Introduction
Neuropeptides are molecules comprising 5 to 50 amino acids that promote intercellular communication (Vishwanatha et al., 2017). Neuropeptides promote the connection between the nervous and endocrine systems (Klavdieva, 1995). The physiological processes and behaviors of starfishes such as locomotion, feeding, and reproduction are controlled and regulated by neuropeptides or hormone peptides (Birenheide et al., 1998; Byrne et al., 2019; Kato et al., 2009; Thorndyke & Carnevali, 2001; Tinoco et al., 2021). To date, the several muscle relaxant and contractile neuropeptides were isolated or identified within the echinoderm phylum (Kim et al., 2016; Semmens & Elphick, 2017; Semmens et al., 2013).
One of the most studied neuropeptides from echinoderms are SALMFamide peptides, which are present in starfish, sea urchin, and sea cucumber (Elphick, 2014; Elphick et al., 1991). The SALMFamide peptides were purified from Asterias rubens and Asterias forbesi using FMRFamide antibody (Elphick et al., 1989). SALMFamide is classified into two types: L-type and F-type. The L-type has a pentapetide motif of SxLxF at the C-terminus, and the F-type has an SxFxF motif at the C-terminus (Elphick et al., 2013). The most studied L-type of SALMFamides are two peptides S1 and S2. SALMFamide-1 (SALMFa-S1) peptide is an octapeptide composed of 8 amino acids, and the amino acid sequence is GFNSALMF. SALMFamide-2 (SALMFa-S2) peptide is a dodecapeptide consisting of 12 amino acids, and the amino acid sequence is SGPYSFNSGLTF. SALMFamide-S1 and SALMFamide-S2 cause the relaxing effect on tube feet, cardiac stomach, and induce the inversion of the cardiac stomach in starfish (Elphick et al., 1991; Elphick et al., 1995; Melarange & Elphick, 2003). SALMFamide-S1 was proposed to take part in promoting growth during regeneration (Thorndyke & Carnevali, 2001). In a recent study, SALMFamide-S1 transcriptional expression during the regeneration process was reported (Byrne et al., 2019). The precursor protein of A. rubens SALMFamide comprises additional putative peptides that have different sequences as shown in Fig. 1 (GenBank: ALJ99974.1). Studies on the bioactivity of these putative peptides have not yet been conducted.
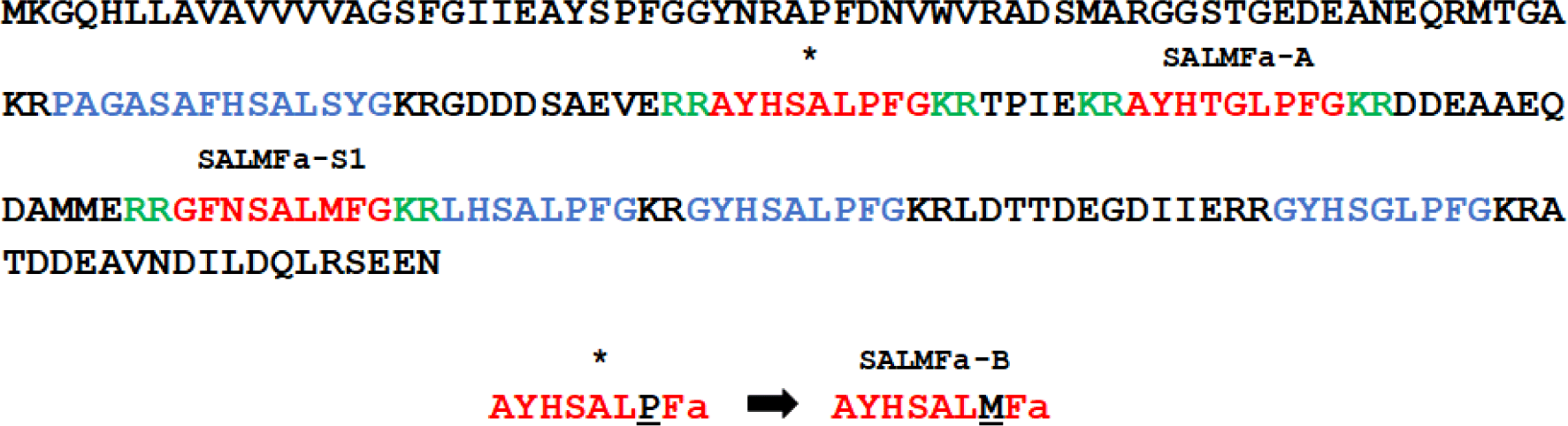
The L-type SALMFamide precursor along with SALMFamide-S1 comprises additional 6 putative peptides, which contain SALMFamide and GLPFamide motifs. The two putative peptides with similar N-terminal motifs and different C-terminal motif were selected for this study. The native AYHTGLPFamide peptide was indicated as SALMFamide-A (SALMFa-A) (Fig. 1). To investigate the effect of C-terminal SALMFamide motif on bioactivity, the proline of the putative AYHSALPFamide peptide was substituted with methionine AYHSALMFamide. This derivative AYHSALMFamide peptide was indicated as SALMFamide-B (SALMFa-B). The SALMFamide-S1 and SALMFamide-S2 neuropeptides were used as control peptides (Fig. 1).
