Introduction
Generally, sexual maturity in fish is affected by conditions in the external environment, such as water temperature, light (photoperiod), and food (Wang et al., 2010). In fish farming, early gonadal maturation in the broodstock is controlled by regulating the rearing environment for economic purposes. However, in an artificial rearing environment, final maturation, ovulation, and spermiation usually do not occur in farmed fish. Therefore, to solve this problem, hormonal manipulation through exogenous hormone treatment is used to induce final maturation and spawning to produce high-quality gametes (Lim, 2016; Mylonas et al., 2010). In general, the early maturation of fish is induced by creating an external environment that accelerates gonadal maturation such that it occurs faster than in nature. Treatment with an exogenous hormone can be used to induce ovulation or spermiation. In fish culture, the hormonal induction of sexual maturation and spawning has been used since the 1930s (Zohar & Mylonas, 2001). Broodstock management and the hormonal manipulation of fish are necessary to achieve successful large-scale aquaculture, and exogenous hormones such as human chorionic gonadotropin (hCG), gonadotropin-releasing hormone agonists (GnRHas), and pituitary gland hormones can be used for reproductive control in many teleost fish. These hormones can have major effects (Zohar & Mylonas, 2001) in both female and male fish. In males, the duration of spermiation can be prolonged such that it lasts longer than in nature through hormonal treatment, and consequently, a greater amount of milt can be collected (Lim et al., 2002, 2016; Mylonas & Zohar, 2001). Furthermore, many studies have used gonadotropin-releasing hormone or hCG to accelerate the male spermiation process and improve sperm quality and yield (Mylonas & Zohar, 2001).
Globally, the family Pleuronectidae contains fish species important to the aquaculture industry, with various flounder species currently being studied. Roughscale sole, Clidoderma asperrimum, belongs to Pleuronectidae in the order Pleuronectiformes. It is a benthic fish that lives at depths of 150 to 1,000 m in sandy places with pearl oysters. Because these fish are caught with trawl nets, the catch is very low compared to other fish species, so they are sold at high prices in the market (Tokranov & Orlov, 2003). The demand for this species has been increasing recently, but it is in short supply because it is only wild caught. Therefore, basic research on reproduction in roughscale sole is needed. In this study, we investigated the effects of exogenous hormone treatment on spermiation by implanting pellets containing a salmon gonadotrophin-releasing hormone analog (sGnRH) into male roughscale soles. Sperm quality was assessed over the spermiation period, and plasma levels of testosterone (T), 11-ketotestosterone (11-KT), and 17α, 20β-dihydroxy-4-pregnen-3-one (17α, 20β-P) were measured. Based on the results, the effects of exogenous hormone treatment on sperm quality and changes in plasma sex steroid hormones were investigated.
Materials and Methods
All procedures were performed in accordance with guidelines for the ethical treatment of animals and were approved by the Institutional Animal Care and Use Committee of Mokpo National University (no. 1183; December 17, 2013).
Fishes used in the experiment were captured from the wild off Gyeongsangbuk-do and transported to the Gyeongsangbuk-Do Fisheries Resources Development Institute to acclimatize in fish tanks. The mean length and body weight of the male roughscale soles (n = 32) were 34.3 ± 2.4 cm and 515.6 ± 101.9 g, respectively. The water temperature in the tanks was maintained at 12 ± 1°C until the end of the experiment using a cooler. Frozen shrimp and live lugworms were supplied once a day at 3 pm during the experimental period. Commercially available Ovaplant (Syndel, Ferndale, WA, USA) pellets containing sGnRH as the main component were implanted into fish dorsal muscles to induce spermiation. Within 24 h, 40%–60% of the sGnRH was expected to be released, with the remainder released over the next 7 to 21 days. The fishes were weighed, and pellets with different hormone concentrations were individually prepared according to the weight of each fish. In the control group, the fishes didn’t received the hormone pellets (GnRH 0 group), whereas fishes in the three treatment groups received pellets containing doses of 25, 50, or 100 µg/kg body weight sGnRH (GnRH 25, GnRH 50, and GnRH 100 groups, respectively). To track spermiation in each individual, a radio-frequency identification chip was inserted into the dorsal muscle along with the hormone pellet. Eight fishes were treated in each group, and each group was housed in a 1-ton fiberglass-reinforced plastic tank.
