Introduction
Biodiversity conservation is increasingly important in coral reefs management as anthropogenic stress and climate change threaten their resilience (Descombes et al., 2015). Many reports from previous studies that coral reef fish diversity has strong links with ecosystem resilience (Lagerstrom et al., 2022), ecosystem functioning (Topor et al., 2019), and sustainability of the ecosystem services (Weijerman et al., 2018). Previous studies on coral reefs’ biodiversity have delineated the coral triangle area (CTA), the area with the highest biodiversity in coral reef biota, with more than 1,000 coral reef fish (Allen, 2008) and more than 500 coral species (Veron et al., 2009). The CTA includes six countries, i.e., Indonesia, Malaysia, Philippines, Papua New Guinea, Timor-Leste, and the Solomon Islands. Conservation efforts should be prioritized in areas of the CTA with highly significant biodiversity importance using multicriteria analysis. Within the CTA, Asaad et al. (2018) found that the most significant biodiversity importance was distributed in the 3 (three) sites in the Philippines and 4 (four) sites in the Indonesian Archipelago. It has been long suggested that Indonesia had the highest diversity of coral and coral reef fish (Bellwood & Hughes, 2001).
The Indonesian Archipelago has a diverse geological process (Hall, 2009) resulting in diverse coral reef types and a megadiversity of marine fauna (Allen, 2008). The archipelago is uniquely right in the middle of the equatorial line between the Indian and Pacific Oceans. The northern part of Indonesia is the western side of the Pacific Ocean, while the southern part of Indonesia is the eastern side of the Indian Ocean. This makes Indonesia’s archipelago may host fish diversity from both the Indian and Pacific Oceans. Such overlapping fish fauna has been suggested by many other authors (i.e., Carpenter & Springer, 2005; Hoeksema, 2013) to contribute to high fish diversity. Siqueira et al. (2021) provided further supporting evidence that the Indonesian Archipelago is the center of coral reef fish biodiversity, for all six trophic groups.
As the Indonesian Archipelago spans more than 5,000 km in length and more than 1,700 km wide, with 86,700 square kilometers of coral reefs (Huffard et al., 2012), variability in both the nature of habitat and exploitation pressure is enormous within the archipelago. The next question is to explain where the center of coral reef fish biodiversity within the Indonesian Archipelago. There have been studies to answer such questions. Allen & Adrim (2003) claimed that central and eastern Indonesia is the center of reef fish diversity and endemism in Indonesia. Further, Allen & Erdmann (2009) showed that the Bird’s Head Peninsula of Papua, comprising Raja Ampat, Kaimana coast, and Cendrawasih Bay, had the highest coral reef fish diversity. All the claims were based on their observations and added from a checklist of previously published studies. Such comparison can provide insight as an answer to the question of fish species’ origin or diversity. However, it does not necessarily present empirical evidence that the center was there, since there is no ‘apple to apple’ comparison with other locations.
A study on coral reef fish diversity and abundance is very important for the management of very valuable coastal resources. The objectives of the present study are to determine 1) which Indonesian region shows the highest coral reef fish diversity and abundance, and 2) which location (district) shows the highest fish diversity and abundance across the archipelago. This archipelagic-scale study is the first one to study coral reef fishes in the world’s largest archipelago. The present study not only enables us to see the big picture of coral reef fish in Indonesia but also to compare it from different locations. The previous study on the ecology of Indonesia’s coral reef fishes is limited to islands- or district-levels. Variations among islands hardly show the large-scale ecological process that is important for the coral reef fishes.
Materials and Methods
Data on fish diversity and abundance were collected from 24 locations (districts) across 4 (regions) in the Indonesian Archipelago (Fig. 1). The Indonesia archipelago adjoins two oceans, the Indian and Pacific Oceans. The archipelago was divided into four regions or physiographies based on its oceanography and geological properties. The Indian Ocean region includes all coral reefs on the southern coast of Sumatra, Lombok, and Sumba Islands. It is estimated that coral reefs in this region had been developed about 500 kya (thousand years ago), at the time the Mentawai Islands were separated from the Sumatra mainland (Kingstone, 2009). Between the Indian and Pacific Oceans, there are typically two marine regions. The first region is the Sunda Shelf which was inundated and became the seas during the second glacial era, about 9.5 kya (Voris, 2000). The second region is very much older seas, formed about 10-5 mya (million years ago), that consists of coral reefs in the Makassar Strait, Flores Sea, and Banda Sea (Hall, 1997). This Wallacea region is located on the western side of the Sahul Shelf and has never been connected either to the Asia or Australian continents (Hall, 1997; Voris, 2000). This region mostly receives water from the Indonesian Throughflow. The Pacific Ocean region comprises Ternate in the north Maluku Islands, Raja Ampat, and Biak. The last two locations are situated in the western Pacific Ocean and were part of the Sahul Shelf, while Ternate is on the western side of the present Sahul Shelf. The three locations are situated on the same Australian plate (Hoeksema, 2013). The Bird’s head of the Papua microcontinent was already formed in 50 mya, during the early Eocene (Hall, 1997). The distribution of the number of transects in each location and region is presented in Table 1.
