Introduction
The short ninespine stickleback, Pungitius kaibarae, is a freshwater species belonging to the family Gasterosteidae within the order Gasterosteiformes. There are five genera and 18 species reported in total, whilst two genera and five species, including P. kaibarae, Pungitius sinensis (P. sinensis), Pungitius tymensis (P. tymensis), Pungitius pungitius (P. pungitius), and Gasterosteus aculeatus (G. aculeatus), have been reported in Korea (Kim et al., 2005; http://www.fishbase.org). P. kaibarae is a small gasterosteid fish that is distributed in Russia and Japan and is native to the east coast of the Korean Peninsula. They inhabit only some rivers that drain into the East Sea (Bae et al., 2017; Kim et al., 1989; Kim, 1997 ). This species was designated as an endangered species in Korea until 2012 and as a near-threatened (NT) species since 2016 (MEK, 2016).
Sticklebacks have been used as a model species in various studies owing to their short life span, specific reproductive behavior, including building a nest, and territorial behavior (Hahlbeck et al., 2004; Sokolowska & Kulczykowska, 2006). To date, studies on the courtship and territorial behavior, population structure, and genomics of P. kaibarae have been reported (Bae & Suk, 2015; Jang et al., 2006; Hwang et al., 2012; Park & Lee, 1999; Park et al., 2001). However, there exists no detailed ontogenetic profile of the early life history, including egg/larval development, of the short ninespine stickleback. Research on the early life history of fish can be used as important data in breeding ecology and applied to related subjects such as conservation or restoration of endangered or endemic species (Blaxter, 1974).
In the present study, we explored spawning behavior and egg and larval development in order to provide fundamental information on the reproductive biology of P. kaibarae, a Korean endemic species.
Materials and Methods
Short ninespine sticklebacks (P. kaibarae) were collected downstream from Jinhae, South Korea, from April to May, 2019, using a scoop net. Fish (100 males and 200 females) were moved into a continuous flow-through indoor tank (2 m3) and maintained under a photoperiod of 14L:10D at 20.0 ± 0.5°C. Fine sand was placed on the bottom of the fish tank and water weeds from their natural habitat were planted to enable the fish to build nests. Fish were fed a commercial feed and frozen mosquito larvae.
Published literature include drawings of the spawning behavior of short ninespine sticklebacks (P. kaibarae) (Park et al., 2001). However, there are no digital photography data available. In the present study, videos and pictures were created using a digital camera when males had nuptial body coloring, as described by Park et al. (2001), until spawning.
The spawned and hatched larvae in each nest were counted. The bulk area of the nest, the area covered by a portion of the nest through which no basal substratum was visible, was calculated. The number of spawned and hatched larvae and nest area were compared with those of other stickleback species.
After spawning, 10–15 fertilized eggs were carefully separated from the nests. The embryonic development of P. kaibarae was observed using a dissection microscope. After hatching, the total length (TL) of newly hatched larvae was measured at intervals of 5 d using a dissection microscope. During the rearing process, larvae were fed 5–10 Artemia from 3 days post hatching (DPH). Water temperature and photoperiod during the rearing period were 20.0 ± 0.5°C and 14L:10D, respectively.
Several nests were harvested after spawning, and their bulk area (mm2), the area covered by a portion of the nest through which no basal substratum was visible was calculated by measuring width (mm) and height (mm) according to the method of Barber et al. (2001). Then, the spawned and hatched larvae in each nest were counted.
Results
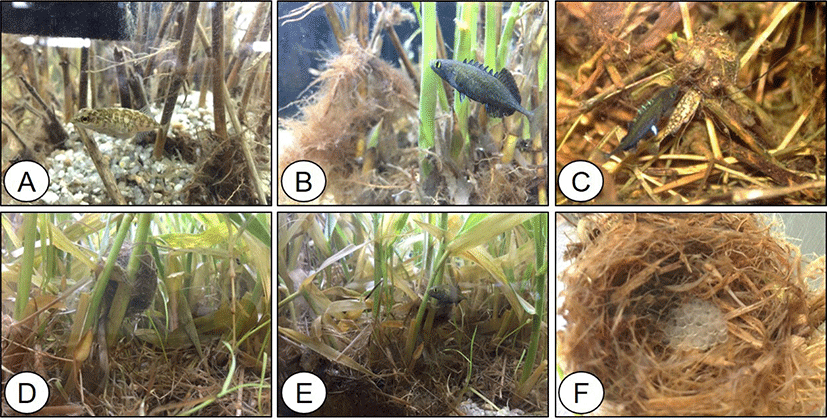
Males had nuptial coloration on their entire black bodies, with blue dorsal spines and yellow eyes, whereas females had a brown spotted pattern on their bodies (Fig. 1A and 1B). Males built nests on the stems of water weeds and attracted females. Fertilization occurred in the nest immediately after spawning (Fig. 1C), and males guarded the eggs until they hatched (Fig. 1D and 1E).
