Introduction
Protein glycation, and advanced glycation end products (AGEs) are increased with hyperglycemia and are generated by irreversible reactions between carbohydrates and proteins. AGEs are increased and accumulated in the extracellular matrix of the patient with diabetes (Singh et al., 2014), and induces self-oxidation to produce other reactive intermediates, leading to diabetes development and its complications (Chou et al., 2015; Park & Kim, 2012). An AGEs accumulation in progressive prediabetic stages of disease may contribute to the onset of type 2 diabetes (Stirban et al., 2014). Recently, glycation inhibition has been considered as an effective strategy to slow down human senescence and disease development (Baig et al., 2017). So, there is a growing interest in AGE inhibitors, including a searching bioresources (Nagai et al., 2014).
Indeed, elevation of methylglyoxal (MGO) contributes to the vascular diseases pathogenesis implicated with diabetes complications (Schalkwijk & Stehouwer, 2020). Dysfunction of endothelial cell (EC) implies several vascular diseases, including impairment of ECs barrier functions, vasodilation, disturbances in proliferative activities, tube formation properties, angiogenic properties, synthetic function reduction, and inhibition of white blood cells from adhesion and diapedesis (Cernuda-Morollón & Ridley, 2006; Goligorsky, 2005). ECs control the immune system of the vascular layer and have a critical role in the regulation of the metabolic system (Schalkwijk et al., 2020). Therefore, prevention of diabetes complicated with vascular anormalies is much important for maintaining human health.
Loliolus beka is an edible mollusk that is widely used in several countries particularly in Korea as a food. However, it is not known about the advantageous effects of L. beka on the managing vascular-related diseases (Lee et al., 2019). In current study, therefore, we determined whether extract of L. beka meat protects MGO-induced human umbilical vein endothelial cells (HUVECs) damage.
Materials and Methods
Loliolus beka were obtained from market and identified by Chonnam National University and washed with tap water to remove salt and other impurities. The water extract prepared according to the previous study (Lee et al., 2019).
HUVECs line, was purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). HUVECs were maintained in DMEM (Welgene, Gyeongsan, Korea) containing 10% FBS (Welgene) and maintained in 5% CO2 humidified atmosphere and 95% air at 37°C.
A cell viability was determined using D-PlusTM CCK (Dongin LS, Seoul, Korea) kit. For the assay, cells were seeded onto 96-well plates with 1 × 104 cells/well. After 16 h hot water extract of Loliolus beka meat (LBM-HWE) and/or MGO were treated to the cells. And, the MGO treated to the cells with the indicated concentrations (0.2, 0.4, 0.8, 1, 2, and 4 mM) for 24 h and then LBM-HWE (125, 250, 500, and 1,000 µg/mL) for 24 h. To determine the protective effects of LBM-HWE, cells were treated with 125, 250 µg/mL LBM-HWE for 1 h, prior to addition of 1 mM MGO for 24 h at 37°C.
To prepare cDNA, we used PrimeScriptTM cDNA synthesis kit (Takara Bio, Shiga, Japan) and total RNA was extracted from the cells using Trizol solution (Takara Bio) prior to cDNA synthesis and all the procedures were performed according to the manufacturer’s instructions and the previous study protocol (Cha et al., 2018). Table 1 listed sequences of primers.
To determined AGEs contents the HUVECs were seeded onto 6-well plates as 4 × 106 cells/well, and the cells were incubated with 250 µg/mL of LBM-HWE for 1 h and then further incubated with/without 1 mM MGO (Sigma, St. Luis, MO, USA) for 24 h. The detail procedure followed as described in the previous study (Cha et al., 2018).
HUVECs (4 × 106) were seeded in each well onto 6-well plates, and the cells were incubated with 250 µg/mL of LBM-HWE for 1 h and then further incubated with/without 1 mM MGO for 24 h. Carbonylation of protein casing oxidative damage was determined using a Assay Kit for Protein Carbonyl Content (Abcam, Cambridge, UK) accoding to the company’s instructions.
Results
In order to determine whether LBM-HWE has a protective effect on MGO-induced toxicity in human blood vessel cells, HUVECs were treated with either LBM-HWE or MGO alone, or were preincubated with LBM-HWE for 1 h and then further incubated with various doses MGO for various doses. The concentration range tested (125–1,000 µg/mL) of LBM-HWE alone did not show any cytotoxicity in HUVECs, even it induced cell proliferation in dose-dependent manners (Fig. 1B). Cytoviability was significantly lower in MGO-treated HUVECs over 0.8 mM dose (Fig. 1A). Pretreatment with 125, 250 µg/mL LBM-HWE increased the cell viability in dose-dependently, even the viability similar to that of the control at 250 µg/mL LBM-HWE treated cells in the presence of 1 mM MGO for 24 h (Fig. 1C), suggesting that LBM-HWE possesses a protective effect against MGO-induced damage in HUVECs.