Data used in the present study are mostly from the Reef Health Monitoring Program 2018 which was regularly performed by the Research Center for Oceanography (RCO), the National Board for Research and Innovation (BRIN, formerly LIPI) of Indonesia. The archipelagic scale data were collected by several teams involving researchers from six state universities and the RCO, in the period from July–November 2018. All team members are certified data collectors by the National Board for Professional Certification (BNSP). Data collection in the fields was also supervised by RCO staff to ensure controlled quality. Data collection in two locations, Sumbawa and Luwuk, was carried out for non-monitoring purposes but with the same methods.
Data on the fish diversity and abundance were collected underwater (in situ) at 5–7 m depth using the underwater visual census methods (Wilson et al., 2018) on a 5 × 70 m2 size transect. There were 321 transects in total. Trophic levels of the coral reef fish were grouped into three categories, i.e., corallivore, herbivore, and carnivore. The corallivore fish is comprised of the family of Chaetodontidae. The herbivore fish consisted of three families, Siganidae, Scaridae, and Acanthuridae. The carnivore fish included four families, Lutjanidae, Lethrinidae, Serranidae, and Haemulidae. Major fishes (Pomacentridae, Labridae) were not included in the monitoring program due to its high species diversity and lack of reef fish expertise. The exclusion also happened for Blennidae and Gobiidae for its frequently cryptic appearance.
Data analysis was done using non-parametric statistics since all the diversity and abundance data did not conform with the normal data distribution and homogenous variance assumptions. Data transformation did not sufficiently improve data normality distribution and homogenous variances. Among regions comparison of coral reef fish diversity and abundance were carried out using the Kruskal-Wallis test. The same data analysis was applied to compare coral reef fish diversity and abundance among 24 locations (districts). Pairwise comparison among locations used the Wilcoxon method. The results of this analysis are presented only for the top 10 highest-rank locations on the eight fish variables within the archipelago. All the data analysis used statistical software JMP-Pro 13.0.0, SAS Institute (Cary, NC, USA).
Results
The present study showed that within the Indonesian Archipelago, coral reef fish of the Pacific region shows its supremacy over the other three regions. The highest coral reef fish species diversity was found in the Pacific Ocean region (Fig. 2A). The difference in the species diversity among the four regions was significant (Table 2). The number of species was more than twice higher in the Pacific Ocean region than in the Indian Ocean region. This pattern is consistent for all three trophic levels, i.e., corallivore, herbivore, and carnivore fishes. The diversity of coral reef fish in the Indian Ocean was also lower than in the Wallacea region, for all three trophic levels. The rank of coral reef fish diversities was in the order of Pacific Ocean, Wallacea, Indian Ocean, and Sunda Shelf regions. In the Indian Ocean, however, carnivore fish diversity is about the same as in the Sunda Shelf region. Among the three trophic levels, the diversity of herbivore fish is generally higher than the diversity of the other two trophic levels. The diversity of herbivore fish is about twice as higher as that of carnivore fishes, except in the Sunda Shelf region. A comparison between the diversity of herbivores and corallivores does not show any specific pattern.
