Introduction
Lake Shala is known for its extreme environmental condition and is characterized by a high surface water temperature (26°C) (Ogato, 2015), pH (9.65), saline-alkaline conditions (18.1 g/L, 224 meq/L) and high phosphate content (2.73 mg/L1) (Kebede et al., 1994), but with remarkably low nitrogen levels (NH4+, 4.3 μg/L) (Kebede et al., 1994). Despite this extreme nature of the environment, Lake Shala supports Oreochromis niloticus and Aplocheilichthys sp. (Golubtsov et al., 2002).
Nile tilapia, O. niloticus, is a popular fish species in Africa for commercial and subsistence fisheries (Opiyo et al., 2018). In Ethiopia, O. niloticus constitute a bulky percentage of the capture fishery and the commercial inland fish catch (Degsera et al., 2020; Teame et al., 2018). O. niloticus also constitutes an irreplaceable piece of the food chain for local populations residing around lakes (Degsera et al., 2020) and supports substantial populations of fish-eating birds in East African saline-alkaline lakes (Kavembe et al., 2016).
Studies on the length-weight relationships (LWR) and condition factor (CF) of the species are vital in fisheries science (Murua et al., 2017). The LWR can give evidence on the growth patterns and used to forecast weight from length measurements made in the yield assessment (Zuh et al., 2019). It has been widely used to evaluate index of well-being of fish and providing information on growth patterns (Degsera et al., 2020). But the CF indicates the appropriateness of a specific aquatic ecosystem for growth of fish and the physiological state of the fish (Mondal & Chakravartty, 2016). Information on the LWR and CF of O. niloticus has been reported by Hirpo (2012) in Lake Beseka, Ethiopia, Mortuza & Al-Misned (2013) in Wadi Hanifah, Riyadh, Saudi Arabia, and Tessema et al. (2019) in Lake Hayq, Ethiopia. Conferring to these studies, LWR and CF are affected by various factors like stress, age, season, sex, sexual maturity, availability of food sources, and other water physico-chemical parameters. The study of morpho-metric relationship, growth patterns, CFs, and related environmental issues of fish species are the most significant biological parameter for the management and conservation of fish inhabitants (Mondal & Chakravartty, 2016).
O. niloticus can reproduces incessantly throughout the year, but in some, breeding more exhaustive during periods of strong sunshine and rainfall (Tessema et al., 2019). However, all fish species have different passions of breeding time in the year, which governed by the accessibility and quality of food, water level fluctuation, and seasonal differences in water temperature (Teame et al., 2018). Therefore, information on the influence of season on the reproductive biology of O. niloticus can provide basic knowledge for the appropriate management and conservation of the fish populations.
In Ethiopia, the morphometric relationship, growth patterns, reproduction strategies and breeding seasons of O. niloticus in lakes, including the Rift Valley Lakes, are well documented (Admassu, 1990; Kebede et al., 2018; Teferi & Admassu, 2002; Tesfaye & Tadesse, 2008). Almost no such data is available for the same species in Soda Lakes (Kavembe et al., 2016). O. niloticus of Lake Shala have been reported by Golubtsov et al. (2002) and Klemperer & Cash (2007); the latter reported Aplocheilichthys species retrieved after seismic explosions in the lake. Therefore, our objectives were to study the LWR, growth performance, sex proportion, CF, fecundity rate and breeding seasons of O. niloticus in Lake Shala. By analyzing the aspects of the growth pattern and reproductive biology of O. niloticus, we are generating information fundamental for conservation and management of the fish in Soda Lake, Lake Shala, Ethiopia.
Materials and Methods
Lake Shala is on Ethiopian Central Rift Valley, lie approximately between 7°24’ to 7°33’N and 38°23’ to 38°39’E at an altitude of 1,558 m above sea level within the Abijata-Shala Lakes National Park (Fig. 1), 207 km South of Addis Ababa. The lake is around maximum 266 m deep and has an area of 329 km2, with a massive catchment range (3,920 km2) (Baxter, 2002). The lake region has high evaporation rate (781 million cubic meters) that surpasses the annual mean rainfalls (232 million cubic meters) (Ayenew & Legesse, 2007). The climate is mostly classified by alternating wet and dry seasons following the annual activities of the Intertropical Convergence Zone (Ayenew & Legesse, 2007), with the dry season from October to February and a rainy season from March to September. The rainy season is characterized by a bimodal rainfall pattern, with a minor rainy period ranging from March to May and a heavy rainy period from June to September.