After implantation of the hormone pellets, the experimental fishes were periodically anesthetized using tricaine methanesulfonate (Syndel), milt was collected under abdominal pressure, and the amount of milt collected was measured. The spermatocrit (Sct) was measured using a micro-hematocrit reader (Hawksley, Lancing, UK) in the milt collected from all groups. The movable sperm ratio (MSR) was determined as the ratio of the number of moving sperm to the total number of sperm observed under a microscope with 200× magnification (Olympus, Tokyo, Japan).
Blood samples were taken from the caudal region using a 3 mL syringe (23G) treated with heparin sodium. The collected blood was centrifuged at 7,000×g for 10 min to separate the plasma, and the supernatant was transferred into a 1.5 mL tube and stored in an ultra-low temperature freezer at –80°C until analysis. The plasma level of T was measured using a fish T enzyme-linked immunosorbent assay (ELISA) kit (CUSABIO, Houston, TX, USA), the plasma 11-KT concentration was measured using a fish 11-KT ELISA kit (MyBioSource, San Diego, CA, USA), and 17α, 20β-P was analyzed using a fish 17α, 20β-dihydroxy progesterone ELISA kit (MyBiosource). For all ELISAs, the absorbance at 450 nm was measured using a SpectraMax 190 microplate reader (Molecular Devices, San Jose, CA, USA).
The measurements obtained from the experiment are expressed as the mean ± SD, and the significance of differences among the treatment groups was tested using one-way ANOVA and Duncan’s multiple range test (p < 0.05) with SPSS software (version 24; IBM, Armonk, NY, USA). Two-way ANOVA was used to assess the interaction effect between the days elapsed during the experiment and treatment type.
Results
The volume of milt measured in each experimental group is shown in Fig. 1. In all experimental groups, spermiation commenced at 8 days post-implantation (dpi). In the GnRH 0 group, the highest milt volume of 4.99 ± 1.45 mL/kg was recorded at 18 dpi, but no spermiation was observed after that. In the GnRH 25 group, the milt volume increased to 8.51 ± 2.34 mL/kg at 18 dpi, the largest volume released during the experimental period. Then, until 35 dpi, the amount of milt released gradually decreased. In the GnRH 50 group, the highest milt volume of 4.95 ± 0.10 mL/kg was collected at 28 dpi, but no milt was collected thereafter. The GnRH 100 group produced the most milt, 8.29 ± 1.49 mL/kg, at 18 dpi, with the volume subsequently decreasing until 35 dpi.
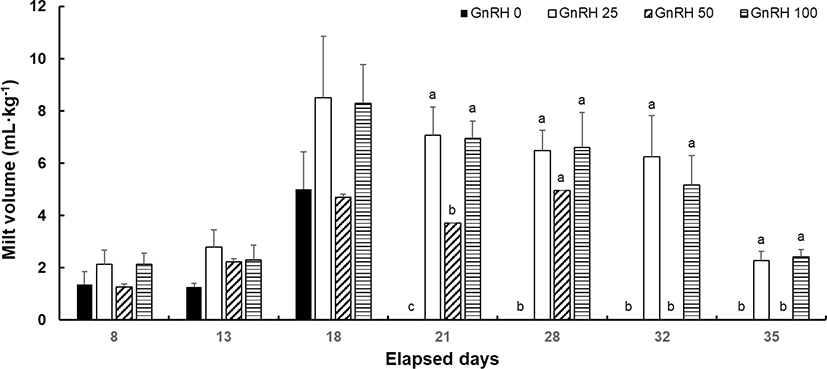
The MSR in the GnRH 0 group decreased after 8 dpi, whereas that in the GnRH 25 group gradually increased until 18 dpi before decreasing thereafter. In the GnRH 50 group, the MSR peaked at 28 dpi. In the GnRH 100 group, the MSR did not follow a consistent trend and increased and decreased repeatedly (Fig. 2). The Sct tended to decrease over time from day 8 after milt was first collected in all groups. However, the Sct in the GnRH 100 group remained at 40%–50% until the end of spermiation.