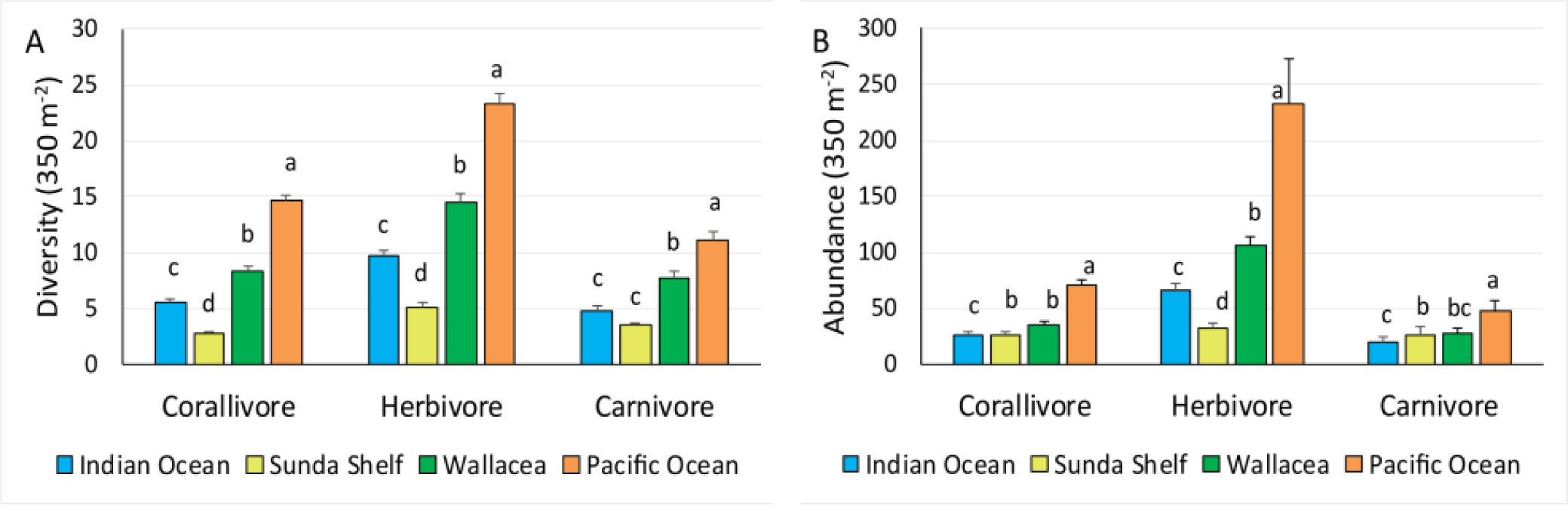
Coral reef fish abundance was also the highest in the Pacific Ocean region (Fig. 2B). The pattern of the fish abundance comparison was also the same as afore-mention in the fish diversity. Coral reef fish abundance in the Pacific Ocean region was significantly higher than in the Wallacea, the Indian Ocean, and the Sunda Shelf regions. This high fish abundance in the Pacific Ocean region was consistent for the three trophic levels. The abundance of corallivore fish was about the same in the three regions, Wallacea, Sunda Shelf, and the Indian Ocean. The Wallacea region has a higher abundance of herbivore and carnivore fishes than the Sunda Shelf region. Between Sunda Shelf and Indian Ocean regions, the Indian Ocean only has a higher abundance of herbivore fish.
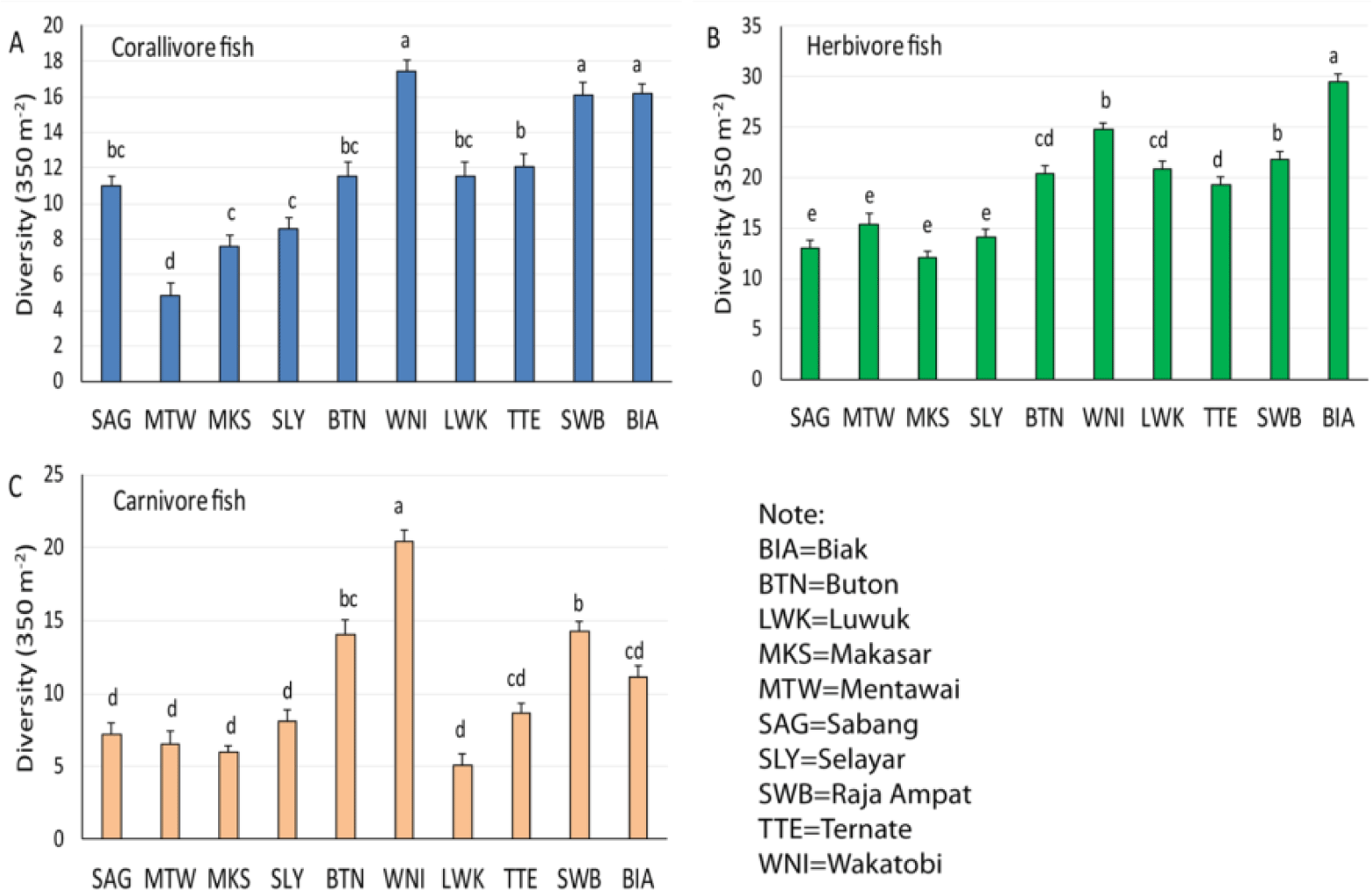
Multiple pairwise comparisons among 24 locations revealed that coral reefs with high-rank order of diversity and abundance were not only found in the Pacific Ocean region. Coral reefs in the Indian Ocean region also contributed to the top ten highest-rank coral reef fish groups, i.e., Sabang and Mentawai (Table 3). Coral reefs in the Wallacea region had four representatives in this high-class group, i.e., Makassar, Selayar, Wakatobi, and Luwuk. Makassar and Selayar are located in the Makassar Strait and the Flores Sea, while Wakatobi and Luwuk are both located in the Banda Sea. All three locations in the Pacific Ocean region, Ternate, Raja Ampat, and Biak, were among the top ten highest-rank coral reef fish diversity. There were no locations from the Sunda Shelf region included in the shortlist. Among the ten best locations in Table 3, the differences were all significant for all six fish variables (Table 4).
The present study also revealed for the first time that the coral reefs of Wakatobi showed the best in the diversity and abundance of coral reef fish in the Indonesian Archipelago. Biak and Raja Ampat reefs followed in the second and the third rank orders. The coral reef of Wakatobi was predominant for corallivore and carnivore fish (Fig. 3A and 3C), while Biak was prevalent for herbivore fish (Fig. 3B). Wakatobi, Biak, and Raja Ampat share about the same species diversity on corallivore fish. Biak and Buton had about the same carnivore fish species diversity. It is also interesting to include Sabang from the Indian Ocean region which shares a similar corallivore fish diversity with Buton, Luwuk, and Ternate (Fig. 3A).