Materials and Methods
The starfishes Patiria pectinifera and Asterias amurensis were collected in Busan, Korea. This study did not require local agency/ethics committee approval as experimental work on starfish is not regulated.
The four neuropeptides used in this study were synthesized and purchased from Peptron (Daejeon, Korea).
To measure synthetic peptides’ in vitro activities and pharmacology, three muscle preparation of muscle tissue from the starfishes P. pectinifera and A. amurensis were used (Elphick et al., 1995). All measurements were performed in 55 mM Mg2+ artificial seawater (ASW) with the following compounds: (mM) NaCl 445, KCl 10, CaCl2·2H2O 10, MgCl2·6H2O 55, Glucose 10, Tris-HCl 10 (pH 7.8). The muscle preparations were connected to a physiography system equipped with a force-displacement transducer (Type 45196A; NEC-Sanei Instrument, Tokyo, Japan). The relaxing activity of muscle was monitored and registered by a recorder (WR7300; GRAPHTEC, Yokohama, Japan) through an amplifier (AS1302; NEC-Sanei Instrument).
Prior to testing the SALMFamide peptides’ relaxing activity, the apical muscle was precontracted by acetylcholine (Ach) 5 × 10–6 M while tube feet and cardiac stomach were activated by carbachol (Carb) 5 × 10–6 M. To measure the pharmacological values, synthetic peptides were dissolved with a calculated amount of distilled water to concentration of 1 × 10–2 M and were logarithmically diluted. Then 20 µL of peptides were applied in the 2 mL polypropylene chamber to obtain the proper final concentration. The activity was observed and measured for 2 min after the application. After each application muscle was washed by ASW buffer and stabilized for 15 min prior to the next application.
The muscle preparation starts with the starfish dissection by the removal of starfish eyespot followed by the separation of the aboral and oral side using scissors. To prepare the starfish apical muscle, the pyloric caeca were removed from the center of arms. Then each arm was cut, and the apical muscle was removed from the inner side of the body wall. Lastly, approximately 10 mm of apical muscle was cut, and both ends were tied with cotton threads. The muscle preparation was vertically fixed in a 2 mL polypropylene chamber with aeration, where the bottom end is connected to a silver wire on the bottom of the chamber, and the top end is connected to a physiography system.
To prepare the starfish tube feet, the ambulacral plate was cut using scalpel. Then, the tube feet were cut together with an ambulacral ossicle and ampulla. Both ends of the muscle were tied with cotton threads, where on the bottom side, the loop was previously made. The top end was tied with a tube feet sucker, whereas the bottom side was tied with an ambulacral ossicle. And finally, the tube feet preparation was vertically fixed in a 2 mL polypropylene chamber in the same manner with apical muscle.
To prepare the cardiac stomach muscle, the extrinsic retractor strands that are attached the cardiac stomach to the ambulacrum were cut carefully to separate the stomach from the inner side. Both ends of the muscle were tied with cotton threads. The bottom end of the loop was tied with the aboral part whereas the top end was tied with the oral opening part of the cardiac stomach. Finally, the muscle preparation was vertically fixed in a 2 mL polypropylene chamber in the same manner with previous muscles.
For the statistical analyses, one-way analyses of variance (ANOVA) followed by the Bonferroni multiple comparisons test were performed by GraphPad Prism 8.4 for Windows (GraphPad Software, CA, USA). The means denoted with different letters at the top of the columns indicate statistically significant differences (p < 0.05). To determine the pharmacological properties the sigmoidal-shaped four-parameter dose-response curves were constructed using GraphPad Prism 8.4. The peptide efficacy (Emax) is based on the best-fit top value on a dose-dependent curve constructed by the in vitro bioassay values, which is calculated as a relative percentage of the muscle maximum contraction induced by Ach or Carb. All values are shown with mean SD. The statistically significant differences (p < 0.05) were determined by one-way ANOVA or two-way ANOVA followed by the Bonferroni multiple range test using GraphPad Prism 8.4.