Specimens of O. niloticus were collected using Gillnets (4, 6, 8, 10, and 12 mm stretched mesh size) monthly during the period between January and December 2018 at fixed sampling site of Lake Shala (Fig. 1). Gillnets was used parallel to shallower part of the lake in the evening (about 5:00 PM) and lifted in the next morning (around 7:00 AM). Captured O. niloticus were measured the total length (TL, cm) and total weight (TW, g). Each fish specimen was dissected to determine their sexes and gonad maturity stage following Holden & Raitt (1974) procedures. The ovaries were gently detached and the gonad weight (GW, g) were recorded. Ovaries containing eggs were well-preserved in 10% formalin solution and transported to Addis Ababa University for extra laboratory examination.
The relative growth of O. niloticus was assessed using LWR following Le Cren (1951):
where, TW = total weight (g), TL = total length (cm), ‘a’ is the coefficient associated to the fish body shape (intercept), and ‘b’ is an exponent correlated with changes in body shape (slope) (Le Cren, 1951).
The assessment of ‘b’ from the length-weight relationship (Murua et al., 2017) was used to compute the relative CF. The CF was calculated conferring to the approaches of Nehemia et al. (2012), following the formula:
where, CF = condition factor, TW = total weight (g), TL = total length (cm) and ‘b’ = the value obtained from the length-weight equation.
Where, FCF = fulton condition factor, TW = total weight (g) and TL= total length (cm).
The sex ratio of individual fish specimen was determined after dissection and gonad maturity rank was assessed by inspection of gonads. The maturity status of individual fish specimen was determined and classified as stages I, II, III, IV, V, and VI following procedures stated by Armstrong et al. (2004). Ovaries of the matured fishes was cross-sectioned and preserved in 10% formalin. The preserved ovaries were transported to Addis Ababa Fishery Laboratory for further analysis.
The gonado-somatic index (GSI) for every fish specimen was calculated for each sex using the equation (Peña-Mendoza et al., 2005):
Where, GSI = gonado-somatic index, GW = gonad weight (g), TW = total body weight (g), where the weight of the gonad is the weight of the fresh gonad blotted on absorbing paper.
GSI was calculated for monthly sampled fish specimens to govern the breeding season of fishes in the lake (Peña-Mendoza et al., 2005).
Fecundity (F) of mature eggs in the ovaries was assessed by straight counts of the hydrated eggs (Shoko et al., 2015). Fecundity was estimated from total counts of eggs in the ovaries of ripe female fish in IV and V stage development (Shoko et al., 2015). Sub-samples of eggs in the spawning phase were weighed and counted from triplicates of 1-g sub-sample of the eggs. Individual mean fecundity was back-calculated by the gravimetric method:
where ‘n’ is the number of eggs in the sub-sample, and ‘m’ is the weight of the ovarian sub-sample. The association of fecundity with TL (cm) and TW (g) of fish specimens was investigated by regression analysis and expressed by the following formula:
where F = fecundity; TL = total length (cm); TW = total weight (g); m, n, a, and b are constants.
The LWR data, the relation between absolute fecundity and the TL, fecundity and TW of fishes were subjected to linear regression analysis using SPSS version 20.0 statistical package (IBM, Armonk, NY, USA). To test the ‘b’ value against the ideal growth value of 3 (isometric), Student’s t-test was employed to test any significant deviation. Deviations from the hypothetical distribution of sex a 1:1 ratio were statistically tested using the Chi-square goodness (χ2) with a 5% significance level. One-way analysis of variance, non-parametric Kruskal-Wallis test (p < 0.05) was used to compare absolute fecundity, CF and GSI of O. niloticus.
