Introduction
Free radicals and other reactive oxygen and nitrogen species can cause oxidative damage and tissue dysfunction, but they also are molecular signals that activate stress responses beneficial to an organism (Venditti & Di Meo, 2020). In some cases, the body’s antioxidant system can stabilize some of these free radicals. However, when free radicals are excessively produced or induced by stress, they trigger oxidative stress to lipids, proteins, and DNA. This oxidative stress results in structural and functional damage to cells, which consequently leads to chronic and degenerative diseases like cancer, rheumatoid arthritis, chronic inflammation, and aging (Ahmadian et al., 2020; Uttara et al., 2009). Similarly, inflammation is a protective response for maintaining equilibrium and balance in the body against tissue damage and infection by external stimuli (Kim et al., 2012). However, continuous inflammation eventually results in cell tissue damage by increasing the generation of cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), interleukin-6 (IL-6), and IL-1β. Excess production of COX-2, iNOS, IL-6, and IL-1β can cause asthma, brain disease, Alzheimer’s disease, arteriosclerosis, cancer, and aging (Graça et al., 2020; Kwon et al., 2015).
Hyaluronic acid (HA) is an essential component of the extracellular matrix and it is involved in cell adhesion, cell proliferation, cell differentiation, and cellular signaling. HA has been extensively used in biomedical applications, such as abdominal surgery, ophthalmology, dermatology, rhinology, and osteoarthritis (OA) therapy (Ondrésik et al., 2017; Ürgeová & Vulganová, 2016). HA and its derivatives have been mostly used in the development of several carrier systems for cancer diagnosis and treatment because they act as a signal molecule in various immunological processes. Long chains of HA effectively regulate immune responses, stimulate angiogenesis, and initiate proinflammatory cytokine synthesis. The insoluble form of HA is a potential drug matrix and it is used in medicine (Illiás et al., 2011). HA can be extracted from a variety of sources. For example, marine animals with a gelatinous body matrix such as the leptocephali (larvae stage) of eels and bonefish are rich in sulfated glycosaminoglycans (GAGs) and HA (Pfeiler et al., 2002).
Recently, the search for bioactive compounds with pro-health functions has turned its attention towards other lower forms of marine life such as seaweed. Furthermore, studies have shown that it is possible to increase the stability and shelf life of HA by grafting it with synthetic aliphatic polyesters such as poly lactic acid Son et al., 2014) and poly caprolactone (Chen et al., 2017). In this study, HA extracted from cubed snailfish (Liparis tesselatus) eggs was grafted with caffeic acid (CA), ferulic acid (FA), gallic acid (GA), and nisin. The antioxidant and anti-inflammatory properties of the grafted samples were investigated for their potential in cosmetic, nutraceutical, and pharmaceutical applications.
Materials and Methods
GA, CA, FA, N-hydroxy succinimide (NHS), N-ethyl-N’-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC), phosphate buffered saline (PBS) tablets, 4-morpholineethanesulfonic acid, sodium chloride (NaCl), sodium carbonate (Na2CO3), hydrochloric acid (HCl), adipic acid dihydrazide, and ethylenediaminetetraacetic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). Nisin Z (90% purity) was purchased from Anhui Minmetals Development (Anhui, China). All other chemicals and reagents used in this study were high performance liquid chromatography-or analytical-grade.
RAW 264.7 murine macrophage cell lines were purchased from the Korea Cellular Bank (KCLB, Seoul, Korea). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), L-glutamine, penicillin, and streptomycin were purchased from Thermo Fisher Scientific (Waltham, MA, USA). A CellTiter 96® Aqueous One Solution Cell Proliferation Assay (MTS) kit was acquired from Promega (Madison, WI, USA) and a PGE2 EIA Monoclonal kit was acquired from Cayman Chemical (Ann Arbor, MI, USA).
