Introduction
Fish reproductive studies include physiological aspects such as, sex ratio, gonadal development, size at first maturity, fecundity, spawning periods and spawning patterns (Adewumi et al., 2014). Increasing studies on induced spawning and hybridization has led to investigations of gonad stages of maturation which is important in fish production (Omotosho, 1993). Synodontis schall belongs to the family Mochokidae. The Mochokidae is a family of African catfishes commonly known as “squeakers” and “upside-down” catfishes. In Nigeria, the family Mochokidae, consists of about 30 species distributed in 5 genera namely; Brachysynodnotis, Chiloglanis, Hemisynodontis, Mochokus and Synodontis. Of these genera, Synodontis is the largest with about 23 species (Synodontis nigrita, Synodontis resupinatus, Synodontis clarias, Synodontis membranaceus and Synodontis eupterus) in Nigerian waters (Idodo-Umeh, 2003). Although investigations on varied aspects of the biology of S. schall and related species exist (Adedeji & Araoye, 2005; Akombo et al., 2011; Akombo et al., 2014; Akombo et al., 2015; Idodo-Umeh, 2003; Lalèyè et al., 2006; Olele & Etim, 2011; Olojo et al., 2003; Olojo et al., 2012; Shinkafi & Daneji, 2011; Shinkafi et al., 2010); there exist a paucity of reported literature on the reproductive biology of S. schall in Nigeria (Araoye, 2001; Lalèyè et al., 2006; Mekkawy & Hassan, 2011; Oboh et al., 2013). Therefore, there is need for detailed studies on their gonad morphology, maturation stages, sex ratio and fecundity. The objective of this study is to examine the reproductive biology of S. schall from River Siluko, to provide recent information needful for the fisheries resources utilization and management in Nigeria.
Materials and Methods
This study was carried out in River Siluko which rises from the Owena River, and empties into the Atlantic Ocean as a tributary of the Benin River in Edo State, Nigeria (Arimoro et al., 2006). The vegetation along the river is that of forest swamp and consists of trees (Khaya senegalensis, Pycnanthus angolensi, Astonia congoensis and Khaya ivorensis), grasses (Pennisetum purpureum, Megathyrsus maximus and Leersia hexandra), and weeds (Pistia stratiotes, Nymphea lotus and Salvina nymphellula). The river bank is composed of sharp sand, clay as well as alluvium materials with a few collections of allocthonomus materials derived principally from fallen leaves of surrounding vegetation (Fig. 1).
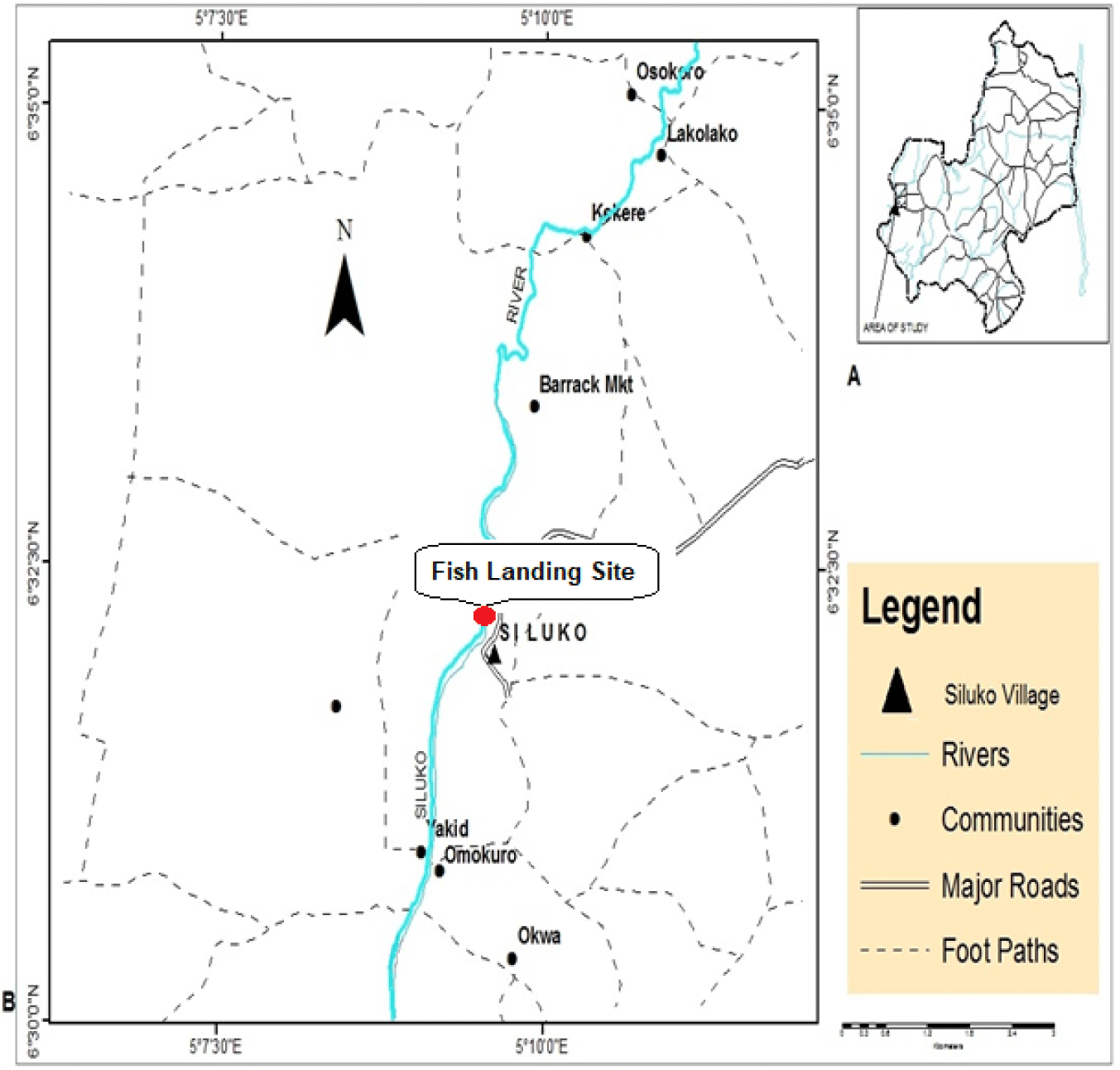