The fertilized eggs of P. kaibarae were spherical, demersal, adhesive, and transparent, and a single egg measured 1.43 ± 0.07 mm in diameter. Ten minutes post fertilization, accumulation of cytoplasm took place, and the blastodisc was formed from the animal pole (Fig. 2A). The eggs reached 2, 4, 8, and 16 cell-stages (Fig. 2B–2E) at 0.5, 1, 1.5, and 2 post fertilization (HPF), respectively.
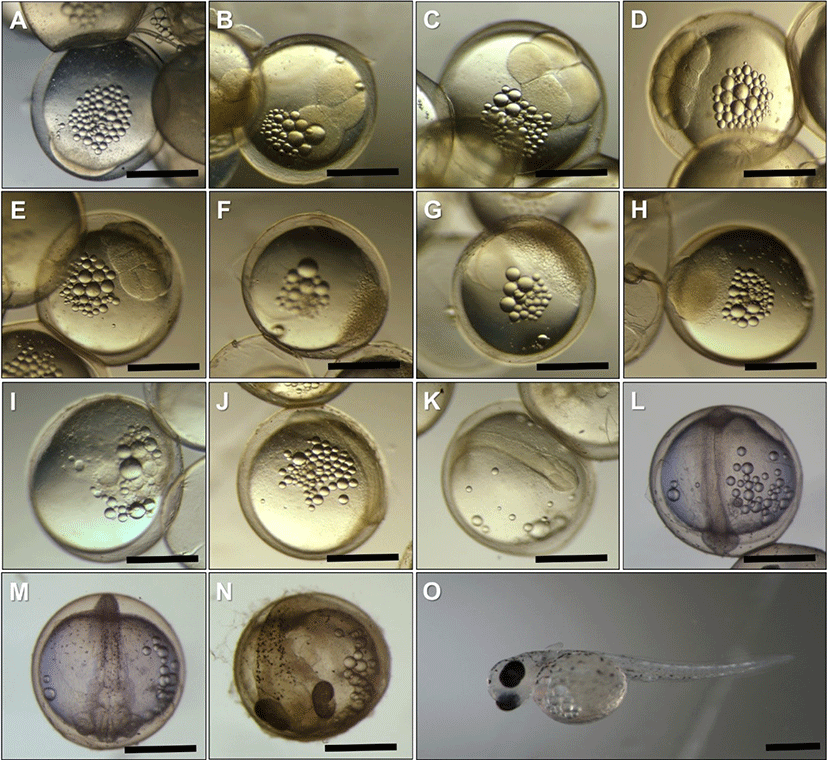
At 4 HPF, eggs reached the morula stage and the cells of the blastomere became smaller (Fig. 2F). The morula cells continued to reduce in size and appeared as a cell mass. At 9 HPF, eggs reached the blastula stage, continuous cell division took place, and there was an increase in the number of blastomere cells (Fig. 2G).
At 16 HPF, eggs reached the gastrula stage (Fig. 2H). At this stage, epiboly and blastoderm were expanded over the yolk surface, covering < 25% and < 50% of the surface (Fig. 2I) at 16 and 18.5 HPF, respectively. At 21.5 HPF, embryo formation and elongation were observed (Fig. 2J). At 24.5 HPF, paired optic lobes were formed at the posterior head (Fig. 2K). At 28 HPF, 6–8 somites appeared in the body, and optic lobes were differentiated as optic vesicles (Fig. 2L). At 42.5 HPF, the optic vesicles were further differentiated as optic cups, and accumulation of pigment was observed in the dorsal part of the body and near the yolk sac (Fig. 2M). During this period, the heartbeat was found to be 70–80 movements per min. At 75.5 HPF, the pigment accumulated in most areas of the eyecups and appeared not only in the dorsal part of the body but also in the mid-brain (Fig. 2N). In addition, the mouth and tail were formed at this stage. At 96 HPF, the embryo hatched (Fig. 2O). Newly hatched larvae had visible pairs of the pectoral fin and measured 5.67 ± 0.50 mm in TL. The hindguts and anuses were formed, and a rudimentary small fin was observed between the yolk and anus.