HALTE was extracted following the method of Ticar et al. (2015). First, a 10 mg/mL aqueous solution of crude polysaccharide from LTE was filtered with a 0.2 μm membrane. Then the solution was passed through a DEAE-Sepharose CL-6BTM(Sigma-Aldrich, Inc., St. Louis, MO, USA) column (2.5 × 30 cm), which was equilibrated with 0.5 M sodium phosphate buffer (pH 6.5) with a linear gradient of 0 to 2.0 mol/L NaCl in the same buffer at a flow rate of 0.5 mL/min. The fractions were checked for sugar content and combined. The three obtained fractions were labeled as F1, F2, and F3. Then they were dialyzed with a Pellicon UF membrane (molecular weight cut-off [MWCO]: 10 kDa; Millipore, Burlington, MA, USA) against deionized water to remove dissolved solids and then they were lyophilized. The F2 and F3 fractions contained 20.2% to 26.8% sulfated GAGs, 34.5% to 45.0% uronic acids, and 7.5% to 23.5% hexosamines. Electron spray ionization-mass spectroscopy confirmed the composition of the F3 fraction to be mainly HA, as reported by Ticar et al. (2017).
Phenolic acids (GA, CA, and FA) and nisin were grafted onto HALTE as described by Nguyen et al. (2020). Reaction mixtures for each phenolic acid were prepared by adding a 30-fold molar excess of GA, CA, and FA to HALTE (3 mg/mL) solutions. The reaction mixtures were adjusted to pH 6.8 and then supplemented with EDC (1 mmol) and 1-hydroxybenzotriazole (1 mmol) in a dimethyl sulfoxide (DMSO)/H2O (1:1, 1 mL) solution. The pH was maintained at 6.8 while the reaction continued for 12 h. Then the pH was neutralized, and the reaction mixtures were dialyzed (MWCO: 14 kDa) against distilled water for 72 h. For nisin, a 2 mg/mL HALTE solution was made by dissolving HALTE in 40 mL of PBS solution (pH 7.4) and constantly stirring overnight. Next, 500 μL of NHS and EDC were added and continuously mixed for 30 min. Then the nisin (1.75 mg/mL in 0.02 M HCl) solution was added to achieve a final concentration of 0.01 equivalent nisin for each carboxylic acid of HALTE (approximately 5 mL). The pH was adjusted to 7.4 and a final volume of 50 mL was obtained using PBS as a diluent. The reaction mixture was stirred constantly under a continuous flow of oxygen-free nitrogen gas for 24 h at room temperature. After stirring, a NaCl (0.1 M) solution was added to the reaction mixture, which was then dialyzed (MWCO: 14 kDa) against distilled water. Finally, the dialysates were lyophilized to obtain phenolic acid-grafted HAs, which were labeled GA-g-HALTE, CA-g-HALTE, FA-g-HALTE and Nisin-g-HALTE. The grafting ratios were measured, and their structure were determined by Fourier transform infrared, and proton nuclear magnetic resonance (Nguyen et al., 2020).
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity (RSA) was determined according to Liu et al. (2010) with slight modifications. First, GA-g-HALTE, CA-g-HALTE, FA-g-HALTE, and Nisin-g-HALTE solutions were prepared at various concentrations (0.1, 0.5, 1.0, 5.0, and 10.0 mg/mL). Next, 50 μL of each sample solution was added to a 200 μL methanolic DPPH solution (0.4 mM). The reaction mixture was left in the dark for 30 min at room temperature. The absorbance was measured using a microplate reader (SpectraMax M2, Multi-Mode Microplate Reader, Molecular Devices, Sunnyvale, CA, USA) at 517 nm. The DPPH RSA was computed using the equation below:
where, Abs0 is the absorbance of the control, which is water instead of sample, Abs1 is the absorbance of the sample, and Abs2 is the absorbance of the sample under identical conditions as Abs1 but with methanol instead of DPPH solution.
The 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) RSA was evaluated with modifications to the method presented by Rapta et al. (2009). First, 17.2 mg of ABTS was mixed with an aqueous solution of K2S2O8 (3.3 mg/5 mL H2O) and allowed to react overnight in the dark at 4°C. Next, 1 mL of the resulting radical cation solution was diluted with H2O to 60 mL. For the analysis, 100 μL of each sample solution at different concentrations (0.1, 0.5, 1.0, 5.0, and 10.0 mg/mL) was mixed with 2 mL of the diluted ABTS radical cation solution and incubated in the dark for 1 h at room temperature. The absorbance was measured at 734 nm using a microplate reader (SpectraMax M2). The ABTS RSA was calculated with the following equation:
where Abs0 is the absorbance of a standard prepared under the same conditions but without any sample, and Abs1 is the absorbance of the sample solution.