Fresh samples of S. schall were collected forth-nightly for a duration of fourteen (14) months–March 2015 to April, 2016 at the fish landing site in Siluko town (latitude 06° 17 ′ 20 ″ N and longitude 5° 00 ′ 25 ″ E) in Ovia South-West local Government Area of Edo State, Nigeria (Fig. 1). Fish samples were collected with the assistance of artisanal fishermen utilizing fishing traps, cast and gill nets of several mesh sizes. A total of 130 specimens of S. schall were collected throughout the study. The fish samples were immediately stored in an ice chest and transported to the laboratory in the University of Benin were the morphometric measurements were carried out. All fish specimens collected were identified to species level using the taxonomic keys of Idodo-Umeh (2003) and each specimen was tagged and numbered for easy reference and retrieval (Fig. 2).
After taking routine measurements, each fish was dissected to expose the gonads. The shape, size, colour, texture of the gonads and degree of occupancy of the visceral cavity were recorded. The whole length of the reproductive system was traced out and a diagrammatic representation was made. Testes and ovaries were carefully removed for each fish specimen, and weighed to the near 0.01 g using a Griffin’s top loading balance. Immature testes/ovaries and mature testes were preserved in 4%–5% formalin, while the mature ovaries were split longitudinally and preserved in Gilson’s fluid (Bagenal, 1978). After about a week, the mature ovaries were vigorously shaken at intervals to enable the eggs (oocytes) separate properly from each other and the ovarian tissue. The eggs were then thoroughly cleaned by rinsing with preservative and estimated by direct-count.
Information on the size of first sexual maturity is vital in the management of fisheries resources (Dadebo et al., 2003). To calculate the size at which 50% of individuals are mature, individuals were ranked in size classes and determined by finding the median for the ogive curve for males and females respectively using Excel (Windows 2013). All individuals in maturity stages II, III and IV were taken as mature individuals for both sex (Brown-Peterson et al., 2011).
After dissection, the sex of each fish specimen was ascertained by macroscopic examination of the fresh gonads. The sexed specimens were categorized into males and females for each species. The total number of each sex for the various species was pooled monthly and the ratio of males to females was determined for each species.
The chi-square analysis was calculated using the equation:
Where,
fi = Observed frequency
Fi = Expected frequency
The maturity stages were determined based on macroscopic examination of the fresh gonads of and were classified as one of the following stages according to Nikolsky & Birkett (1963).