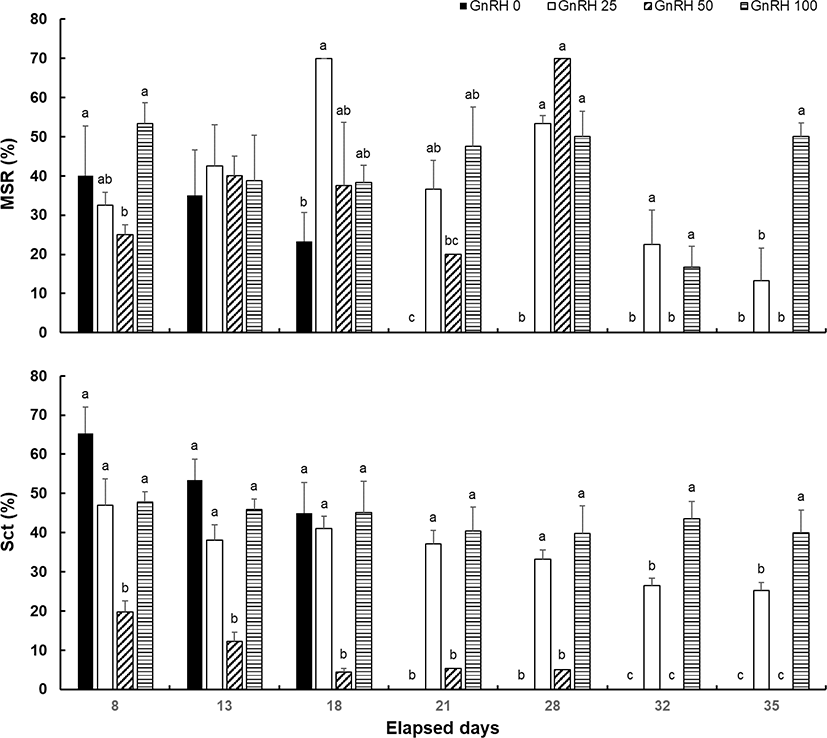
Plasma levels of T did not differ significantly between the control and treatment groups on the day the hormone pellet was implanted, but significant differences were found at 8 dpi. The lowest T level of 9.15 ± 2.64 ng/mL was observed in the control group. The highest level for each of the treatment groups was observed during the spermiation period, with values of 15.8 ± 0.9, 14.21 ± 2.0, and 16.95 ± 0.35 ng/mL recorded for the GnRH 25, –50, and –100 groups, respectively. There were no significant differences in T level among the hormone-treated groups. In these groups, the plasma level of T tended to decrease over time, whereas the T level in the control group remained relatively constant on all days except at 18 dpi (Fig. 3).