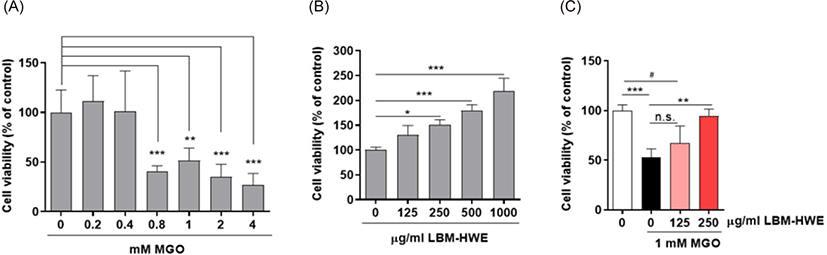
The higher levels (2–4-folds) of MGO is found in diabetic patients plasma (Berlanga et al., 2005; Senda et al., 2015) and exposure of MGO caused inflammation (Berlanga et al., 2005). So, we determined whether LBM-HWE can reduce the production of MGO-induced pro-inflammatory cytokines mRNA expression. As expected, the pro-inflammatory cytokines mRNA levels including IL-1β, TNF-α, and IFN-γ were induced by MGO exposure and its overproduction were reduced as similar to that of the control by LBM-HWE treatment (Fig. 2). These results suggest that LBM-HWE protects MGO-induced pro-inflammatory cytokines activation.
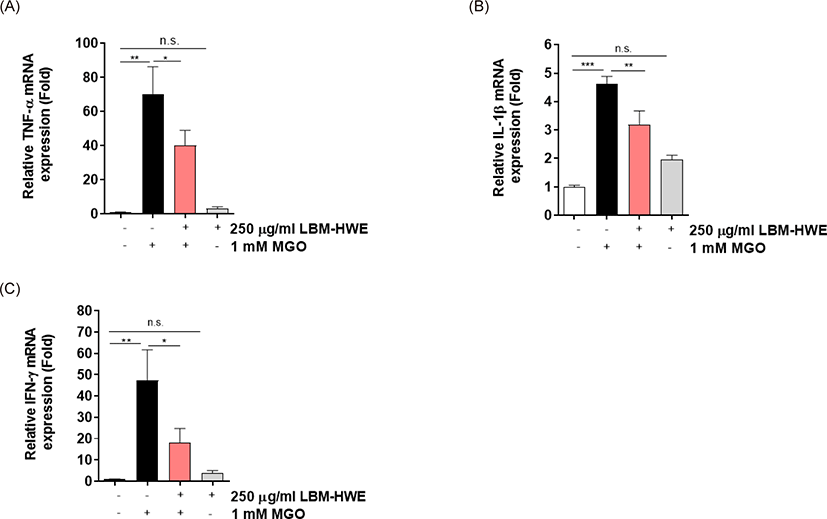
AGE formation is a crucial in complicating with diabetes (Ahmed, 2005). So, we determined whether LBM-HWE reduces AGE formation caused by MGO in HUVECs. A significantly increased (2.5-folds) AGE formation was observed in MGO-treated cells, whereas the induced AGE formation was prohibited by pretreatment with LBM-HWE (Fig. 3A). Due to the harmful effects of AGEs usually interact with its receptor, RAGE (Wang et al., 2019). So, the mRNA expression level of RAGE was also determined. The mRNA expression of RAGE was also significantly induced by MGO exposure, whereas it was reduced by treatment with LBM-HWE prior to MGO (Fig. 3B), suggesting that LBM-HWE induces a protective effect of protein glycation caused by MGO.