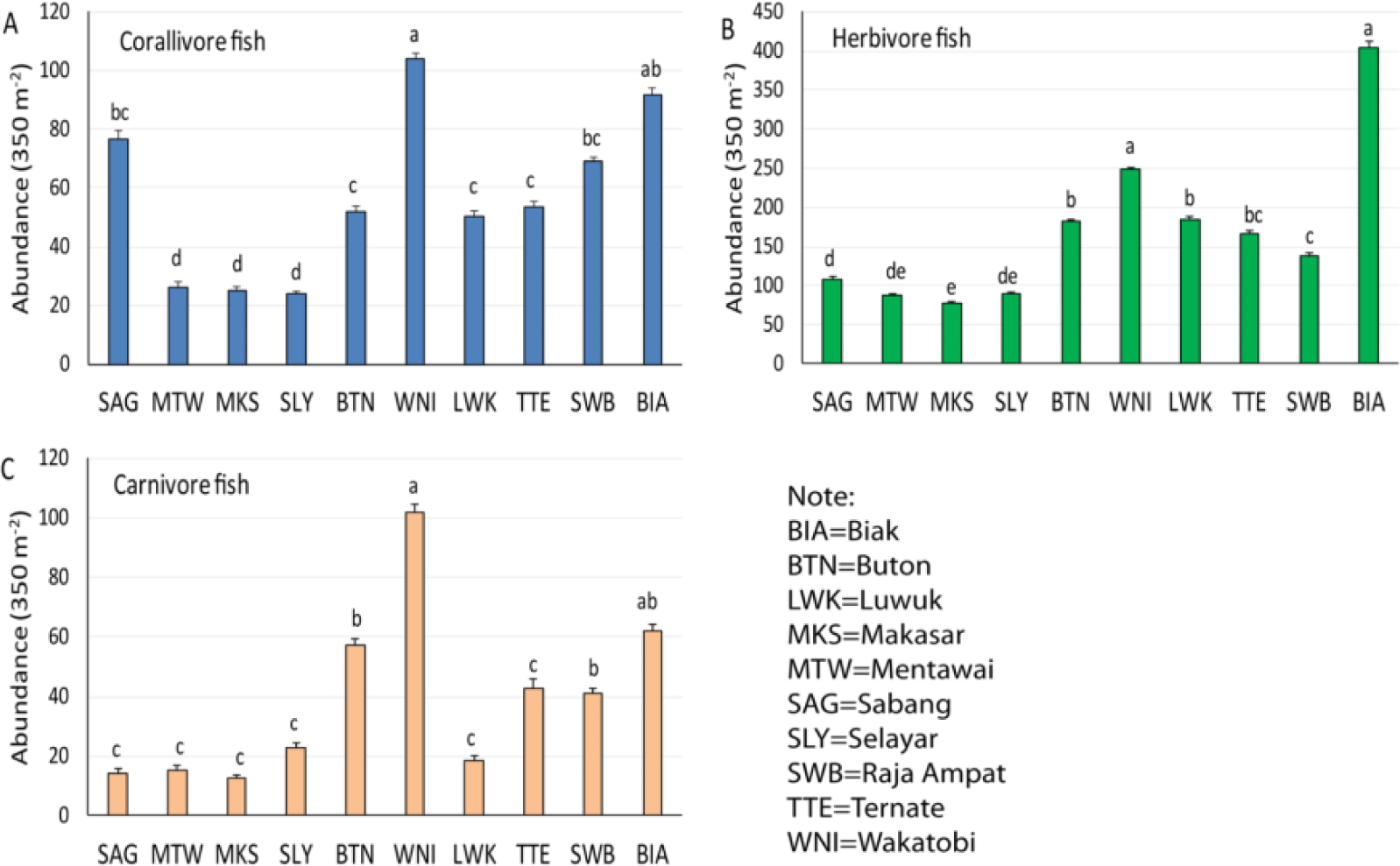
The pattern of fish abundance comparison was similar to the species diversity pattern. Coral reefs of Wakatobi and Biak showed their predominance in the first group on the abundance of all corallivore, herbivore, and carnivore fish (Fig. 4). Coral reefs of Raja Ampat belong to the second group of corallivore and carnivore fish but to the third group of herbivore fish. The coral reefs of Sabang belong to the second group of corallivore fish, along with Raja Ampat and Biak (Fig. 4A). Buton, Luwuk, and Ternate were part of the second group of the herbivore fish abundance, and the third group of the corallivore fish abundance. In Biak, herbivore fish abundance was 62% higher than in Wakatobi (Table 3). In return, carnivore fish abundance in Wakatobi was also 64% higher than in Biak. The difference in corallivore fish abundance between the two locations was 13% in favor of Wakatobi.