Results
The pharmacological activity of SALMFamide peptides and a derivative was investigated. The investigation conducted using apical muscle of A. amurensis revealed comparable relaxing activity of SALMFa-A and SALMFa-B (Fig. 2A). However, SALMFa-A and SALMFa-B showed lower relaxing activity compared to the control SALMFa-S1 and SALMFa-S2 peptides (Fig. 2B). The weakest relaxing activity was exhibited by the native SALMFa-A peptide (Fig. 2C). The results revealed following Emax values: the SALMFa-S1, SALMFa-S2, SALMFa-A and SALMFa-B had Emax (%) values of 19.92 ± 0.99%, 22.10 ± 2.77%, 11.41 ± 0.61% and 15.32 ± 0.98%, respectively. The order of relaxing effect on A. amurensis apical muscle was: SALMFa-S2 > SALMFa-S1 > SALMFa-B > SALMFa-A. The threshold concentration (TC) relaxing response for SALMFa-S1, SALMFa-A, and SALMFa-B were observed at 10–8 M. Meanwhile, the TC of SALMFa-S2 was observed at 5 × 10–8 M concentration (Table 1).
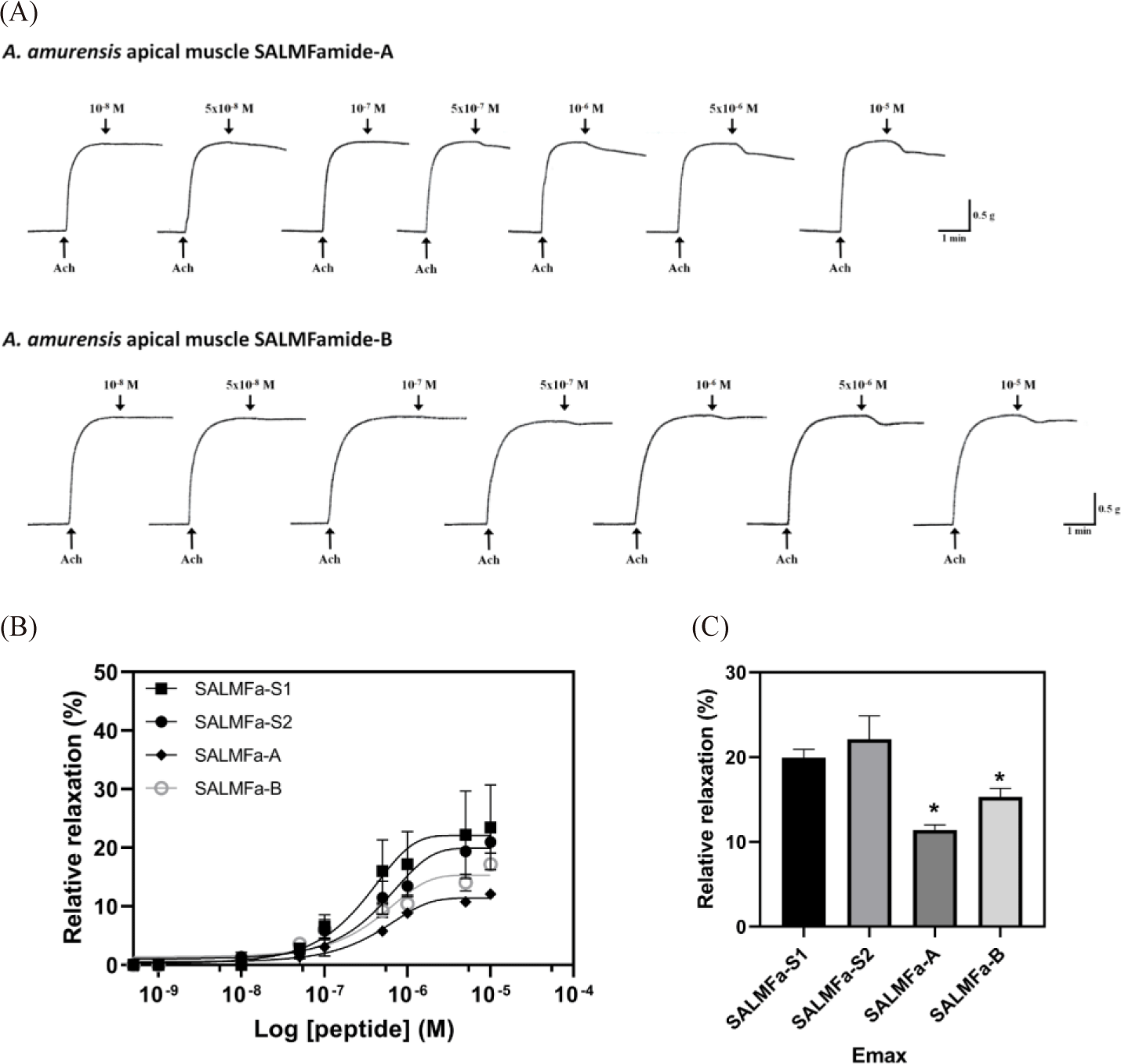
TC is the concentration at which each neuropeptide started to exert myoactivity. The Emax is based on the best-fit top value on a dose-depend curve constructed by the in vitro bioassay and estimated as a relative percentage of the muscle maximum contraction induced by acetylcholine 5 × 10–6 M on apical muscle. TC, threshold concentration; Emax, peptide efficacy.