RAW 264.7 macrophage cells were cultured in DMEM supplemented with 10% FBS, 4 mM L-glutamine, 100 U/mL penicillin, and 100 U/mL streptomycin. Then they were incubated at 37°C in humid conditions with 5% CO2 until they reached 80% to 90% confluency. After that, the cells were treated with GA-, CA-, FA-and Nisin-g-HALTE for 24 h. Next the cells were treated with MTS solution (0.5 mg/mL, CellTiter 96® Aqueous One Solution Cell Proliferation Assay kit) for 4 h (5% CO2 at 37°C), and then the purple formazan crystals were solubilized with 100 μL of DMSO. Finally, the cell viability was measured with a microplate reader (SpectraMax M2) at 490 nm absorbance.
RAW 264.7 macrophages (1 × 106 cells/mL) were grown in 96-well plates with 100 μL of medium (phenol red-free DMEM medium supplemented with 10% FBS) containing sample solutions and lipopolysaccharide (LPS) (1 μg/mL) at 37°C for 24 h. The nitrite content of the cell cultures was determined using the Griess reaction. In this assay, 50 μL aliquots of supernatants and 50 μL of Griess reagent were mixed in 96-well plates at room temperature for 10 min. The absorbance at 540 nm was determined by an automated plate reader (SpectraMax M2). Then the concentration of nitrite was calculated according to a standard curve made with known concentrations of sodium nitrite prepared in phenol red-free DMEM medium (Kwon et al., 2015).
Prostaglandin E2 (PGE2) was measured using a PGE2 EIA Monoclonal kit. First, 50 μL aliquots of diluted spent supernatant were added to a 96-well goat anti-mouse immunoglobulin G coated plate and incubated for 18 h at 4°C. Each well in the incubated plate was washed with a commercial wash buffer. Then 200 μL of Ellman’s reagent (Cayman Chemical) was added and the plate was shaken for 90 min in the dark to develop color. The PGE2 quantity was calculated according to a PGE2 standard curve (Micali et al., 1996).
Results and Discussion
Variations between the DPPH and ABTS RSA of the grafted HALTE samples were observed. These variations may be due to the chemical structures of the grafted components or the influence of free hydroxyl groups surrounding the molecule. Phenolic acids like GA, CA, and FA are known antioxidants that form resonance-stable phenoxy radicals based on their structures (Illiás et al., 2011). The scavenging activities of the grafted HALTE samples against DPPH radicals are shown in Fig. 1. Results showed that their activity increased as the concentration of grafted HALTE increased, and the GA-g-HALTE sample exhibited the highest DPPH RSA followed by CA-g-HALTE, FA-g-HALTE, and Nisin-g-HALTE. According to Eom et al. (2012), the DPPH RSA of CA-conjugated chito-oligosaccharides (CFA-c-COS) was highest, followed by sinapinic acid-c-COS, ferulic acid-c-COS and p-coumaric acid-c-COS, where CFA-c-COS demonstrated over 80% scavenging against DPPH radical at concentrations of 100 μg/mL.