Results
Three hundred forty-three specimens of O. niloticus ranged from 7.70 cm to 33 cm in TL and TW between 7.8 g and 708.21 g were used to determine LWR by linear regression analysis and scatter diagrams were plotted (Fig. 2). The LWR of O. niloticus representing male, female and the combined sexes (both sexes) are presented in Table 1 and Fig. 2.
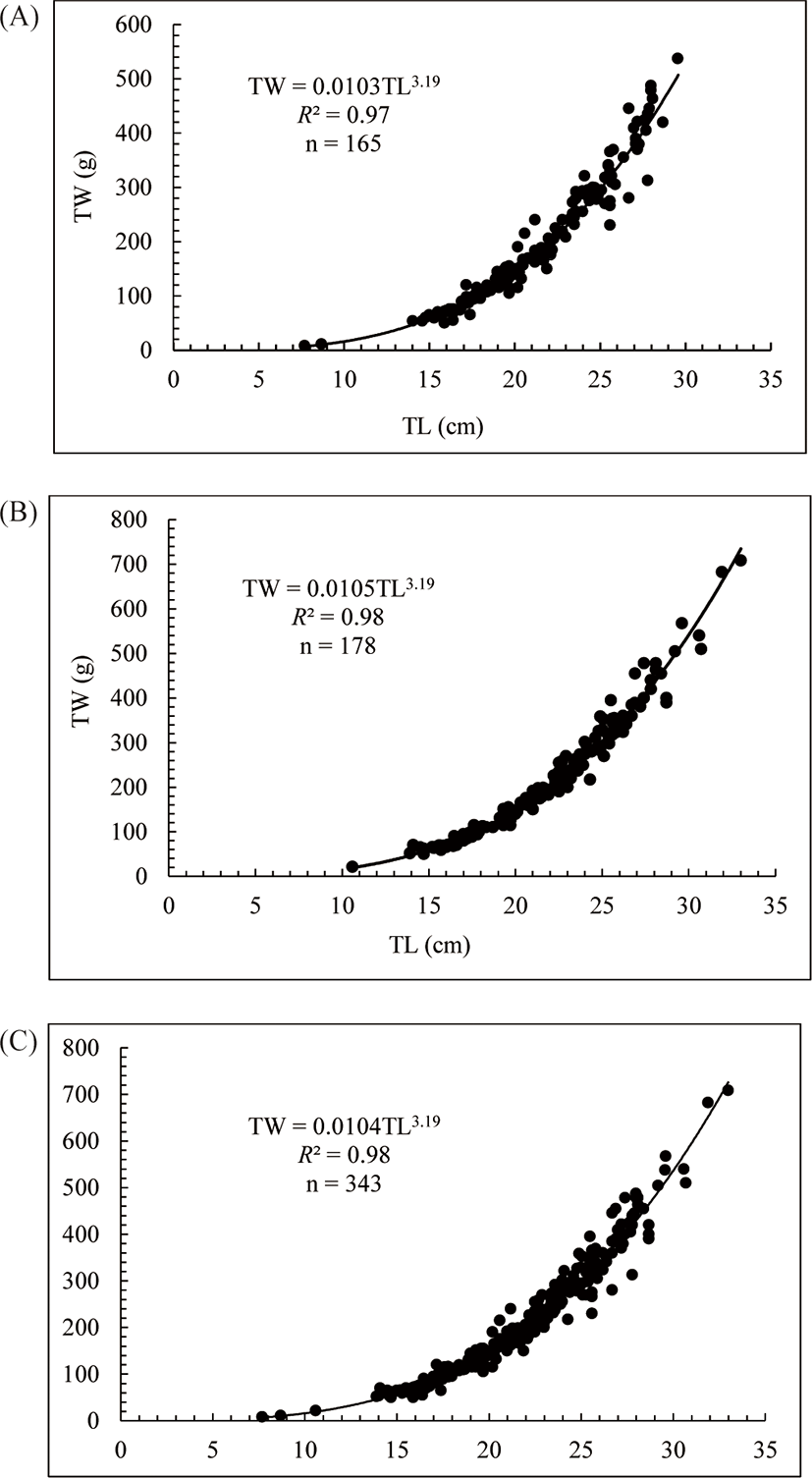
| Sex | N | Regression equations | a | b | R 2 |
|---|---|---|---|---|---|
| Male | 165 | TW = 0.0103TL3.19 | 0.0103 | 3.19 | 0.97 |
| Female | 178 | TW = 0.0105TL3.19 | 0.0105 | 3.19 | 0.98 |
| Combined | 343 | TW = 0.0104TL3.19 | 0.0104 | 3.19 | 0.98 |
Table 2 displays the variation in the mean CF of O. niloticus in Lake Shala. The CF of O. niloticus varied from 0.72 to 1.51 with a mean of 1.05. The CF for males and females ranged from 0.72 to 1.41 and 0.83 to 1.51, respectively (Table 2). The CF O. niloticus exhibited insignificantly variations between males and females (p > 0.05), but the value was to some extent higher in females (1.06) as compared to males (1.04).
