Introduction
Fisheries management must be directed to maintain the fish populations remain sufficiently abundant to minimize extinction risk and sustain intact ecosystems (Freshwater et al., 2020). Fish reproduction is an important aspect in maintaining the equilibrium of the fish stock population in the water since stock recovery is highly dependent upon reproductive success that is closely related to environmental changes particularly temperature, photoperiod, and food supply (Jonsson & Jonsson, 2014). Thus, fecundity, sexual maturity, and spawning habits must be understood to explain the variation of the population level to increase the amount of fish harvest and maintain the recovery rate (Brown et al., 2003; Das et al., 1989).
Dolphinfish, Coryphaena hippurus(Linnaeus, 1758) (Coryphaenidae), known as mahi-mahi, is a commercially important species in tropical and temperate waters worldwide (Benjamin & Kurup, 2012) in line with the tuna catch decline in the Indian Ocean (IOTC, 2012). This species has a sufficiently large size, the young one is about 30 cm long and the adults can reach 200 cm long with bodyweight up to 50 kg. The individual weight of fish caught ranges from 7 to 13 kg and rarely reaches 15 kg. The species is caught as bycatch in several types of fishing gears, such as purse seine, longline, and trolling (Chodrijah & Nugroho, 2016).
C. hippurus is a long-range and fast swimming fish that displace with time and is an opportunistic epipelagic predator and preys on biota associated with a fish aggregating device (FAD) and floating debris, such as fish, squids, and shrimps (Malone et al., 2011; Whitney et al., 2016). C. hippurus can stay several days in association with a raft (Taquet et al., 2007). Dolphinfish spend > 80% of daytime activity and 40% of nighttime activity near the surface (Lin et al., 2020) and inhabit warmer seawater temperatures of 24°C–30°C (Palko et al., 1982; Schlenker et al., 2021). When surface sea temperature rises, dolphinfish use behavioral thermoregulation by moving deeper up to 250 m, and the nighttime activity increased with increasing lunar illumination (Schlenker et al., 2021). The IUCN status of dolphinfish is the least concerned (Carlson et al., 2020).
This study is aimed at estimating the size at first maturity of dolphinfish C. hippurus caught in the Molucca Sea, North Sulawesi. Size at first maturity is the smallest size of mature legally taken, the size at which 50% of the individuals are sexually mature (Farley et al., 2013). Knowledge of length at maturity and spawning season is important for the proper management and conservation of fish stocks (Nandikeswari, 2016). Size at first maturity is commonly evaluated for wild populations as a point of biological reference to ensure that a sufficient number of juveniles reaches maturity (Roa et al., 1999) because only fishing the individuals which have reached maturity is one of the basic rules that should be followed to ensure sustainability (Ilkyaz et al., 2018). It has been utilized in various exploited animals, such as crustaceans (Peixoto et al., 2018), fish populations (Tesfahun, 2018), and mollusks (Galimany et al., 2015), to protect juveniles, let them grow into adults, and spawn at least once before being caught. Proper estimation of size at first maturity is very useful for fish stock management (Karna & Panda, 2011). These data provide basic information on fish biology that is crucial for dolphinfish fisheries management in Indonesian waters and other neighboring countries.
Materials and Methods
Dolphinfish C. hippurus samples were mainly collected from fishermen in the Kalinaun coast, East Likupang district, North Minahasa, North Sulawesi. The fish samples were obtained from May to July 2021, because there was no catch after this period. Fishing activity was conducted near a man-made FAD in the Molucca Sea located in the northeastern part of the village between 125°11’24’’ E and 125°13’48’’ E and 1°35’24’’ and 1°35’24’’ N. Local fishermen usually used live bait-handline. Live baits were obtained in the multi-hooks handline fishing before daybreak. Trolling was also carried out around the FAD to obtain more samples. The fish were sexed on the beach. The fork length (FL) and weight were also recorded, then the gonads were removed and brought to the Laboratory of the Faculty of Fisheries and Marine Sciences, Sam Ratulangi University, Manado, for further observation. The estimation of sex ratio used a non-parametric comparative test Chi-square (x2, α = 0.05). Gonadal maturation was observed under a dissecting microscope. The fish maturity stage was identified following Effendie (2002) (Table 1).