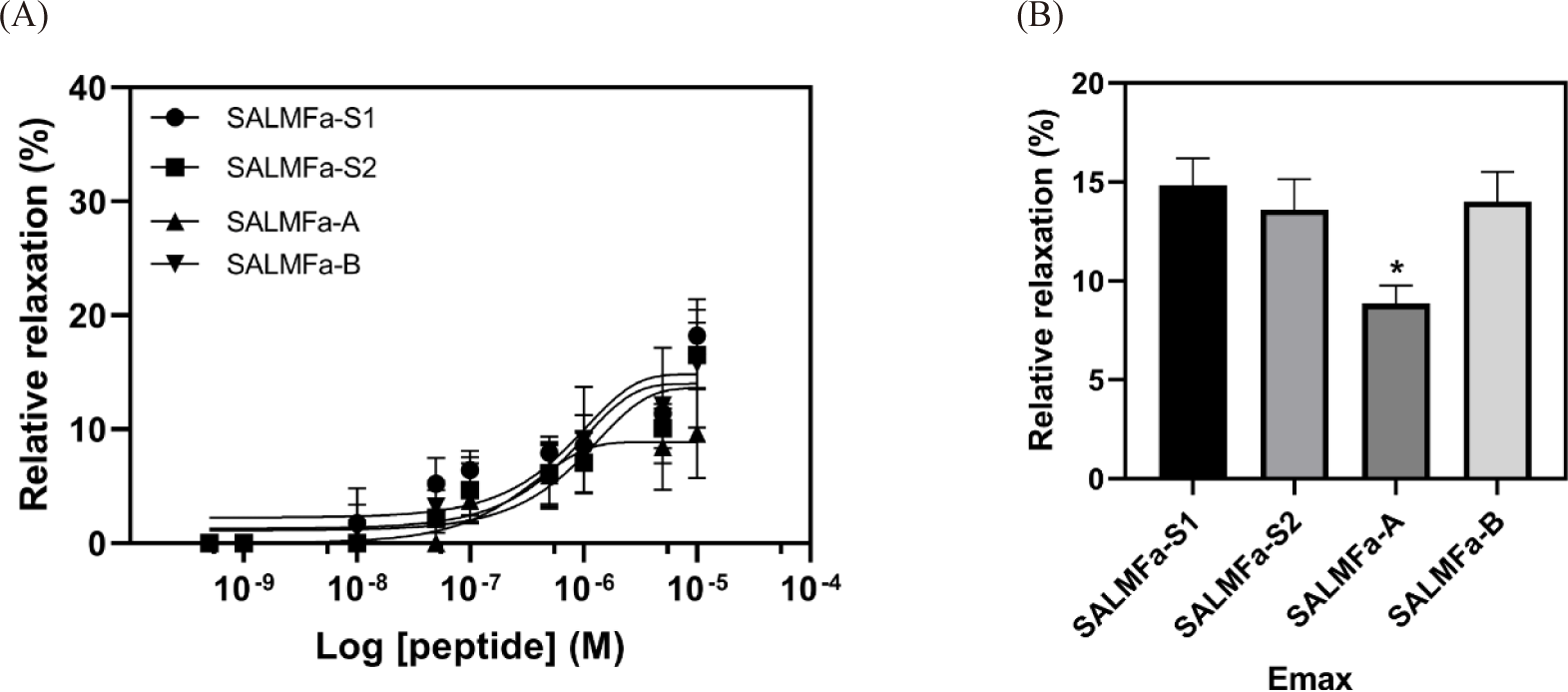
The investigation of SALMFa-A and SALMFa-B peptides’ relaxing activity, performed on apical muscle of P. pectinifera, showed a similar pattern of potency. However, the relaxing effect of native SALMFa-A was dramatically lower than those of control SALMFa-S1 and SALMFa-S2 peptides (Fig. 3A). The efficacy of SALMFa-A was roughly 2-fold lower than those of SALMFa-S1 and SALMFa-S2 peptides (Fig. 3B). The pharmacological activity of the SALMFa-S1, SALMFa-S2, SALMFa-A, and SALMFa-B showed 14.82 ± 1.38%, 13.61 ± 1.53%, 8.86 ± 0.90%, 14.00 ± 1.50%, respectively. The TC for SALMFa-S1 and SALMFa-B were observed at 10–8 M. On the other hand, the TC of SALMFa-S2 and SALMFa-A was observed at 5 × 10–8 M and 10–7 M concentration, respectively (Table 1). The order of relaxing effect on P. pectinifera apical muscle was: SALMFa-S1 > SALMFa-B > SALMFa-S2 > SALMFa-A.