Mean fulton CF (FCF) values of O. niloticus varied from 1.23 to 2.52 for males with a mean value of 1.85. Whereas female O. niloticus the mean FCF values are 1.5 to 2.5 with the mean value of 1.88. For combined sexes of O. niloticus, the mean FCF value was 1.87 ± 0.197 (Table 2). There was insignificant variation in mean FCF (p > 0.05) between female and male O. niloticus in Lake Shala.
Of 343 collected O. niloticus specimens, 165 male (48.1%) and 178 female (51.9%) were obtained from Lake Shala. The sex ratio (male:female) was 0.93:1 which did not deviate significantly from the ideal distribution of 1:1 (χ2 = 0.47, p > 0.05) (Table 3).
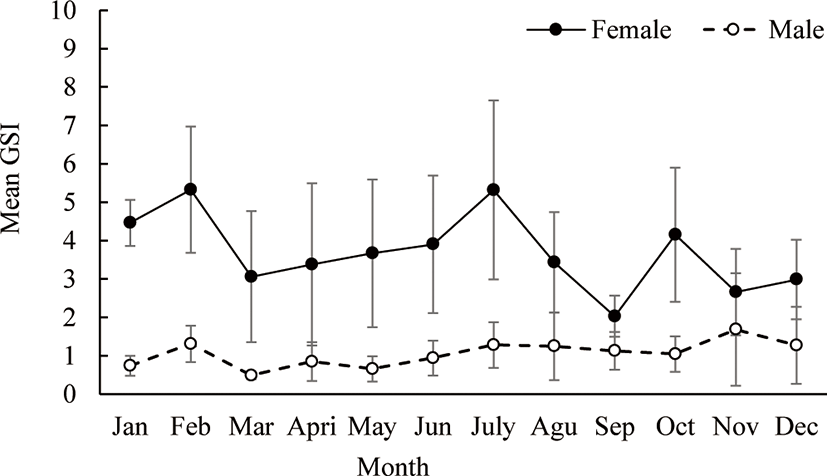
Monthly variations in GSI exhibited that both male and female O. niloticus followed nearly the same trend (Fig. 3). In females, the mean monthly GSI values ranged from 2.02 to 5.32 and showed a significant temporal variations (p < 0.05). Peak points of female GSI values were observed during January, February, June, July and October (Fig. 3). This indicates that female O. niloticus may breed more than once in the season. While, GSI value of males O. niloticus varied from 0.49 to 1.68 with a mean of 1.0 ± 0.64 (Fig. 3) and did not vary significantly among months (p > 0.05).
