Introduction
Sea cucumber (Echinodermata), especially species Holothuria scabra is a potential marine commodity with domestic and international value in the fisheries sub-sector (Hair et al., 2020). This organism contains bioactive substances, including steroid compounds. These are important forms of hormones in the aquaculture industry used as aphrodisiacs (vitality enhancers) (Riani et al., 2013) and sex reversal (genital reversion) agents (Dewi et al., 2011; Susanto et al., 2018; Susanto et al., 2021). Lieberman-Burchard test results and chick bioassay positively identified the androgen hormone, testosterone in the extract (Meydia et al., 2016). Furthermore, 1 kg of wet sea cucumber innards consists of 21.28 g (2.128%) of steroid compounds that were contained in the dried form (Riani et al., 2013; Susanto et al., 2021). The evaluation of the meat and innards, using thin layer chromatography recognized the presence of testosterone and cholesterol (Meydia et al., 2016; Susanto et al., 2018). Therefore, the use of sea cucumber (H. scabra Jaeger) as a natural steroid in aquaculture is very convincing.
The purpose of administering steroid hormones was to stimulate metabolic activities, such as those showing an increased aggressiveness toward eating in fish and crustaceans (Tatang et al., 2020), stimulate animal reproduction, growth, and sex reversal (Megbowon & Mojekwu, 2014; Thongbuakaew et al., 2021). In addition, one of the biotechnology applications was in the male monosexual system which is often used in increasing aquaculture production (Aflalo et al., 2006; De Bock & López Greco, 2010). Since the male freshwater crayfish Cherax quadricarinatus specimen was larger in size and weight, compared to the female counterpart of similar age (De Bock & López Greco, 2010; Rodgers et al., 2006). Furthermore, sex reversion to males was expected to accelerate growth, and increase production as well as economic value, due to the diversion of reproductive energy to somatic growth activities (Ferdous et al., 2014).
The sex reversal process requires the use of many synthetic hormones, which face difficulty in decomposing within the body. In addition, there have been some reports of carcinogenicity, pollution of the environment, and other dangerous side effects (Hoga et al., 2018; Lee et al., 2018). The most widely used type is the synthetic androgen hormone, 17α-methyltestosterone (MT) (Abduh et al., 2020), assumed to improve digestion, food absorption, and conversion, alongside regulating sexual development and other physiological processes (Abduh et al., 2020). There has been a decline in the use of MT because of the rising incidence of pollution and cancer amongst humans. According to Contreras-Sánchez et al. (2001), Hemmat et al. (2015), and Karimi et al. (2014), MT anabolic residues remain in pond sediments after three months of use and usually tend to damage the liver of treated animals. Therefore, the discovery of a natural hormone extracted from the visceral organ of sea cucumber is important as a more environmentally friendly substitute for MT (Dewi et al., 2011).
Based on biotechnological application, genital reversion is recognized as one of the monosex formation techniques usually performed through the administration of natural hormones (androgens and estrogens) to stimulate the formation of the desired fish sex (Dunham, 2011; Hoga et al., 2018). In addition, a relationship was established between the beginning and end of differentiation with the length of hormone activity. Besides that, administering small amounts of testosterone in individuals with undeveloped gonads directly influences the hypothalamus during the critical stages of gonadal development and male characterization. Also, testosterone is suspected to have an effect on neurons through the preoptic part of the hypothalamus, with gonadotropin-releasing factor secreted in synapses (Karimi et al., 2014). Yet, this study showed less effective male fish formation after the administration of smaller doses (2–3 mg/L). In addition, success depended on the type (species) and dose of hormones, as well as fish age, method and duration of administration, and time of contact (Chatain et al., 1999; Hunter & Donaldson, 1983).