Discussion
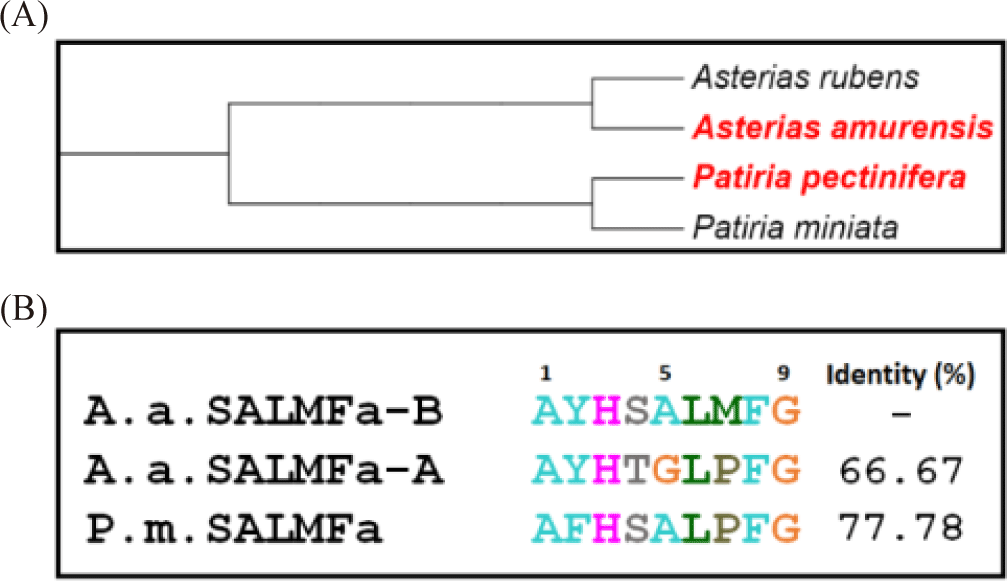
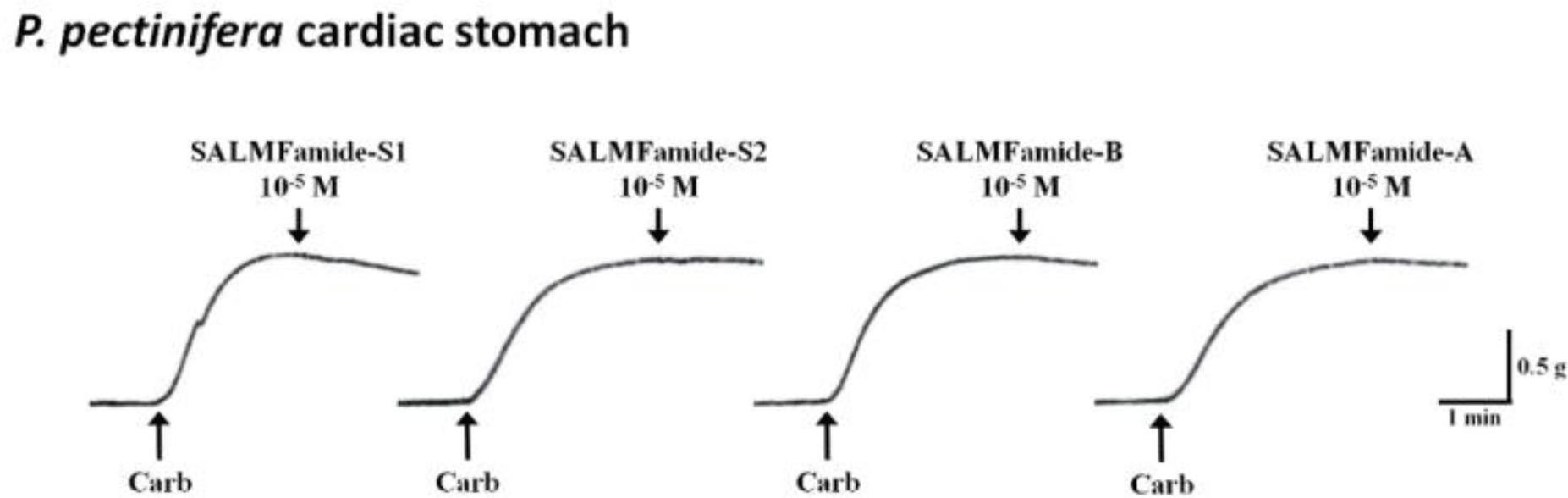
In this study, the SALMFamide peptide isotype and its analog bioactivity was described for the first time. All these obtained results suggest that derivative peptide SALMFa-B has equal or potent relaxing activity compared to native SALMFa-A peptide activity on apical muscle of the starfishes A. amurensis and P. pectinifera, respectively. The starfish A. amurensis is phylogenetically close to A. rubens compared to starfish P. pectinifera species. Meanwhile, P. pectinifera is phylogenetically close to Patiria miniata (Fig. 4A). Therefore, the sequences of derivative SALMFa-B peptide were compared with native SALMFa-A peptide of A. rubens, and SALMFa peptide of P. miniata (Fig. 4B). Firstly, comparison revealed the 66.67% identity between the derivative SALMFa-B and native SALMFa-A peptide of A. rubens. The lower similarity of these two peptides may explain the relatively equal lower relaxing effect on A. amurensis apical muscle preparation. Secondly, the comparison between the derivative SALMFa-B peptide of A. rubens and SALMFa peptide of P. miniate showed the 77.78% identity. These relatively higher sequence similarities may elucidate the relatively higher relaxing effect of derivative SALMFa-B of A. rubens on apical muscle of P. pectinifera compared to those of native SALMFa-A peptide. On the other hand, the SALMFa-A and SALMFa-B peptides did not show any activities on tube feet preparation of both starfishes A. amurensis and P. pectinifera (data not shown). Whereas, the slight relaxation activities were noticed on P. pectinifera cardiac stomach preparations at 10–5 M concentration (Fig. 5). All these results suggest that native SALMFa-A