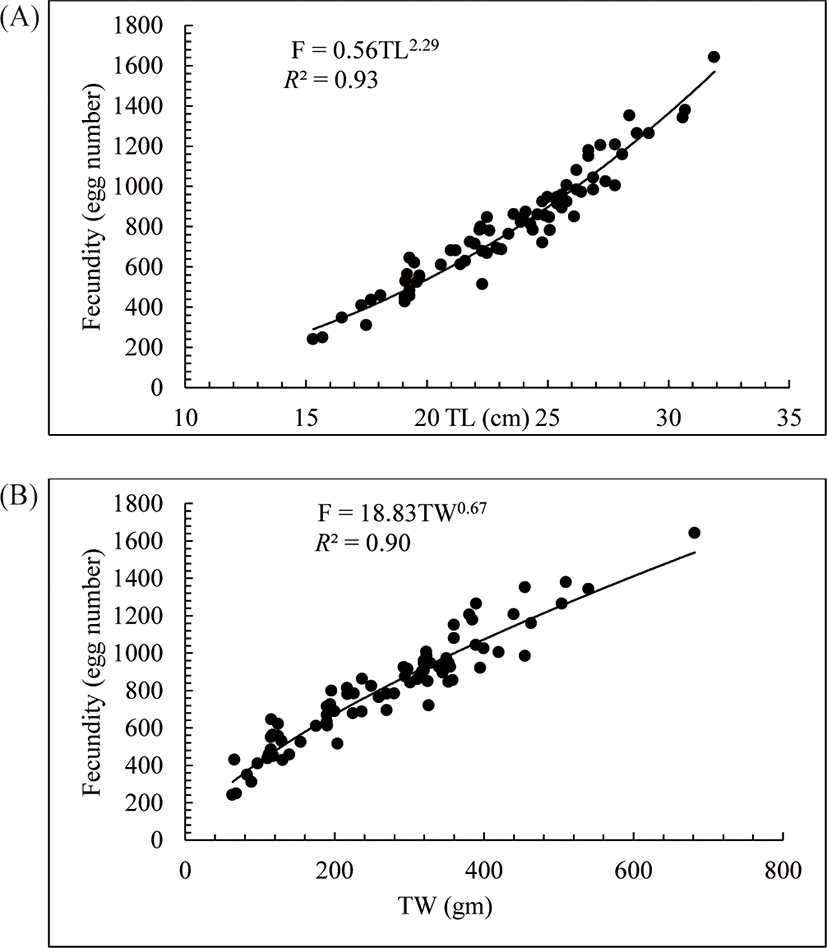
The fecundity of O. niloticus was estimated from 79 ripe females ranging in TL from 15.3 cm to 31.9 cm and TW from 63.7 g to 682.1 g (Fig. 4). The estimated mean fecundity was found to vary from 240 and 1,642 eggs with the corresponding fish size and weight. The mean fecundity of O. niloticus in Lake Shala was 806 eggs per fish. The fecundity of O. niloticus in Lake Shala was strongly correlated with TL (F = 0.56TL2.29, R2 = 0.93, p < 0.05) (Fig. 4A) and TW (F = 18.83TW0.67, R2 = 0.90, p < 0.05) (Fig. 4B).
DISCUSSION
In the present study, O. niloticus in Lake Shala exhibited positive allometric growth pattern with regression analyses exponent ‘b’ (3.19) values (Table 1 and Fig. 2). For an ideal isometric growth pattern, the ‘b’ value is 3.0, and populations in which the exponent varies from 3.0 show allometric growths (Allen, 1966). The correlation coefficients (r) of O. niloticus which ranged between 0.97 and 0.98 showed positive correlation between their TL and TW of the fish. The implication is that the weight of the fishes increased with increasing in TL (Allen, 1966; Njiru et al., 2006). Our results follow to the result reported by Stewart (1988) and Njiru et al. (2006) in their study conducted in the Lake Turkana and Lake Victoria, respectively. These researchers reported the ‘b’ value for O. niloticus as 3.17 and 3.20, respectively. Conversely, this ‘b’ value is higher than for the same species reported by Hirpo (2013) in Lake Beseka (b = 2.69), Lake Hayq (b = 2.95) (Assefa & Getahun, 2014) and Lake Langeno (b = 2.88) (Kebede et al., 2018). These variations possibly because of variance in fatness and gonad development stage, sex, health, amount of feed and feeding habits, habitat type, different stages in the ontogenetic development, spawning season, water temperature, preservation techniques and differences in a geographical location with the related ecological factors (Alhassan et al., 2015; Froese, 2006).