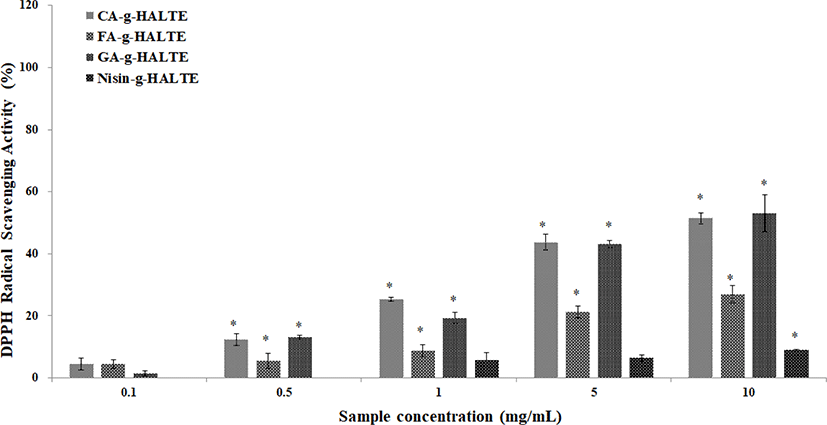
The ABTS RSA of the grafted HALTE samples are shown in Fig. 2. The ABTS RSA showed concentration-dependent increases, and these increases were higher than those of the DPPH RSA. As showed in Fig. 2, FA-g-HALTE had the highest ABTS RSA followed by CA-g-HALTE, GA-g-HALTE, and Nisin-g-HALTE. ABTS RSA measures the hydrogen-donating capacity of lipophilic and lipophobic antioxidants, especially phenolic compounds, to combat free radicals, which are related to oxidative stress, lipid peroxidation, and neurodegenerative diseases (Oliver et al., 2016).
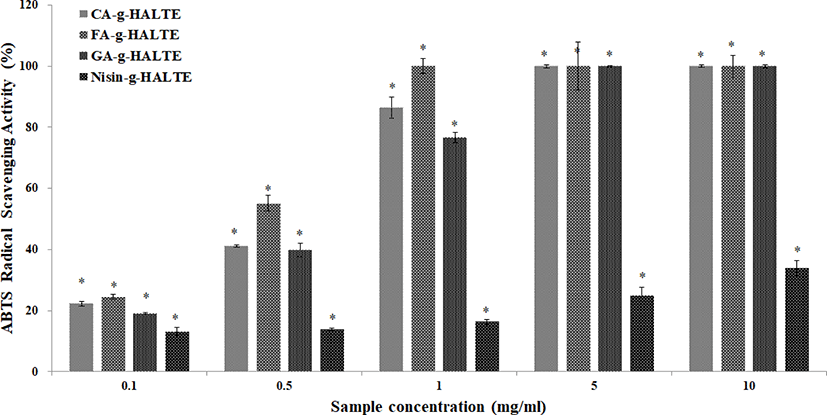
GA-g-HALTE is a hydro-benzoic acid derivative with three hydroxyl groups in its phenol constituent while CA-g-HALTE and FA-g-HALTE are hydro-cinnamic acid derivatives with two and one hydroxyl groups, respectively. The minimal DPPH RSA and lower ABTS RSA shown by Nisin-g-HALTE could be the result of the nisin constituent’s bulky peptide structure and hydrophobic nature. Nisin is a unique cationic and hydrophobic antimicrobial peptide consisting of 34 amino acids with carboxyl end groups and five internal thioether ring structures bridged by disulfide bonds (Cheigh & Pyun, 2005). It is mainly known for its effective inhibition of gram-positive bacteria (Shin et al., 2016). Comparatively, GA-g-HALTE, FA-g-HALTE, and CA-g-HALTE have smaller structures and are more hydrophilic due to additional hydroxyl groups from the phenolic acids.