At 1 d posthatching (DPH), the TL of the larvae was 6.01 ± 0.11 mm and approximately 50% of the yolk was absorbed (Fig. 3A). At 5 DPH (TL = 7.93 ± 0.95 mm), the yolk was completely absorbed. At this stage, the swim bladder was observed, and larvae began to feed voraciously on Artemia with developing jaws. In the ventral part of the head, gill filaments were observed. The membranous fin was folded. The terminal part of the notochord grew upward, and rudimentary caudal fin rays (n = 10–12) had developed, forming a heterocercal tail (Fig. 3B). At 10 DPH (TL = 9.65 ± 0.41 mm), the area of the digestive tract increased in the abdominal cavity. Dorsal, anal, and caudal fins were separated by folding of the membranous fin, and fin rays were formed. The ventral lobe in the caudal fin grew and increased in number (n = 16–18), forming a homocercal tail (Fig. 3C). At 15 DPH (TL = 10.40 ± 1.05 mm), the dorsal spines began to appear and the caudal fin was completely formed (Fig. 3D). At 20 DPH (TL = 10.61 ± 0.63 mm), dorsal spines were developing, but gill filaments were not observed owing to formation of the operculum. The snout had become elongated (Fig. 3E). At 25 DPH (TL = 18.54 ± 1.20 mm), the dorsal spines were formed completely, the snout was more elongated, and the lower jaw was longer than the upper jaw. At this stage, it showed typical morphological characteristics of an adult fish (Fig. 3F).

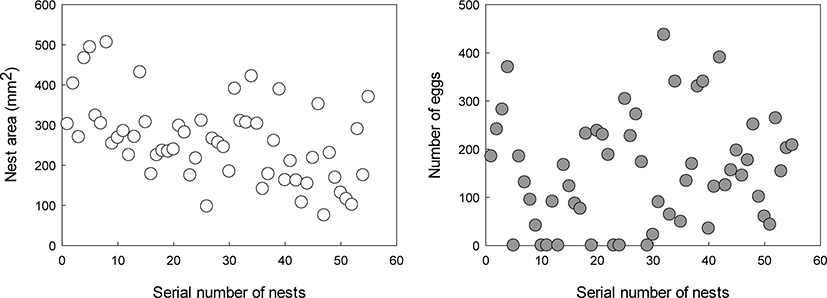
During the spawning trial in the indoor tank, 55 nests were built from 21st April to 30th June (Fig. 4). The average area of the nest and number of eggs were 259.56 ± 101.39 mm2 (75.18–506.04) and 155.33 ± 114.12 (0–437), respectively. There were 8 nests without eggs.
Discussion
The spawning period of P. kaibarae has been reported to be between February and June, and the peak period is from early March to late April (Chae & Yang, 1993). Our results indicated that reproductive performance continued until late June. This prolonged spawning could be due to our study being conducted in the indoor tank rather than in the wild environment, as in the previous study. Moreover, the different latitudes of their habitats or different populations may also be a factor for prolonged spawning of the fish. The diversity in spawning period among different populations of the threespine stickleback Gasterosteus aculeatus (G. aculeatus) and the ninespine stickleback Pungitius pungitius (P. pungitius) has been previously reported (Heins et al., 2003; Ishikawa & Kitano, 2020). In this regard, future studies on the reproductive cycle of P. kaibarae in various habitats could provide more detailed information.
We aimed to illustrate the spawning behavior and describe the embryonic and larval development of P. kaibarae reared in indoor tanks. Coste (1848) first reported the nest building of stickleback fish with G. pungitius. Since then, several studies have reported the nest building of various other stickleback species (Cunningham, 1887; FitzGerald, 1993; Hancock, 1852; McKenzie & Keenleyside, 1970; Morris, 1958). Park & Lee (1999) reported that P. kaibarae with a longer TL could occupy territories more easily than smaller ones. In G. aculeatus, similar results have been reported: fish that are larger, older, and have a brighter nuptial color are dominant in reproductive behavior and occupying their territories (Fraipont et al., 1993; FitzGerald, 1993). Moreover, P. kaibarae show a hetero-sexual territorial system; both males and females build nests in their territories during the reproductive period (Morris, 1958; Park & Lee, 1999). However, the female’s territory is lost by the male’s aggressive expelling behavior just after spawning and fertilization; the aggressive behavior of males could be due to their strong parental care. Interestingly, Chae & Yang (1993) reported that males built two nests in an indoor tank. We suspect that the male built two nests because of territorial competition among males within a cramped tank. In the present study, we did not observe two nests built by a male.