Discussion
The present study revealed that among the four regions, coral reefs in the Pacific Ocean region have the highest fish diversity and abundance. Its supremacy occurs on the three trophic levels of the coral reef fish. Zoogeography explains biodiversity in at least four theories, i.e., the center of origin, the center of accumulation, the center of survival, and the center of overlap (Hoeksema, 2007; Sanciangco et al., 2013). The Pacific Ocean region reef fish diversity may be explained by the first three theories. The Pacific Ocean region is part of the Australian plate (Hoeksema, 2013) and have been formed during the early Eocene (Hall, 1997). This very old geology may serve as the center of origin. This west Pacific Ocean region may also be the center of accumulation. In an open recruitment system, the Pacific Ocean region may accumulate drifted fish larvae from the Mindanao Current, Southern Equatorial Current, and New Guinea Coastal Current (Schiller et al., 2008). Speciation may occur outside the region but accumulate in the region. The region of the Pacific Ocean could also serve as the center of survival since tropical waters provide all year long optimum resources and conditions. A complex geological process of the region increased habitat heterogeneity that supports the center of survival. The center of overlap may explain diversity in the Wallacea region, but not in the Pacific Ocean region. The degree of overlapping currents in the Pacific Ocean is lower than that in the Wallacea region.
The present study was the first one to compare coral reef fish among locations (district level) in the Indonesian Archipelago and found that the coral reefs of Wakatobi, Biak and Raja Ampat were the best three hotspots in coral reef fish diversity and abundance. Carpenter & Springer (2005) claimed that the center of shore fish biodiversity was in the Verde Island Passage, located between Mindanao and Luzon, and Pulau Bintan (Sunda Shelf-Indonesia) was the secondary center of the fish biodiversity. In contrast, the coral reef fish of Bintan enlisted on the 16th rank in the present study. The shore fish in Carpenter & Springer’s study (2005) was common fish that included many soft-bottom fish but excluded small coral reef fish. Allen (2008) provided maps of coral reef fish biodiversity in the CTA that are in agreement with the finding of the present study. Marwayana et al. (2022) found that coral reef fish diversity in Raja Ampat is higher than in Wakatobi based on an eDNA study. The present study did not include the planktivore fish which is not comparable with Marwayana’s study.
The level of biodiversity should be interpreted to its spatial and temporal scales. Using different spatial scales comparison of coral reef fish diversity from the same data may result in different rankings, for example between 100,000 km2 and 500,000 km2 scales (Allen, 2008). In the present study, the fish diversity was presented at a transect scale, of 350 m2. Therefore, the finding of this study should be interpreted by considering its scale. Allen & Erdmann (2009) reported that coral fish diversity in Raja Ampat was 1,320 species. This high number of species included previous studies from 1975 to 2008. There was no comparable study with similar space- and time-scales. Therefore, the claim that Raja Ampat had the highest reef fish biodiversity in the CTA may be exaggerated. In the present study, coral reef fish diversity was compared in an ‘apple to apple’ manner. All districts’ fish diversity was compared at the transect level, with a replicate of 7–21 transects. Using the fairly district-to-district comparison placed Raja Ampat on the third rank of coral reef fish diversity and abundance in the Indonesian Archipelago. Raja Ampat has six locally managed marine protected areas (Carvalho et al., 2021). The finding that the Pacific Ocean region host higher coral reef fish diversity and abundance was also supported by observable evidence.
The high diversity and abundance of herbivore fish at Biak reefs while carnivore fish at Wakatobi does not necessarily mean that both functional groups have different centers of biodiversity. The geological formation of the Wakatobi Islands is relatively recent, less than 1 mya (Nugraha & Hall, 2018), which may exclude Wakatobi as the center of origin. However, its position in the center of the Sunda Banda Seascape makes Wakatobi potentially to be the center of overlap, the center of survival, and the center of accumulation. In contrast, Biak had developed pinnacle reefs during the middle of Miocene, ~15 mya (Gold et al., 2014). Therefore, Biak may potentially serve as the center of origin, the center of survival, and the center of accumulation. Biak may not serve as the center of overlap due to its location in the Pacific Ocean.
The difference in coral reef fish diversity and abundance between Biak and Wakatobi reefs may be explained from the coral reef substrate compositions. Biak coral reefs had more dead coral algae (DCA) coverage than Wakatobi, while Wakatobi reefs had more non-acroporiid coral coverage than Biak (unpublished data). The DCA cover is an important predictor of herbivore coral reef fish abundance and diversity (Tootell & Steele 2015), whereas hard coral cover is the strong predictor for carnivore fish (Russ et al., 2021). The contrasting coral reef habitat conditions were likely responsible for the difference in fish composition between the two locations.