The studies on juvenile freshwater crayfish C. quadricarinatus, aged 2–3 weeks immersed in 2 mg/L dose of sea cucumber steroid extract (SCSE) for 18 h showed 79.86% males (Susanto et al., 2018) and a combination of steroid gamma of sea cumber dose of 2 mg/L and with the addition of honey bee dose of 20 mL/L showed 83.75% male after 18 h immersion at the same species (Susanto et al., 2021). The steroid, testosterone was easily metabolized and rapidly inactivated in the digestive tract before absorption. Partial accumulation of testosterone was in the hepatopancreas tract, followed by metabolizes, partial absorption through the intestinal lumen, and the subsequent movement to target organs (Connell & Miller, 1984). In addition to having androgenic properties, there have been reports on the anabolic characteristics, responsible for the stimulation of muscle growth. This further plays a role in libido enhancement and spermatozoa formation (Dewi et al., 2011). The most significant testosterone levels in male prawns were observed following the administration of sea cucumber extract at a dose of 25 mg/L and after a 36 h immersion time of 0.16 ng/mL (Triajie, 2010). This treatment was known to have instigated up to 66.67% of male genital reversal. Therefore, it is necessary to perform further research on the male production and testosterone content of juvenile freshwater crayfish (C. quadricarinatus) after immersing in extracts of sea cucumber steroids. The aim of the research, therefore, is to identify the male formation as well as the level of testosterone in the hemolymph of juvenile red claw crayfish, after exposure to SCSE.
Materials and Methods
The extracted sea cucumbers were categorized based on length and weight, in terms of species and age. These conditions were to determine the presence of testosterone. The raw materials obtained from fishermen at Lampung bay, comprised approximately 12 kg of mature sea cucumber (H. scabra) cultures, with each weighing around 200–700 g, and a length range of 17–33 cm. The sea cucumber with a size of > 400 g was recommended with the high steroid contents. In addition, various solvents were used to collect the powdered sea cucumber extracts. These include n-hexane, where the yield was concentrated at 30°C, and under low pressure, after a 24 h exposure period, using rotary evaporation. The product of diethyl ether solvent extraction was ready after 48 h, and the subsequent evaporation was performed at 35°C. The methanol extract was complete after 72 h, and the solvent was removed at 40°C. The separation of methanol-aqueous extract required the formation of ether-methanol, after adding ether. The upper phase separation was conducted, using a separating funnel. This compartment was further combined with n-butanol, and another separating funnel was used to separate the aqueous extract. Each yield then was mechanically shaken at room temperature (25°C), and subsequently retained in a freezer compartment. The process of sea cucumber extraction was performed at the Integrated Laboratory and Technology Innovation Center, University of Lampung, and all processes were accomplished under dark conditions (Golestani et al., 2016).
This research was conducted at the Aquatic Biology Research Laboratory, University of Lampung. The processes include the morphological selection of juvenile crayfish, based on length, body color, level of organ completeness, as well as age. About five hundred approximately 1–2 weeks old juvenile crayfish with 2–2.5 cm body length, were acquired from Gemma Farm cultivators, Klaten, Central Java. Healthy samples morphologically comprising complete extremity organs (encompassing chelipeds and pleopods) were well-selected from broodstocks. The juvenile crayfish were initially weighed before placing in a container at the laboratory for acclimatization. This was performed over a 2–3 d period, before being transferred to the tub or aquarium volume 50 L.
The samples were subjected to a hormone dose of 2 and 4 mg/L, including a natural steroid collected from the extracts of sea cucumber innards, and the synthetic type termed 17α-MT, acquired from Biotech Argo-Lab (Sidoarjo, Indonesia). All the treatments were immersed for 18 h and 30 h. Each treatment was performed in three replications. The specimens were fed twice a day with silkworms and fish pellets ad libitum within a 50 d rearing period. The juveniles were evaluated under a stereoscopic microscope to determine sex during nursery rearing. This assessment required determining the presence/absence of genital papillae at the base of the fifth pair of walking legs (males), genital openings at the base of the third pair of walking legs (females), or both structures in an individual (intersex) (De Bock & López Greco, 2010; Susanto et al., 2018; Susanto et al., 2021). Water quality was sustained at an optimal level and the containers were properly aerated, in order to ensure the growth of crayfish. Subsequently, about 30% to 50% of the total water volume was substituted every 3 d with fresh water, using basic dirt settling or siphoning techniques. The water parameters evaluated include pH, temperature, hardness, and dissolved oxygen content (DO).