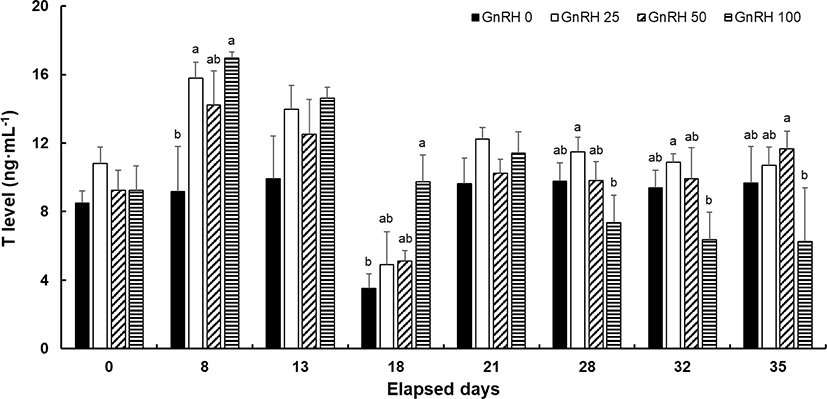
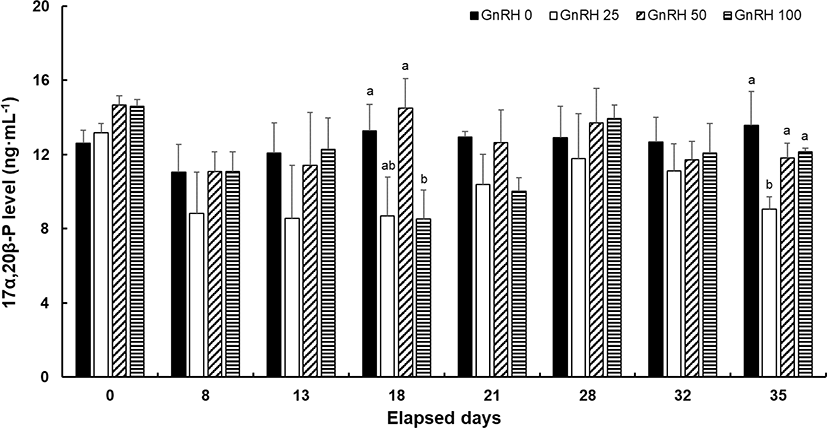
No significant differences in plasma level of 11-KT were found between the control and hormone-treated groups except at 35 dpi. On that day, fishes treated with 50 μg/kg sGnRH had a significantly lower 11-KT level. The plasma level of 11-KT tended to be higher in the latter half of the spermiation period than at the beginning in all groups (Fig. 4). Significant differences in the plasma 17α, 20β-P level were found among groups at 18 and 35 dpi, with significantly lower values recorded in the GnRH 100 group at 18 dpi and the GnRH 25 group at 35 dpi. In addition, the pattern of change in 17α, 20β-P according to spermiation stage was not consistent among all hormone-treated groups (Fig. 5).
Two-way ANOVAs were conducted to assess whether there were significant interaction effects between the days elapsed and the sGnRH concentration used. For the milt volume, MSR, Sct, and T level, the interaction effect was significant (p < 0.001; Table 1).
Discussion
Methods employing exogenous hormones to induce maternal sexual maturation, ovulation, and spermiation have been used successfully in fish for a long time (Lim, 2016; Zohar & Mylonas, 2001). In many flounder species, an increase in milt has been frequently observed regardless of the treatment method used (Clearwater & Crim, 1998; Harmin & Crim, 1993; Lim et al., 2002, 2004, 2007, 2016; Moon et al., 2003; Pankhurst & Poortenaar, 2000; Shangguan & Crim, 1999; Zohar & Mylonas, 2001). Roughscale sole is widely distributed in the East and West Seas of Korea, north of Hokkaido, Japan, the East China Sea, the East Pacific, and off the coast of Canada (NFRDI, 2011). It is a popular fish in Korea, but the natural fish populations are rapidly declining, and the technical know-how for farming this species is lacking. Because proper broodstock management and a breeding method for obtaining high-quality gametes from broodstock have not yet been developed, fertilized eggs have to be obtained from wild fish. When using wild-caught male broodstock to obtain sperm for artificial insemination, problems such as spermiation failure, a low milt volume, and the production of abnormally dilute milt are often encountered.