In our study, the CF computed for the O. niloticus was 1.04 ± 0.11 and 1.06 ± 0.09 for males and females respectively (Table 2). This result is similar to the other study of O. niloticus by Ighwela et al. (2011), who reported that CF value which is greater than one signifies that a fish is in good condition. The CF O. niloticus in Lake Shala were near to those reported by Olurin & Aderibigbe (2006) in Sanni Luba Fish Farm, Ogun state, where male CF was 1.14 and 1.08 in females O. niloticus. In this study, female O. niloticus (1.06) was heavier compared to male CF (1.04), but statically insignificant (p > 0.05). The higher CF of female O. niloticus may be accredited to the better fat accumulation (Mortuza & Al-Misned, 2013), higher state of maturity and GW (Mondal & Chakravartty, 2016).
The mean values of FCF were 1.85 and 1.88 for male and female O. niloticus, respectively (Table 2). Findings of this FCF values are in line with those reported for O. niloticus by Assefa & Getahun (2014) in Lake Hayq, Kebede et al. (2018) in Lake Langano and Teame et al. (2018) in Tekeze Reservoir. In this study, the FCF of O. niloticus was ranged between 1.23 and 2.52 with a total mean of 1.87 (Table 2). These mean FCF values are comparable to those reported by Tesfaye & Tadesse (2008) in Lake Koka (1.87), but the value was higher than the reports made in Lake Ziway (1.81) and Lake Langeno (1.84) (Tesfaye & Tadesse, 2008) and Lake Hayq (1.81) (Assefa & Getahun, 2014), which showed that O. niloticus in Lake Shala had a good body condition. The current mean FCF value is also very lesser as compared to the same species reported in other Ethiopian water bodies, like Lake Awassa (2.03) (Admassu, 1990), Lake Tana (1.89) (Tadesse, 1997), Lake Chamo (2.35) (Teferi & Admassu, 2002) and Lake Babogaya (2.13) (Hirpo, 2012). Variations in mean FCF obtained in several water bodies could also be credited to the differences in environmental factors, food accessibility and feeding regime, season and water level fluctuation, and water quality. The CF of fishes has also been reported to be influenced by many factors like spawning periods, maturity stage, sex difference (Degsera et al., 2020), and pollution (Alhassan et al., 2015).
Sex proportion for O. niloticus (0.93:1) in Lake Shala shows that males and females are equally distributed in the lake and the variation from the predictable hypothetical distribution of 1:1 (male:female) was insignificant. This is supported by other studies reported on O. niloticus in Lake Nyamusingiri and Kyasanduka (Bwanika et al., 2004), and in Itapaji Dam (Omotayo et al., 2019). However, studies on O. niloticus have reported the predominance of female O. niloticus in other Ethiopian lakes and reservoirs like Lake Beseka (Hirpo, 2013), Lake Hayq (Assefa & Getahun, 2014) and Tekeze Reservoir (Teame et al., 2018). The preponderance of male O. niloticus population over females was reported in Lake Tana (Tadesse, 1997), Lake Victoria (Njiru et al., 2006), Lake Babogaya (Hirpo, 2012) and Wadi Hanifah (Mortuza & Al-Misned, 2013). Teame et al. (2018) mentioned that the sex ratio show variation significantly from the same species in different water bodies, but usually it is close to one, and predominance of sex may differ due to sexual segregation during spawning period, difference in habitat preference, behavioral variances between the sexes, vulnerability to fishing gear nature and fishing site.
Studies on reproduction period and fecundity potential and their related influences are regularly used to guard the recruits (Nyakuni, 2009) and forecast recruitment variability (Shoko et al., 2015). Fish life history parameters, like spawning period, gonad status and fecundity amount differ among fish species (Peña-Mendoza et al., 2005). During this study, GSI values for female O. niloticus exhibited significant temporal variations, with a biannual cycle between January–February and July–September (Fig. 3). Tadesse (1988) in Lake Ziway, Admassu (1996) in Lake Awassa and Teame et al. (2018) in Tekeze Reservoir also reported for O. niloticus a bi-modal breeding pattern. However, in the present study, matured ovaries and the breeding period (GSI) of females of O. niloticus exhibited several peaks all year round. This extended period showed that female O. niloticus breed more than once in the year. Similarly, Njiru et al. (2006) and Teame et al. (2018) pointed out that, there is more than one breeding time per year for the majority O. niloticus.