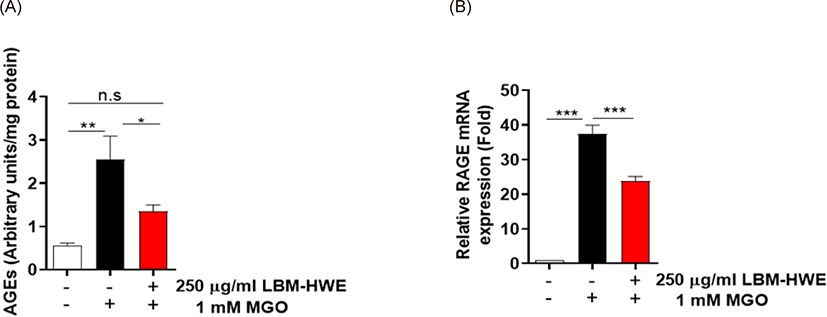
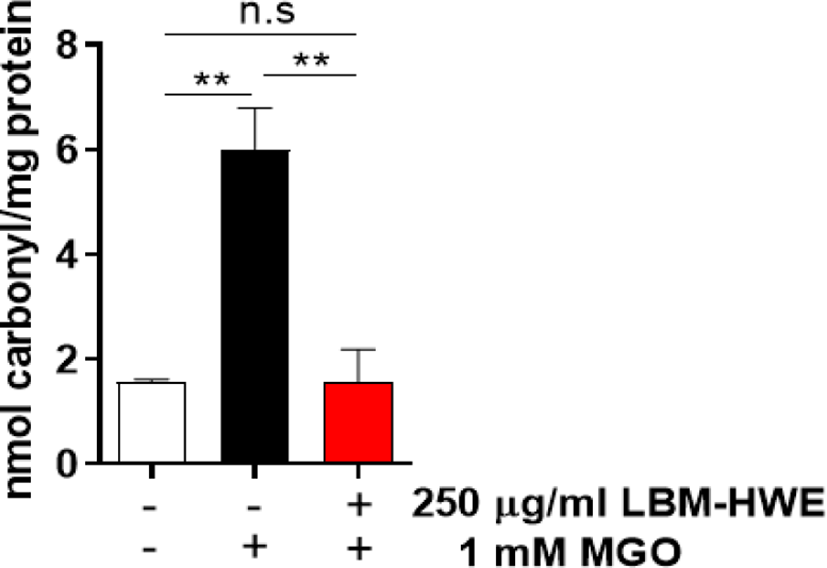
MGO is oxidatively modified to form carbonyl proteins (Allaman et al., 2015; Hanssen et al., 2019). So, we determined whether LBM-HWE protects MGO-induced formation of protein carbonyl, and this content was significantly induced in MGO-treated cells, whereas pretreatment with LBM-HWE prevented MGO-induced protein carbonyl formation (Fig. 4).
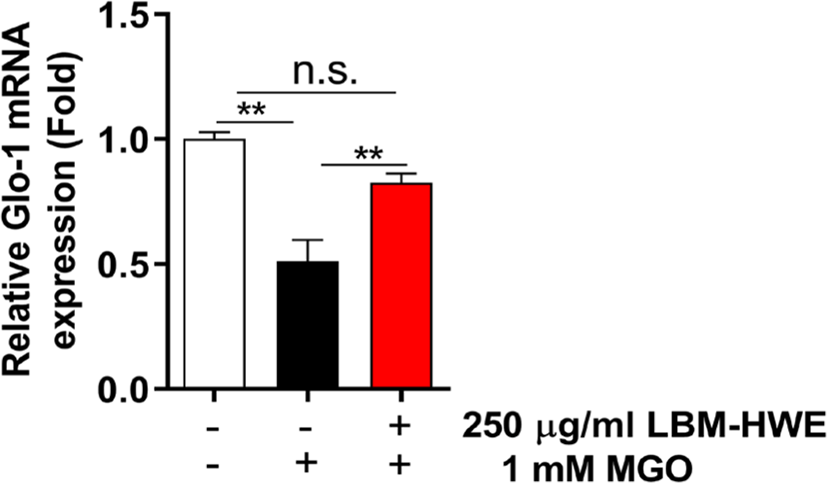
Glycated protein, particularly MGO are detoxified by glyoxalase-1 (Glo-1) (Hanssen et al., 2019). So, we determined glo-1 mRNA expression and found that the expression levels were increased in LBM-HWE-pretreated cells (Fig. 5). These results suggest that LBM-HWE induces a protective effect against MGO-induced protein glycation by increasing the expression of Glo-1 mRNA.
Discussion
A major dicarbonyl compound, MGO, found in higher concentrations of diabetes patients, has been known as a critical player in the development of diabetes complications, including diabetic vascular disease (Cha et al., 2018; Goligorsky, 2005). Recently, in addition, the number of reports is incresing that reactive carbonyls including MGO cause diabetic complications (Pastor-Belda et al., 2017; Senda et al., 2015). Reactive carbonyl responsible for AGE formation is recorded in high concentrations in most patients with diabetes, which is a major factor in the pathogenesis of diabetes mellitus (DM) (Gkogkolou & Böhm, 2012; Kopytek et al., 2020; Pastor-Belda et al., 2017; Senda et al., 2015; Uribarri & He, 2015). Therefore, elimination of accumulated and/or overproduction of AGE or inhibition of its formation is extremely important in treating DM.