The first gonad maturity was estimated by setting the size class intervals, from the smallest to the largest one. Length distribution analysis followed Sturges (1926) as follows:
where k is number of classes and n is number of data. The class interval was estimated as
where C is class interval, Xn is the largest data value, X1 is the smallest data value, and k is number of classes.
Spearman-Karber equation was applied to estimate the size at first maturity of the fish (Udupe, 1986) as follows:
where xk = log last size in which 100% fish are fully mature
x = log size increment = xl+1 – xl, l = 1, 2, …, k–1
and xo = log last size in which no fish are fully mature
rl = number of fully mature fish in size group i
pi = proportion of fully mature fish in size group i
pl = rl/nl, if nl ≠ nl+1 for i = 1, 2, …, k–i
and pl = rl/n, if n = nl = nl+1 for i = 1, 2, …, k–i.
Size at first maturity was obtained with antilog (m) = M.
Results
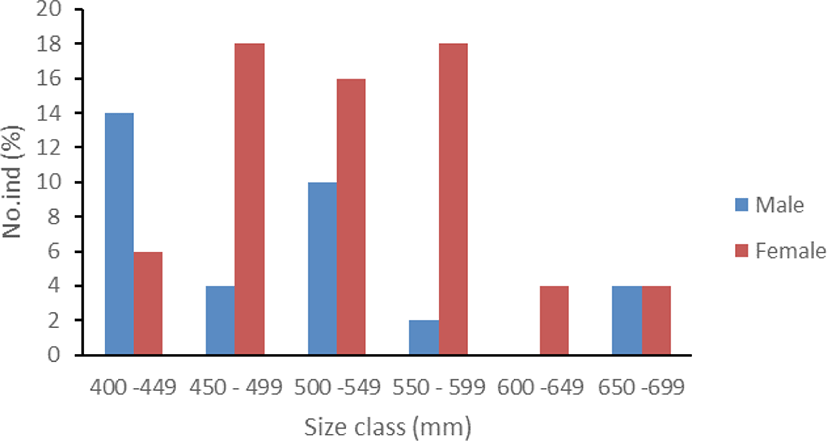
During the study 50 fish individuals were collected from local fishermen in Kalinaun coast, East Likupang district, North Minahasa, North Sulawesi. Males had a size range of 405–674 mm FL with a weight range of 670–1,640 g, and females were at a length range of 431–687 mm FL with a weight range of 725–2,650 g. Based on Sturges (1926), the length distribution was divided into 6 size classes (Fig. 1).
Sex ratio information is useful to maximize reproduction. The present study found a sex ratio of 1:1.94 (p < 0.05) represented by 17 males and 33 females. Gonad maturity of this species shows that more females mature at a smaller size than males (Table 2).
Size at first maturity was estimated as 529 mm FL for males with a range of 475–588 mm and 405 mm FL for females.
Discussion
This low number of catches could result from that C. hippurus is not a target species. Local fishermen in this area go fishing for yellowfin tuna, marlin, and sharks, whereas C. hippurus is optional when the target fish are not found due to the low local market value of this species. Field observations also revealed that the occurrence of C. hippurus in this region is seasonal. Besides, although the fish are around, they did not bite at all in trolling or live bait fishing. Only a few individuals of C. hippurus are caught, usually 1–5 individuals per boat. However, there is still no study on the fishing season of C. hippurus, particularly in this area.
A previous study on dolphinfish landing in the Bitung Fisheries Port found 4,160 individuals of C. hippurus in the size range of 300–1,210 mm FL with a mean length of 598 ± 13.9 mm FL (Chodrijah & Nugroho, 2016) reflecting small size dominance. The fish samples came from catches of many kinds of fishing gears, such as purse seine, longline, and trolling. The present study found narrower size distribution, and it could result from less number of samples obtained due to high dependence on local artisanal fishermen who rely on hand-line fishing.