We observed two batches of fertilized eggs with different developmental stages in a few nests. Pianka (1983) suggested two types of sexual selection. One is intrasexual selection, which represents competition for the best combination among members of the same gender, and the other is epigamic or intersexual selection, which is operated by the preference between different genders. In this regard, epigamic or intersexual selection may have occurred in P. kaibarae, as the nest-guarding males could mate with other females.
The fertilized eggs of P. kaibarae were spherical, demersal, adhesive, and transparent. The average diameter of each fertilized egg was 1.43 ± 0.07 mm, which was similar to that of G. aculeatus and P. pungitius eggs (Table 1). Most of the characteristics of egg and larval development were in accordance with the general view by Swarup (1958) on the study of G. aculeatus, the representative species of the family Gasterosteidae. However, there were differences in hatching time and the number of oil droplets.
| Species | Diameter of fertilized egg (mm) | Elapsed time for hatching (water temperature) | Total length of hatched larvae (mm) | Place of nest | Nest area (mm2) | References |
|---|---|---|---|---|---|---|
| Gasterosteus aculeatus | 1.2–1.7 | 192 h (18°C–19°C) | 4.71 | Bottom | 1,901 ± 842 | Barber et al. (2001) Swarup (1958) |
| Pungitius pungitius | 1.42 | Not measured | Not measured | Stems/branches of waterweed off bottom | Not measured | Heins et al. (2003) |
| Pungitius kaibarae | 1.43 ± 0.07 | 96 h(20.0 ± 0.5°C) | 5.67 ± 0.50 | Stems/branches of waterweed off | 259.56 ± 101.39 | Present study |
| 1.6 ± 0.11) | Not measured | Not measured | 866.30 ± 129.512) | Chae & Yang (1993) |
In G. aculeatus, the elapsed time for hatching was 192 h at 18°C–19°C. However, the elapsed time for hatching in P. kaibarae was 96 h in 20.0 ± 0.5°C water temperature. Although the water temperature in the present study was higher than that in the previous study with G. aculeatus, the elapsed time for hatching of P. kaibarae was approximately half that of G. aculeatus. The reason for this difference is not yet known. In addition, the TL of newly hatched P. kaibarae was more than that of G. aculeatus, although adult G. aculeatus was larger than P. kaibarae. The elapsed time for hatching in fish is associated with survival rate in the early developmental stage; fast hatching reduces predation during the most vulnerable period (Kim & Zhang, 1994). A comparison of elapsed time for hatching and survival rate among stickleback species will be conducted in a future study. In P. kaibarae, approximately 20–30 oil droplets were observed during embryonic development, and newly hatched larvae had less than ten oil droplets in their yolk. However, in G. aculeatus, the number of oil droplets was less than 20 during embryonic development, and newly hatched larvae had single oil droplets in their yolk (Swarup, 1958). This is a useful characteristic for the identification of species belonging to Gasterosteidae.
We measured the nest area of P. kaibarae from an indoor tank, and the average nest area was found to be 259.56 ± 101.39 mm2. In a previous study on G. aculeatus, the average nest area was larger than that of P. kaibarae. However, Chae & Yang (1993) reported that the nest area of P. kaibarae was 866.30 ± 129.51 mm2, which was larger than that in the present study (Table 1). The reason for this difference could be the calculation of nest area; the bulk area was calculated in the present study, whereas the total area was calculated in the previous study. Through the trial in the indoor tank, spawning was observed until late June; observations suggested that the reproductive period in captivity could be longer than that in the wild environment. Moreover, this prolonged spawning could be used as a technology for improving artificial seed production.
Reproductive and early development characteristics are used as fundamental data for the classification and conservation of certain fish species (Seo et al., 2006). This study is the first illustration of the spawning behavior and egg and larval development of P. kaibarae using photographs. The study findings would be useful as preliminary data for future studies on the reproductive biology of P. kaibarae.