Natural products have been emerging as an alternative treatment for diabetes and diabetes complications (Grosso et al., 2017; Heo et al., 2009; Kulkarni, 2014; Xiao & Högger, 2015) in many countries. Loliolus beka is known to rich with taurine and antioxidant effects are found (Han et al., 2017). However, it is not known whether L. beka, might ameliorate diabetic complications. In the present study, we elucidated that the protective effects of hot water extract of L. beka meat (LBM-HWE) on MGO-induced AGE formation in HUVECs.
One of the major causes of the progressive cellular damage in diabetic complications is inflammation (Berlanga et al., 2005). We found that MGO exposure led to toxicity and inflammation and these effects were significantly attenuated by pretreatment with LBM-HWE. A powerful precursor of AGEs, MGO has distinguished as a key signaling molecule mediating pathophysiology of diabetes (Sena et al., 2012). Activation of RAGE induces production of proinflammatory cytokines (Bezold et al., 2019). Administration of MGO to rats causes microvascular changes similar as diabetes symptom (Berlanga et al., 2005), contributing to endothelial (Sena et al., 2012). In our study, MGO exposure induced not only formation of AGEs but RAGE mRNA expression, and this induction reduced by pretreatment with LBM-HWE. Several groups have initiated studies of diabetes etiology and physiology for MGO the management of diabetes complications. Metformin, an antihyperglycemic agent, is popular drug to cure of diabetes patients and known as effective scavenger of MGO by reducing formation of carbonyls (Ruggiero-Lopez et al., 1999).
The glyoxalase (Glo) is a core system for detoxification of MGO and the other reactive aldehydes such as carbonyls (He et al., 2020). Abnormal level of MGO and dysfunction of the Glo system are associated with various diseases, and Glo-1 is an enzyme of Glo system. Our study demonstrated that MGO treatment increased carbonyl protein formation, and this induction was significantly attenuated by pretreatment with LBM-HWE by elevating Glo-1 mRNA expression. Glo-1 prevents hyperglycemia-induced persistent increases DM, which points towards a significant induction of MGO (El-Osta et al., 2008). In addition, it is known that glucose and its metabolites including MGO are accumulated in the cell in diabetic patients (Hanssen et al., 2019). It was reported that when Glo-1 was increased in blood vessel, AGEs production by MGO was inhibited, indicating that MGO acts as a critical mediator for AGE production in vascular cells (Wang et al., 2019), implying that MGO is an AGE precursor that the central key of glycation end products in ECs. In accordance, we demonstrated that induction of Glo-1 by pretreatment of LBM-HWE reduces MGO-induced levels of AGEs in HUVECs. In in vivo data, when Glo-1 decreased, a lower cell viability and higher inflammation-related genes expression were observed in vascular cells (Shinohara et al., 1998). Knockdown of Glo-1 induced MGO concentration and inflammation-related gene expression in human vascular ECs (Stratmann et al., 2016), all these are supporting a role of MGO for endothelial dysfunction. According to Hansen (2001), it has been reported that changes in taurine metabolism led to clinical complications diseases such as retinopathy, neuropathy, nephropathy, cardiomyopathy, platelet aggregation, endothelial dysfunction and atherosclerosis observed in diabetes. The point of view of the treatment of these diabetic complications caused by changes in taurine metabolism, a possible therapeutic view might be to supplement other osmolality and/or small molecule compounds, and taurine in combination therapy with aldose reductase inhibitors. It was confirmed LBM-HWE contained about 40% of taurine (Cha et al., 2019) and had an inhibitory effect on AGE production. There is a previous study that AGE accumulation by MGO directly scavenged overproduced radicals by regulating nrf2 transcription factor (Cha et al., 2019). As well as, LBM-HWE directly scavenged radical in insulin-secreting rat beta cells. There, although, are still only a few studies on the association between AGE accumulation and its inhibition in diabetes, our results proven that the LBM-HWE expected to provide diabetes prevention through the inhibition of AGE accumulation.
Accordingly, LBM-HWE may reduce AGE accumulation caused by MGO, suggesting that LBM-HWE can be used for functional food and pharmaceutical agents related to DM.