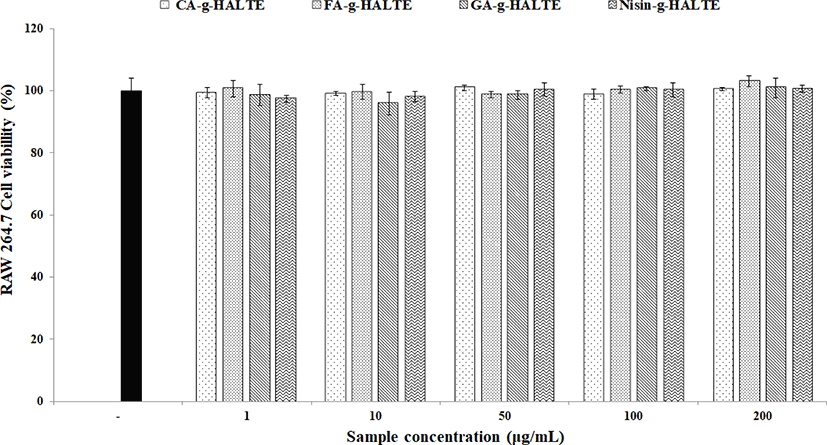
To eliminate the possibility of false anti-inflammatory activities caused by apoptosis, the toxicity of the samples on the RAW 264.7 macrophages was determined via a MTS assay. The remaining viable cells were estimated after treatment with each experimental concentration (1, 10, 50, 100, and 200 μg/mL) of GA-g-HALTE, CA-g-HALTE, FA-g-HALTE, and Nisin-g-HALTE. The results of the MTS assay are displayed in Fig. 3. Although the survival rate of the RAW 264.7 macrophage cells varied in each sample treatment, there were no significant differences compared to the negative control. All concentrations of the phenolic acid-grafted samples, GA-g-HALTE, CA-g-HALTE, and FA-g-HALTE, had no cytotoxic effect on the RAW 264.7 macrophage cells and showed a cell viability of 95.9% to 103.1% after 24 h of treatment. Additionally, no changes were detected in the macrophage cells treated with 1, 10, 50, and 100 μg/mL Nisin-g-HALTE, with 97.4% to 101.1% cell survival after 24 h of treatment.
Inflammation plays a crucial role in the pathogenesis of many diseases including atherosclerosis and arthritis. Nitric oxide (NO) is a small gaseous and multifunctional signaling molecule involved in maintaining metabolic and cardiovascular homeostasis. It is produced by NO synthases in the vascular endothelium. Decreases in NO lead to increases in endothelial dysfunction and cardiovascular risk (Pignatelli et al., 2020). Fig. 4 displays that LPS-stimulated RAW 264.7 macrophages generate less NO as the GA-g-HALTE, CA-g-HALTE, and FA-g-HALTE treatment concentrations increase (1, 10, 50, 100, and 200 μg/mL). Since NO is a free radical that serves as signaling molecule in cells throughout the body, this observed decrease in NO production suggests less macrophage activity and the potential to suppress chronic inflammation. These results imply that the phenolic constituents present in the grafted samples could help regulate inflammation by reducing reactive nitrogen species formation.
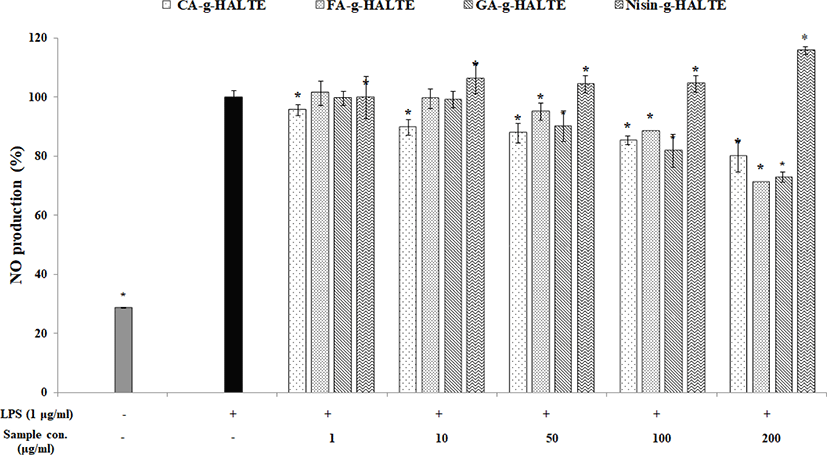
On the other hand, LPS-induced macrophages treated with Nisin-g-HALTE had increased NO content (100%–116%) depending on the treatment concentration. Nisin-g-HALTE displayed the potential to activate NO generation in RAW 264.7 macrophages, which could also contribute to immune enhancement. NO could react with superoxide anions (O2−) to generate peroxynitrite, which is involved in proinflammatory and proapoptotic effects in cartilage. Similarly, another study showed that bovine chondrocytes incubated with IL-1β showed increased intracellular peroxynitrite and superoxide anion generation (Virág et al., 2003). Peroxynitrite exposure enhances cytokine-induced nuclear factor (NF)-κB activation whereas incubation with NO inhibits cytokine-induced NF-κB activation. Peroxynitrite also has proapoptotic effects on cartilage. It can induce mitochondrial dysfunction in cells in a calcium-dependent manner and can trigger caspase-independent apoptosis, which illustrates the influence NO derivatives have on the destructive effects of OA (Abramson, 2008).