The apparently different centers for the two trophic levels may also show the difference in the level of exploitation. The coral reefs of Wakatobi are all inside the marine protected area, the Marine National Park (MNP) of Wakatobi established in 1996. Herbivore fish abundance is usually found higher in the no-take-zone or the gear-restricted site than in the open fishing site, while invertivore and piscivore fish are found higher in the remote site (Campbell et al., 2018; Topor et al., 2019). The MNP Wakatobi reefs may be considered more remote than the Biak reefs. Furthermore, the difference between Biak and Wakatobi reef fish may be explained by their fishermen’s demography. Biak and Wakatobi have about the same population, ~135,000 and ~111,000 people, and also about the same proportion of full-time fishermen, ~6,300 and ~5,700 people respectively (BPS, 2021a; BPS, 2021b). However, the Wakatobi fishermen mostly (60%) use inboard motorized boats, while the Biak fishermen are predominated (75%) by non-powered boats (BPS, 2021a; BPS, 2021b). The fishing vessels showed that the Biak fishermen have the capacity of fishing only in their villages, while Wakatobi fishermen are capable to fish far from their villages. In addition, fishing gears in Biak are mostly hand-line and bottom gill-net. The targeted fish are carnivore demersal fish, such as Lutjanidae, Lethrinidae, and Serranidae (Pattiasina et al., 2021). Carnivore fish are more sensitive to fishing pressure than herbivores (Carvalho et al., 2021). Heavy carnivore fishing pressure in Biak left herbivores predominating coral reefs.
The present study provided a shortlist of the ten best coral reefs with high fish diversity and abundance. The shortlist should be used in consideration for selecting priority locations for conservation and fisheries management purposes. Coral reef management should prioritize coral reefs with high resilience. In the shortlist, there is no representative from the Sunda Shelf. At present, coral reef resilience assessment is still scarcely implemented. There are at least four resilience assessment methods that are available to be used in coral reef management, i.e., Bachtiar et al. (2019), Maynard et al. (2017), Obura & Grimsditch (2009), and Thompson et al. (2020). The second and third resilience assessment methods may not be applicable to developing countries with very large coral reefs, such as Indonesia and the Philippines, since it needs much data and many specialists. The last two methods are more practical in measurement and interpretation. None of them, however, have ever been applied to large-scale monitoring programs. Therefore, a comparison of resilience levels among reefs or locations is barely accomplished. Despite having one of the best coral reef management in the world, Australia has not done any resilience assessment program on its Great Barrier Reefs. At the archipelago scale, an assessment of coral reef resilience was done for the Indonesian reefs (Bachtiar et al., 2011).
High diversity of coral reef fish results in diversity in their response to local and global disturbances. Biodiversity is strongly linked to ecological resilience (Topor et al., 2019). Therefore, it is expected that coral reef with high resilience levels corresponds with high coral and fish diversity. However, Bachtiar et al. (2011) reported that the region with the highest coral reef resilience was the Sunda Shelf. Comparison among districts indicated that the highest coral reef resilience was found at the Natuna and Bintan reefs. Both locations are in the Sunda Shelf. On the opposite, the present study showed that none of the locations in the Sunda Shelf are on the short-list of the ten-best coral reef fish diversity and abundance. These incongruity findings raise the question about the accuracy of the resilience index assessment. The Bachtiar’s resilience index used merely biophysical variables of the coral reef substrate, without integrating recruitment data and fish variables. Both the two important resilience factors were estimated using more practical biophysical substrate metrics. Therefore, further resilience assessment method that incorporates fish variables and coral recruitment data still need to be developed. Secondly, there may be some differential mechanisms for coral diversity and coral reef fish diversity in maintaining the resilience of the coral reefs. Further studies on the complex mechanism of coral reef resilience are also required to improve resilience assessment and resilience-based coral reef management.
As a final point, the present study provided several new findings about coral reef fish diversity and abundance across the Indonesian Archipelago. The Pacific Ocean region had the highest coral reef fish diversity and abundance. At the district level, Wakatobi and Biak are both the hottest hotspots in coral reef fish diversity and abundance, for all three trophic levels. Coral reefs of Raja Ampat are positioned at the third rank. The coral reefs of Sabang showed considerable diversity and abundance of corallivore fish, at the same level as coral reefs at Buton, Ternate, and Luwuk.