In Lake Shala, the peak breeding period of O. niloticus is not restricted to the rainy period (July to September), but several it also breeds during the dry season (January to February). However, other studies on O. niloticus like Hirpo (2012, 2013) found that O. niloticus breed intensively during the wet (rainy) season. Kebede et al. (2018) and Teame et al. (2018) also state the correlation between rainfall and peak breeding periods for O. niloticus in Lake Langano and Tekeze Reservoir, respectively. During the rainy season flooding from allochthonous input which could increase nutrient concentration subsequent in increased accessibility and quality of food resources (Tadesse, 1988), so offsprings are produced at times of better growth and survival (Admassu, 1996). Numerous studies also described precipitation (rainfall), subtle variation in water temperature (Assefa & Getahun, 2014) and water level fluctuation (Kebede et al., 2018) as vital environmental factors allied with exhaustive breeding activities of O. niloticus in Ethiopian lakes and elsewhere (Nyakuni, 2009; Peña-Mendoza et al., 2005).
In this study, the fecundity O. niloticus varied from 240 to 1,642 eggs, with a mean absolute fecundity of 806 eggs. This finding value is lower than that reported by others even among individuals of O. niloticus elsewhere. For example, Nyakuni (2009) recorded a fecundity of 412 to 2,380 eggs (mean of 854 eggs) of O. niloticus in Albert Nile, Njiru et al. (2006) mean of 2,715 eggs in Lake Victoria and Teame et al. (2018) recorded 399–2,129 eggs per fish of O. niloticus in Tekeze Dam, Ethiopia. But, the mean absolute fecundity of O. niloticus in Lake Shala was higher than a report in Lake Beseka (261 eggs) by Hirpo (2013), Lake Langeno (464 eggs) (Kebede et al., 2018) and Lake Hayq (217 eggs) (Tessema et al., 2019). Cuevas-Rodríguez et al. (2017) detailed that the mean absolute fecundity of O. niloticus varies considerably in various factors like abundance and seasonal availability of food and fishing pressure. Body CF and growth of the fish attributed to variation in fecundity of the fish. Fish in poor body condition are reported to possess less fecundity potential than those in better condition (Cuevas-Rodríguez et al., 2017; Peña-Mendoza et al., 2005). Identical fish species also can show variations in mean fecundity from one water body to a different water body because of the TL of the fish examined for analysis. Teame et al. (2018) recorded a maximum fecundity of 2,129 eggs from O. niloticus with size of 37 cm TL in Tekeze Reservoir, while Nyakuni (2009) obtained a fecundity of 2,380 eggs at a size of 42 cm TL of O. niloticus in Albert Nile. In the present study, the maximum fecundity (1,642 eggs per fish) was recorded at 31.9 cm TL. These findings, therefore, confirm that fecundity potential of O. niloticus is variable and correlates with its TL and TW. This finding agrees with other studies within the Ethiopian Rift Valley Lakes (Assefa & Getahun, 2014; Hirpo, 2012; Hirpo, 2013; Kebede et al., 2018; Tessema et al., 2019).
Conclusion
From the findings of this study, it may possibly conclude that the LWR of O. niloticus showed a positive allometric growth pattern. The sex ratio of O. niloticus in Lake Shala was statistically insignificant from the expected 1:1 hypothetical distribution. In Lake Shala, both males and females O. niloticus were in good body condition throughout the study period. The research also revealed that O. niloticus breed throughout the year, with several peaks during January, February, June, July and August. Fecundity was significantly correlated with TL and TW of the fish. Therefore, the present study found a very important database for conservation and the management of the species in Lake Shala.