This research was conducted with a completely randomized design in factorial, comprising 8 treatments of 3 replications. The treatments include the dose of SCSE at 0, 2, and 4 mg/L and 2 mg/L 17α-MT (as control), with 18 and 30 h immersion times. Table 1 shows the experimental and treatment groups.
After immersion, the juvenile freshwater crayfish were reserved for about 50 d or up to the time when the formation of secondary sexual traits, such as genital papillae was visible. The testosterone content in hemolymph was determined at the end of the study at the Laboratory of Pramitra Biolab, Bandar Lampung, using the Enzyme-Linked Immunosorbent Assay method (Hemmat et al., 2015) with Zenix Diagnostics Brand Microplate Reader, Briloner Landstrade, Korbach Germany and Testosterone Enzyme Immuno Assay Test Kit.
The percentage of male crayfish and their survival was obtained as followed.
J: percentage of males (%)
A: number of male crayfish
T: number of crayfish samples
SR: survival rate of crayfish (%)
Nt: number of crayfish at the end of the study
No: number of crayfish at the beginning of the study
Growth rate assessment involved measuring the increase in total length and daily weight gain.
L: increase in total length (mm)
Lt: final average length (mm)
Lo: initial average length (mm)
GR: daily growth rate (g/d)
Wt: average weight of crayfish at the end of observation (g)
Wo: average weight of crayfish at the beginning of observation (g)
t: time between observations (maintenance time) (d)
The effect of treatment dosage and immersion time extracts on testosterone levels was analyzed with one-way analysis of variance at a 5% level of significance and followed by a least significant difference