The present size range is far below the maximum individual size previously reported reflecting that the mean individual size of C. hippurus has been declining. The recovery rate of a population is related to the mortality rate, the closer the mean individual size to the maximum, the lower the mortality rate (ECTF, 2004). The present finding revealed that the dolphinfish population has a high mortality rate. However, so many factors influence fish population availability in the ocean. This condition is supported by Goldstein et al. (2007) that life-history traits are vulnerable to environmental stress and fishing pressure that result in smaller mature fishes as a response for survival. Fish mortality could occur at specific stages and species and the causes may be single or cumulative pressure from a range of sources, such as pollutants, anthropogenic climate change or natural variability (Olsen et al., 2019), and fishing activities. Recruitment patterns with time can influence the population size as well, and therefore, mortality events in the early life stages may have severe and long-lasting effects on the population (Langangen et al., 2017). Climate change is another factor causing changes in fish populations, which can affect the distribution of particular species and the fish susceptibility to particular fishing fleets (Rijnsdorp et al., 2009). This condition could occur because population size has probably fallen below some threshold level of abundance in which the rate of recovery cannot well respond to the fishing rate.
This sex deviation is similar to that reported in the western and central Mediterranean (Benseddik et al., 2019; Potoschi et al., 1999) reflecting sex segregation in C. hippurus until reaching the mature stage. Mature individuals seem to gather in the same area for spawning and feeding around the rafts so that more females were caught than males. This result also agrees with Perle et al. (2020) and Oxenford (1999) that sex segregation occurs in C. hippurus or males are more susceptible to fisheries than females, even though our finding found more females than males. A higher proportion of females from FADs captures could result from greater availability of females, higher natural mortality in males, or differential growth of both sexes (Benseddik et al., 2019). Moreover, males and females show different maturity stages with size class (Table 2). Both sexes show bigger individual sizes than 400 mm FL with more females at mature stages (III and IV). It indicates that males need a bigger size to reach gonad maturity or females reach gonad maturity earlier than males. These data are consistent with Beardsley (1967) that female dolphinfish begin to mature (reach stage II) at about 350 mm FL (about 6–7 months old), 50% are mature at 450 mm FL, and 100% are mature at 550 mm FL, whereas males are mature at a slightly larger size (427 mm FL). Nevertheless, in the Eastern Tunisian coast, Central Mediterranean, Benseddik et al. (2019) found that the first maturity size of C. hippurus occurs at 553 mm FL for females and 605 mm FL for males. In the present study, females above 400 mm FL reached maturity stages III and IV. This difference could result from different environmental conditions in localities. It means that 50% of mature individuals that occurs at this size, particularly in the Molucca Sea population, could be set as the minimum legal size of this species to meet the sustainability criteria and avoid economic loss due to fishing immature individuals. The size range of C. hippurus caught in the Molucca Sea reflects mature individuals and has mostly passed the size at first maturity. Nevertheless, since fishing is a major factor in reducing size and age at first maturity (McIntyre & Hutchings, 2003) and a decline in age and size at maturity may negatively affect the fish recovery (Hutchings, 2002), it needs to be controlled. The individual size decline of C. hippurus far below the maximum size could have indicated a reduced population size and should not be ignored. Earlier maturity can be associated with reduced longevity, increased post-reproductive mortality, and smaller sizes at reproductive age. Populations composed of small individuals will reduce reproductive potential (Scott et al., 1999), increase variance in offspring survival (Hutchings & Myers, 1993), and eventually negatively affect population growth.
Mesh size control and escapement could be an alternative to maintain or increase the individual size range or even increase the longevity, and the reproductive potentiality of dolphinfish. Larger fish have higher fecundity and can produce more eggs. So far, commercial purse seiners (< 30 GT) for small pelagic fish have fished any fish schools encountered in the open sea using small mesh sizes. As a result, small yellowfin tuna, skipjack, and dolphinfish are also caught (field observation). Fishing gear separation should be established for commercial small pelagic and large pelagic fisheries to maintain stock availability and prevent individual size decline. This effort limitation could help reduce the risk of population collapse and become one of the remedies to population recovery. Fish population recovery, therefore, requires institutional structures that either entice fishers to leave the business, through expensive buyout schemes of fishing boats and licenses or force them to reduce fishing activity (Hutchings & Reynolds, 2004).
The present study has contributed to providing important biological information for future management, especially dolphinfish C. hippurus of Molucca Sea. A long-term study on the biology and ecology of this species is required to well describe the population status of C. hippurus so that the management policy could be strengthened. The fisheries committee among neighborhood countries that take advantage of the resources should also participate in sustainable resource utilization programs by maintaining the exploitation level and the ecosystem equilibrium.