at the same condition (Fig. S14). Therefore, 1 and 2 were quantified under optimized LC conditions using 250 × 4.6 mm column.
Method validation included tests of linearity, precision, accuracy, and specificity. Calibration curves for HPLC-MS/MS analyses were linear over a concentration range of 5 to 1,000 ng/mL (regression line: y = 395.39x – 193.52 with a correlation coefficient r2 of 0.9999) for 1, and 5 to 2,000 ng/mL (regression line: y = 370.47x – 606.95 with a correlation coefficient r2 of 0.9999) for 2. On the basis of the calibration curves, LOD and LOQ were calculated to be 0.72 and 2.18 ng/mL for 1, and 1.85 and 5.63 ng/mL for 2, respectively (Table 2).
Method reproducibility was evaluated by intra- (n = 3) and inter-day (n = 9) variability in three replicate analyses of sample solutions. Relative standard deviation (RSD) was less than 2%, showing good precision (Table 3).
The recoveries of 1 and 2 were assessed by spiking samples with high, medium, and low concentrations of each compounds (20, 100, and 800 ppb for 1; 30, 200, and 1,333 ppb for 2). Average recoveries ranged from 90.13% to 108.57% (Table 4). All the peaks of 1 and 2 in CE were identified by comparison of retention time, as well as parent and product ions with those of standards in MRM spectra. As a result, high specificity was shown (Fig. S15). Therefore, this method showed suitable precision, accuracy, reproducibility, and specificity.
Content analysis of 1 and 2 in CE was performed by HPLC-MS/MS (Fig. 1). Average contents of 1 and 2 were calculated to be 3.68 ± 0.07% and 6.14 ± 0.04%, respectively (Table 5). Considering the MICs (62.5 and 250 µg/mL) and contents of the two active compounds (sum of the two compounds: 9.82%), the antibacterial activity of CE with an MIC of 2,500 µg/mL can be explained.
Conclusions
In the present study, five compounds were isolated from a combined extract of 1:1 (w/w) ratio of the roots of A. gigas and aerial parts of Ar. iwayomogi. The antibacterial activities of these isolated compounds against S. iniae, V. anguillarum, and E. tarda were reported for the first time, and among these compounds, 1 and 2 showed strong activities against S. iniae and V. anguillarum. An HPLC-MS/MS method was successfully developed for the simultaneous quantification for 1 and 2 as active compounds. Taken together, these results may be helpful for establishing the chemical profile of CE for commercialization as an antibiotic alternative in aquaculture. In addition, this study will be helpful for quantitative analysis of 1 and 2 as well as standardization of extract using A. gigas.