test using SPSS 19 program by IBM SPSS (Armonk, NY, USA). Regression analysis was used to evaluate the relation between the male crayfish formation and testosterone level.
Results
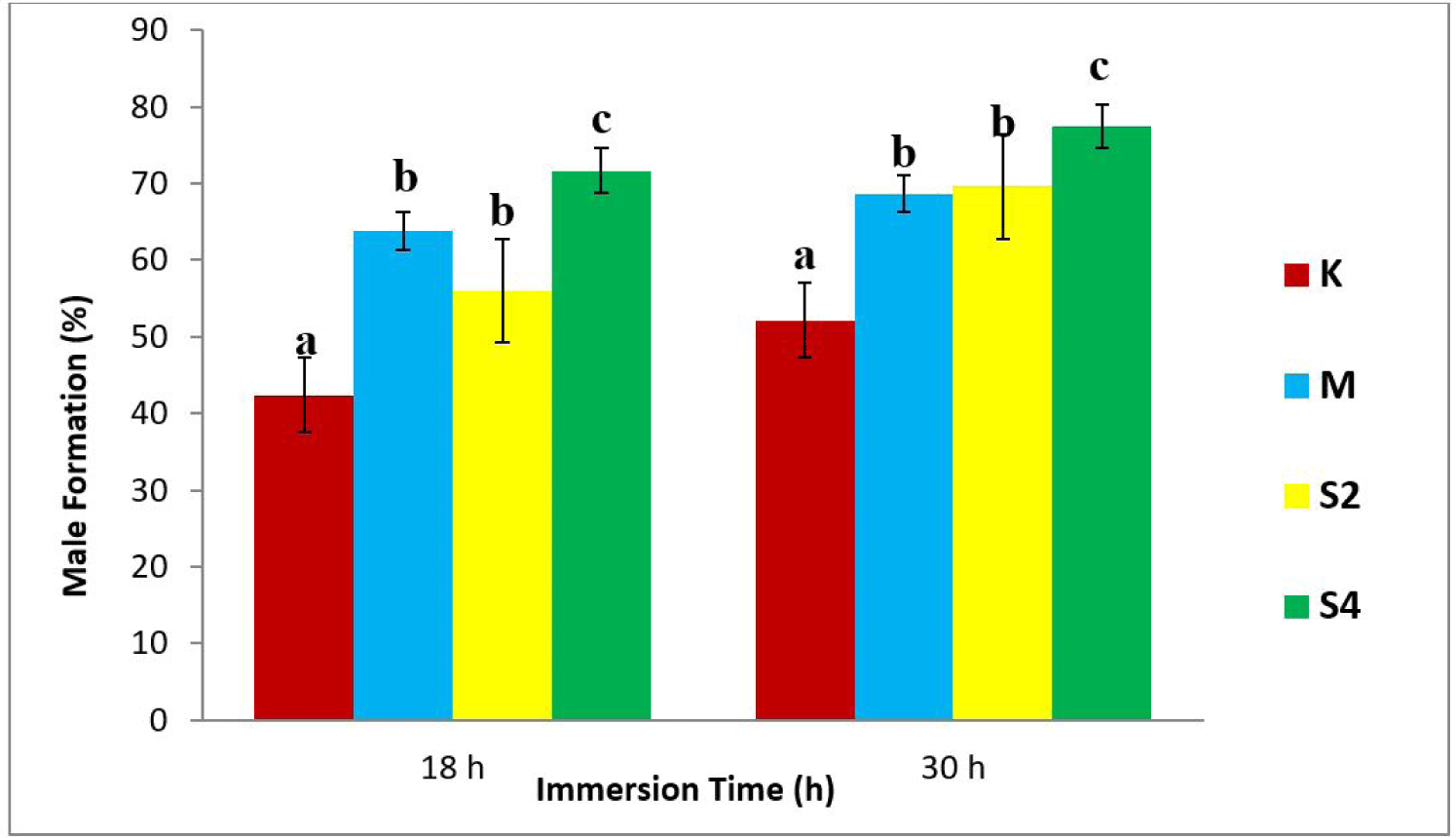
The results showed the highest average percentage of males (77.45%) in the S4-30 treatment, while the least was observed in the K18 treatment (negative control), as shown in Fig. 1.
The study showed that the highest testosterone level, 0.248 ng/mL, was in S4-30 treatment (dose 4 mg/L with a 30 h immersion), while the least value (0.034 ng/mL) was shown in K18 (negative control with 18 h immersion), as shown in Fig. 2.
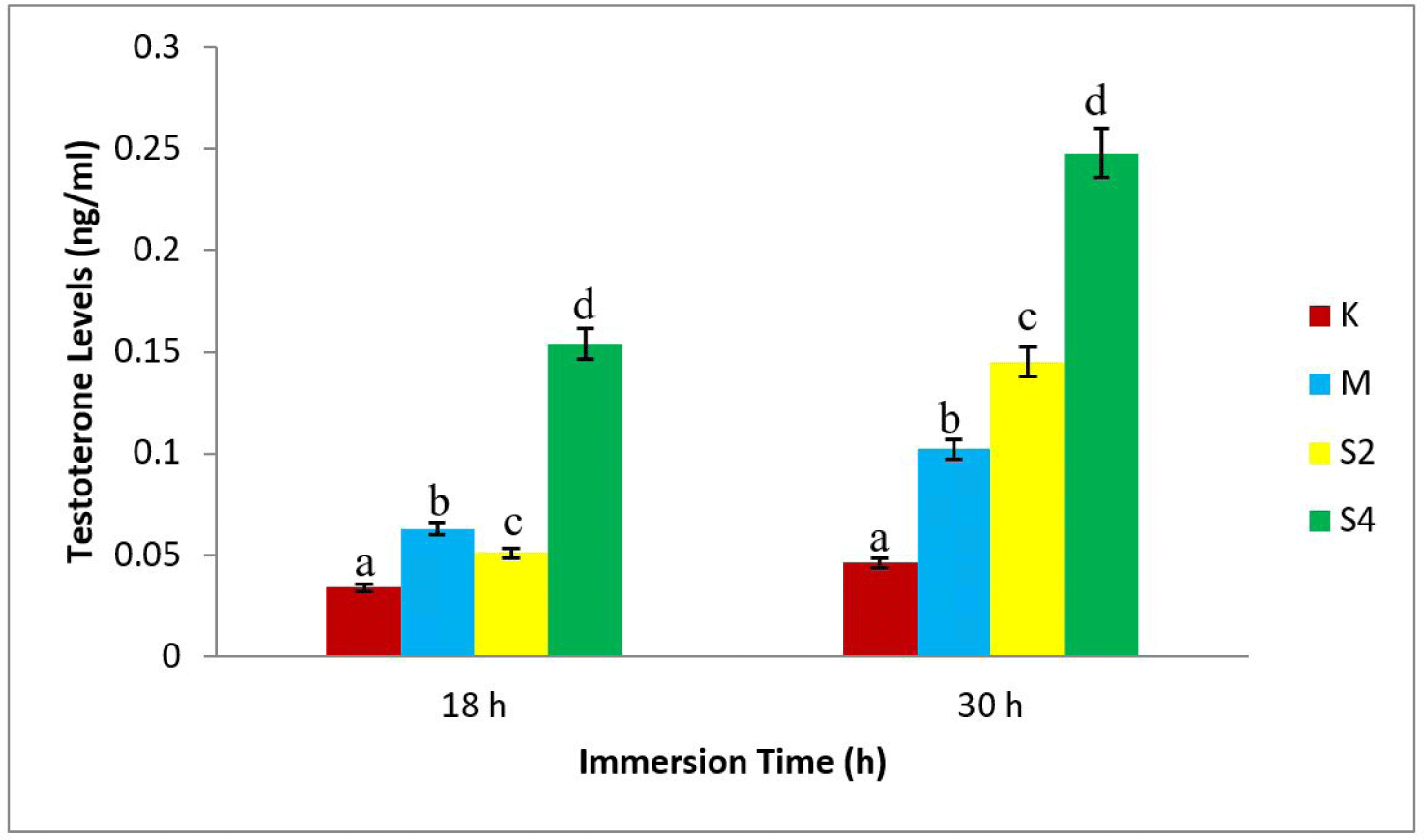
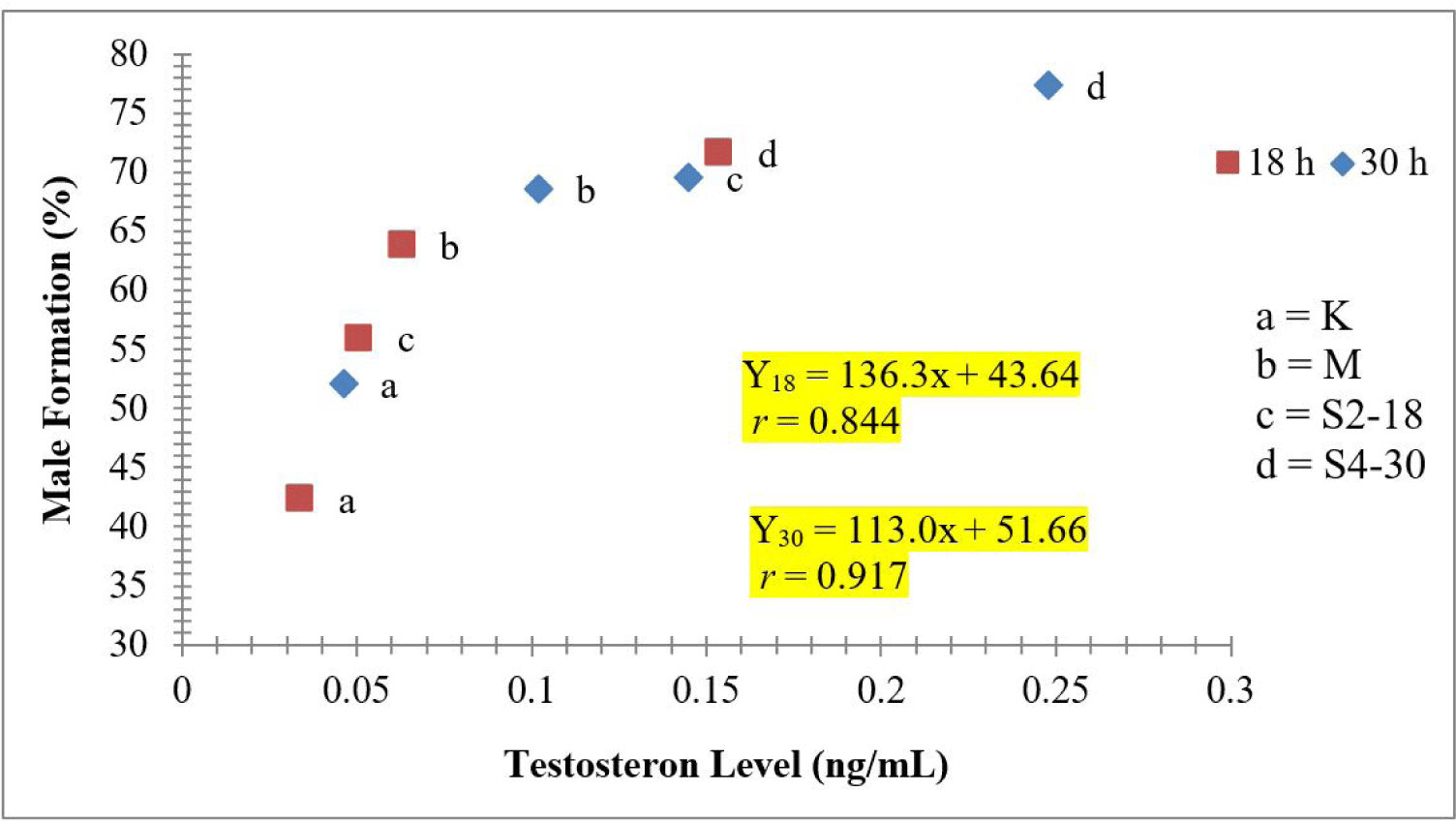
The results of statistical tests showed a relation between the dosage of sea cucumber extract and the formation of males (Fig. 1). Also, there was an increase in testosterone levels, following the increase in hormone doses (Fig. 2). In addition, the 4 mg/L extract treatment immersed for 30 h produced the highest male (77.45%). The regression value between male formation and testosterone level of crayfish was Y = 136.3x + 43.64 with r = 0.844 and Y = 113.0x + 51.66, with r = 0.917 (Fig. 3).
The survival percentage of the whole experiment could be seen in Table 2. The survival percentage of the juvenile crayfish given with 4 mg/L was much higher compared to other treatments either in 18 h or 30 h immersion groups. This highest survival percentage of the juvenile crayfish was also followed by an increase in the average growth length and body weight (p < 0.05). The dose and immersion time of sea cucumber affects survival and increases the length and daily weight of juvenile crayfish.