peptides possess main activity on apical muscle that is used by starfish to movement. The derivative SALMFa-B (AYHSALMFamide) peptide with three amino acid substitutions (Ser4, Ala5, Met7) showed relatively high activity than native SALMFa-A (AYHTGLPFamide), suggesting that C-terminal SALMFamide motif has more effectivity on relaxing activity than GLPFamide motif. Also, it seems that the C-terminal region plays a more important role in the receptor binding of starfish smooth muscles than the N-terminal region of SALMFamide peptides. The both SALMFamide-S1 and SALMFamide-S2 showed 100% relaxing response at 10–5 M concentration on A. rubens cardiac stomach, meanwhile, in this study, SALMFamide-S1 and SALMFamide-S2 did not show significant relaxing response at 10–5 M on P. pectinifera cardiac stomach (Melarange et al., 1999). These results imply that there are dramatic variances on the SALMFamide’s receptor structures in the cardiac stomach of these starfishes. On the other hand, the experiments were carried out under different conditions, which can also affect the results. The relaxing effect of SALMFamide-S1 and SALMFamide-S2 on A. rubens apical muscle preparation was determined as minor effect compare to relaxing effect on A. rubens cardiac stomach. The relaxation activity of SALMFamide-S1 and SALMFamide-S2 on A. rubens apical muscle at 10–5 M was 11% and 32%, respectively (Melarange & Elphick, 2003). These results are comparable to the relaxation activity of SALMFamide-S1 and SALMFamide-S2 on P. pectinifera and A. amurensis apical muscle shown in this study. Therefore, these results suggest that SALMFamide-S1 and SALMFamide-S2 do not show the trivial activity on A. rubens, A. amurensis and P. pectinifera apical muscle preparations.