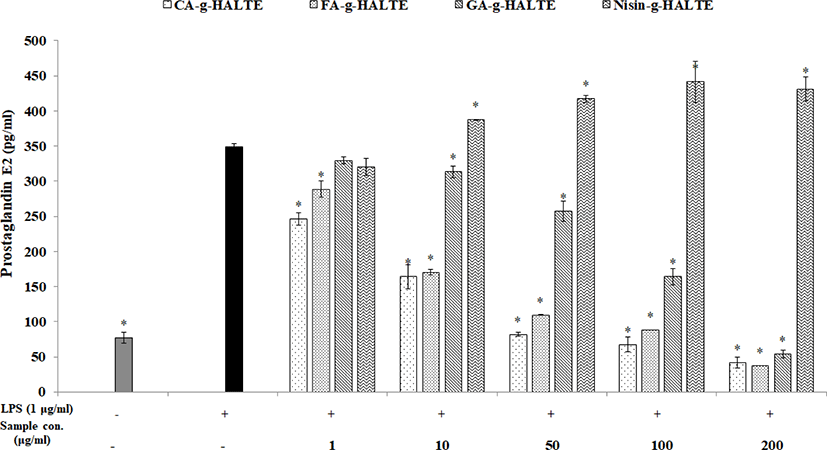
Like NO, nonsteroidal anti-inflammatory drugs are used to treat acute and chronic inflammatory disorders. PGE2 is an endogenous hormone essential for female reproductive functions. However, under pathological conditions, PGE2 can cause endometrial tissue damage by stimulating the secretion of pro-inflammatory cytokines and various chemokines to promote inflammatory responses while acting as an inflammatory mediator (Sheldon et al., 2014). RAW 264.7 macrophages were treated with concentrations of GA-g-HALTE, CA-g-HALTE, FA-g-HALTE, and Nisin-g-HALTE ranging from 1 to 200 μg/mL to determine which concentrations were safe. As shown in Fig. 5, CA-g-HALTE and FA-g-HALTE above 100 μg/mL were effective, although they were active in all the samples used. Increased concentration resulted in increased activity except with 100 μg/mL Nisin-g-HALTE. However, Nisin-g-HALTE showed a low content of 320.4 pg/mL at a low concentration of 1 μg/mL, but with a higher concentration of 10 μg/mL, the concentration was higher than in LPS at 387.7 pg/mL. According to the results, the addition of LPS significantly increased cell death rates and enhanced NO production in RAW 264.7 macrophages. However, treatment with grafted HALTE significantly and dose-dependently reduced LPS-induced toxicity and damages to the cells.
NO has been shown to be a catabolic factor responsible for inhibiting collagen and proteoglycan synthesis, and inducing apoptosis through proinflammatory cytokine mediation, which ultimately perpetuates OA (Abramson, 2008). However, recent studies suggest that NO and its redox derivatives may also have protective effects on cartilage. For example, blocking IL-1 receptors reduced pain and global disability in hand and knee OA patients, though these patients displayed upper respiratory tract infections (Chevalier et al., 2013). IL-1 and tumor necrosis factor-α upregulate COX-2 and iNOS expression, which in turn induce PGE2 and NO synthesis in chondrocytes (Goldring & Berenbaum, 2015).
Conclusion
In this study, we extracted HALTE and grafted it with phenolic compounds (CA, FA, and GA) and nisin. The effect of these grafted HALTEs on the antioxidant and anti-inflammatory activities of LPS-stimulated RAW 264.7 cells were evaluated. The grafted HALTEs showed strong radical scavenging and anti-inflammatory activities in both the in vitro and in vivo models. Results showed that the antioxidant and anti-inflammatory properties of the grafted HALTEs were evaluated, then could be applied as promise solution for any potential biomedical applications.