Discussion
There were differences in the percentage of individual juvenile freshwater crayfish among those treatment groups, provided with the steroid hormone of sea cucumber and 17α-MT. These exposures generated 77.45% and 68.65% of males, respectively. The differences in study results are assumed to have been caused by less optimal dosages alongside soaking time. These variables influence the formation of the male sex in freshwater crayfish, leading to the discrepancy in occurrence across all treatments. Administering a dose that is too low instigates a less perfect sex reversal process (Dunham, 2011). This is due to changes in hormone content entering the juvenile body, through a process of diffusion in the skin and digestive organs, which subsequently reaches the target organ with very low content. The absorption of dissolved components in water through the gills is usually relatively large, and lesser values are recorded through the digestive tract, despite the relatively large entrance. Furthermore, skin-mediated entry generates a comparably small amount (Connell & Miller, 1984). The natural steroid hormone sea cucumber has the ability to produce a higher percentage of males compared to the synthesized 17α-MT. This certainly answers the cultivation problem, where synthetic varieties demonstrate some weaknesses, including the difficulty to decompose within the body, carcinogenic tendencies, and a negative impact on human health (Dunham, 2011). Also, environmental pollution potentials have been estimated, and often cause undesirable side effects, hence the circulation was banned (Director-General of Marine and Fisheries, 2008).
In this study, the testosterone levels of the treatment group were 0.16 ng/mL which was much higher compared to those obtained by another study on the masculinization of giant prawns (Triajie, 2010) that using the steroid extract of sea cucumber at a dose of 25 mg/L and over 36 h duration of immersion. Suryaningsih et al. (2010) also reported that 0.203 ng/mL was the highest testosterone level, during a 1 year study on the hormone changes in bred fish (Puntius orphoides). This was known to have an effect on the significantly high somatic gonadal index and extent of gonadal maturity. Afpriyaningrum et al. (2016) also showed that a 2 mg/L dose of MT stabilized at a temperature of 36°C gave the highest percentage of males, 92.50%, and testosterone levels absorbed by the body of about 23.05–23.86 ng/g after a 4-hour immersion period in tilapia larvae (Oreochromis niloticus) aged 10 days. Moreover, Putra et al. (2012) also indicated an increase in the concentration of testosterone present in the female broodstock of baung fish (Mystus nemurus) for each dose administered.
The administration of steroid sea cucumber at the dose of 2 mg/L significantly affected the testosterone concentration in hemolymph by 283,354 ng/dL and further increased the percentage of male freshwater crayfish by 63.33% which also indicated by a previous study (Susanto et al., 2021). Steroid compounds were one of the inherent bioactive substances, assumed to contain androgen hormones, and was estimated to function as sex reversal substance (Riani et al., 2005). This administration of steroid compounds seemed to directly affect the hypothalamus permanently during male character formation and the critical stages of gonadal development. Presumably, the suspected testosterone affected neurons through the pyrolytic hypothalamus, followed by the secretion of synapses in the form of gonadotropin-releasing factor (Kusmini et al., 2001). These androgen hormones subsequently entered the cytoplasm bound to specific receptors in the cytosol. These steroid ligands formed then traveled to the nucleus and bound to acceptors in the genome (Rougeot et al., 2002).
In order to determine the relationship between the percentage of male formation and the level of testosterone at both times of immersion, simple regression was made and showed that both times indicated their correlation between male percentage and testosterone level. At 18 h immersion, the r value was 0.844 indicating a high correlation, similar to it at those at 30 h immersions. Therefore, indicating that testosterone levels facilitated an elevated male formation percentage in crayfish. The higher average percentage of males leads to an increase in testosterone levels. Riani et al. (2005) also indicated that a higher concentration was recorded in the blood of chicks following the administration of sea cucumber flour. This hormone was estimated to possess anabolic properties, capable of stimulating muscle growth and enhancing libido and spermatozoa formation (Rahman et al., 2011). The control samples (K18) with low content testosterone level, 0.034 ng/mL, were presumably devoid of steroid hormones, and limited hormone types are properly absorbed through the crayfish’s body. In addition to it, testosterone is assumed to be metabolized quickly after absorption, and the possibility of rapid inactivation before absorption in the digestive tract. This leads to partial accumulation in the hepatopancreas tract, followed by metabolizes, and partial absorption through the intestinal lumen, before moving to the target organ (Connell & Miller, 1984).