In this study, hormone pellets were used to deliver sGnRH (Mylonas & Zohar, 2000) to prolong the spermiation period in roughscale sole (Lim et al., 2003, 2016). Regarding treating fish with exogenous hormones, it was previously reported that fish experienced less stress from the implantation of hormone pellets than from repeated injections (Hunter & Donaldson, 1983; Lim et al., 2004, 2016). In a hatchery setting, hormone injections only have a short-term effect on the broodstock; thus, injections have to be repeated several times, which is inconvenient. Moreover, repeated injections are highly stressful to fish and can negatively affect maturation and spermiation (Hunter & Donaldson, 1983; Lim et al., 2004, 2016). The sexual maturation of roughscale sole raised indoors starts from March to April, with full maturation and spawning occurring from May to June (Lim et al., 2012). In this study, pellets containing various doses of sGnRH were implanted in May, when the gonads were most mature, to increase the volume of milt produced by the males and extend the spermiation period. The results showed that the milt volume in all hormone-treated groups increased at 18 dpi. In particular, the GnRH 25 and GnRH 100 groups produced higher volumes of milt than did the control group. After 18 dpi, spermiation stopped in the GnRH 0 (control) group, whereas it continued in the GnRH 25 and GnRH 100 groups until 35 dpi. These results suggest that sGnRH delivery was maintained for several weeks as the implanted pellet slowly dissolved in the fish body, ensuring the continuation of spermiation (Carolsfeld et al., 1988, Lim et al., 2004). The small volume of milt produced by the GnRH 50 group is suspected to be caused by fish selection problems prior to the experiment. The wild-caught males were arbitrarily assigned to treatment groups after acclimatization in tanks, but because the number of experimental fishes in each group was small, the GnRH 50 group might have included fewer mature fishes and more highly stressed individuals.
Based on the MSR (derived from observations under an optical microscope), the control group exhibited a tendency for decreased sperm motility with more days elapsed since the start of spermiation. In the GnRH 25 and GnRH 100 groups, the sperm motility level fluctuated during the spermiation period. Compared to a previous study (Pérez et al., 2000), the induction of spermiation using an exogenous hormone treatment was more successful, with improved sperm motility observed. However, according to Júnior et al. (2017), when spermiation in piapara (Leporinus obtusidens) was induced with gonadorelin, the milt volume increased but sperm quality was not affected, and the same results using GnRHas were reported by Mylonas et al. (2017). As in previous studies, it was difficult in this study to verify whether sperm activity in the hormone-treated groups was high as the sperm-collection time for the control group was short. The GnRH 100 group maintained a constant Sct during the spermiation period, but it tended to decrease in the other groups as spermiation progressed. This means that in all treatment groups except the GnRH 100 group, the concentration of milt decreased toward the latter half of spermiation. In addition, Lim et al. (2004) reported an increase in the total amount of milt due to hydration rather than sperm production.
In this study, treatment with sGnRH-containing pellets increased the milt volume in male roughscale soles but did not significantly affect plasma sex steroid levels. Several previous studies have also reported that GnRHa treatment successfully increased milt volume but had no effect on plasma gonadal steroids (Clearwater & Crim, 1998; Lim et al., 2004; Mañanós et al., 2002). This may indicate that androgens do not play a pivotal role in milt production (Lim et al., 2004). However, some studies have reported positive relationships between GnRHa pellet treatment and sex steroid levels in plasma (Harmin & Crim, 1993; Pankhurst, 1994). These inconsistent results in terms of the relationships between exogenous hormone treatment and steroid levels might have stemmed from differences in the fish species, developmental state of the gonads, type of hormone treatment, and timing of blood collection. In many bony fish, 11-KT is synthesized from gonadotropin hormone secreted from the pituitary gland upon gonadal stimulation. It is secreted into the blood during the development of spermatogonial cells in the testis and is involved in sperm meiosis. In this experiment, the 11-KT level was not significantly correlated with spermiation in roughscale sole. Similarly, in yellowfin sole (Limanda limanda), hCG treatment had no significant effects on plasma 11-KT and T (Canario & Scott, 1991). In teleosts, 17α,20β-P is highly involved in spermiation and ovulation (Lim et al., 2004, 2016). However, as mentioned previously (Lim et al., 2004), increased plasma levels of 17α,20β-P may be due to very rapid conjugation or metabolism after the synthesis and secretion of di-hydroxylated progestins. Although more studies are needed to clarify the effects of exogenous hormones on spermiation induction in fish, including systematic studies on hormone injection methods, the timing of treatments, and the mixed use of dopamine antagonists, it is clear that treatment with sGnRH-containing pellets induces spermiation and is effective for increasing the milt volume in roughscale sole. As a result, the results of this study are judged to disprove that allocation induction using sGnRH is possible from the concentration of 25 µg/kg body weight in roughscale sole. These results may be useful for future research on artificial insemination in roughscale sole for the purpose of cultivation.