The treatment doses and length produced a higher survival value of 81.67%, compared to the controls (55.00%). This survival phenomenon is predicted as an effect of other compounds contained in rough extracts of sea cucumber, which also had the capacity to increase stamina. Dewi et al. (2011) showed a significantly high protein content in sea cucumber at 66.07%. This constituent is characterized by complete amino acids, comprising both the essential and non-essential varieties. Furthermore, these units are estimated to be very useful in protein synthesis, specifically for muscle formation (Huberman, 2000). In addition, the absolute length of freshwater crayfish was also significantly affected by sea cucumber extract, seemingly it showed dose-dependent. Sea cucumber extracts confer a substantial effect on the absolute length of freshwater crayfish. Table 2 shows the highest increase in the 4 mg/L sea cucumber extract group, with 30 h soaking time (1.67 ± 0.09 mm), while the lowest was observed in the negative control group, soaked for 18 h (0.98 ± 0.23 mm). The factors influencing survival rates comprise biotic factors, including population density, age, the organisms’ adaptation ability to the environment, as well as environmental abiotic factors (Dagne et al., 2013; Ferdous et al., 2014). Sea cucumbers have bioactive components known to be beneficial in the medical and health fields. These include triterpene glycosides (saponins), chondroitin sulfates, glycosaminoglycan, sulfated polysaccharides, sterols (glycosides and sulfate), phenolics, peptides, cerebrosides, lectins (Bordbar et al., 2011). In addition, some reports indicated biological and pharmacological activities, including anti-cancer, anti-coagulant, anti-hypertension, anti-inflammation, antioxidant, and anti-fungi (Bordbar et al., 2011; Chludil et al., 2002; Hawa et al., 1999). Table 2 shows the lowest absolute length increase, with an 18 h immersion time of 0.98 mm, as recorded in the negative control. The low growth rate observed in this treatment resulted from the absence of steroid hormones (Tatang et al., 2020), which are needed to influence metabolic activity (Hu et al., 2012). The highest daily weight gain of juvenile crayfish was recorded in samples treated with a dose of 4 mg/L at 30 h immersion (0.032 ± 0.016 g), while the lowest daily value was observed in the negative control group, with 18 h soaking time (0.016 ± 0.001 g). This indicates that steroid hormones are capable of stimulating metabolic activities, and as a result, increase in the growth of crayfish. Hence, the addition of androgen content might activate growth hormones, and eventually causes a higher growth rate compared to the control experiment. This finding is in accordance with the opinion of Tatang et al. (2020), where the steroidal type administered influenced growth in both male and female fish.
From this study, since the laboratory conditions as well as feeding were given homogenously throughout the study for every treatment group, therefore the difference in all parameters was mainly from the treatment given for every experimental group. The data for DO, temperature, and pH of water samples were collected every 3 d, while hardness was evaluated at the termination of the study. The specimens were sustained under continuous aeration and natural photoperiod in 20 L of chlorinated tap water, contained in individual plastic tanks (DO: 3.8 mg/L to 5.2 mg/L, pH: 7.1–7.9, hardness: 85 mg/L as CaCO3 equivalents). Also, the temperature was maintained at 27°C–31°C, while the quality alongside good feed was maintained to support high survival. The results of water quality analysis showed good support for crayfish maintenance (Ani et al., 2022; Viau et al., 2012). Meanwhile, dissolved oxygen, pH, temperature, hardness, and nitrite levels in treatment units were in accordance with the life span required by the freshwater crayfish.
Conclusion
The immersion time and variation in the dose of sea cucumber extract (H. scabra) positively influenced the percentage of male juvenile crayfish (C. quadricarinatus) by 77.45%. The highest testosterone level of 0.248 ng/mL was recorded in the treatment of the SCSE, at a dose of 4 mg/L over a 30 h soaking period. Furthermore, there was also a dose-dependent positive impact on testosterone levels. Yet further study should be done for the size of sea cucumber in order to obtain an optimum level of steroid extract.