I. Immature, II. Resting, III. Ripening, IV. Ripe, V. Spawning, VI. Spent.
Fecundity estimates were made from the matured ovaries in stage IV and V. Only ripe oocytes were estimated by direct-count method. The relationship between fecundity and fish length / body weight / ovary weight was described by the equation (Bagenal, 1978):
Where,
F = Fecundity
X = Body length (cm) or body weight (g) or ovary weight (g)
b = Slope of regression line (regression coefficient)
a = Intercept of regression line with the y-axis (regression constant)
Through logarithmic transformation, the equation becomes
The gonadosomatic index (GSI) was calculated for each gonad as described by Mbu-oben (1995) using the equation:
The Microsoft Excel 2016 and Statistical package for social sciences (SPSS) version 16.0 software were used in the evaluation of data. Chi-square analysis was used to determine the monthly and overall sex ratios of the fish population. Analysis of variance (ANOVA) was utilized in testing for significant differences. Linear relationships was evaluated using regression analysis.
Results
Results from this study focuses on determination of some reproductive parameters of S. schall, based on the gonad morphology, sex-ratio, macroscopic stages of gonad maturity and fecundity.
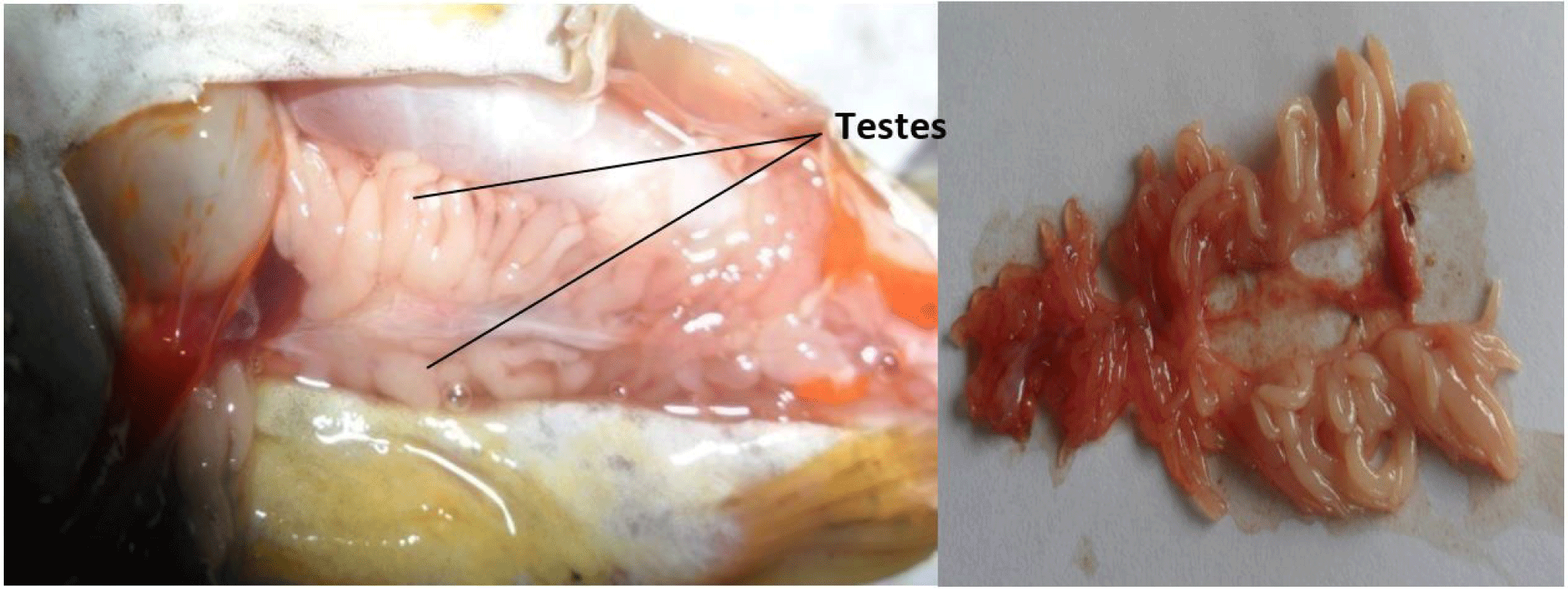
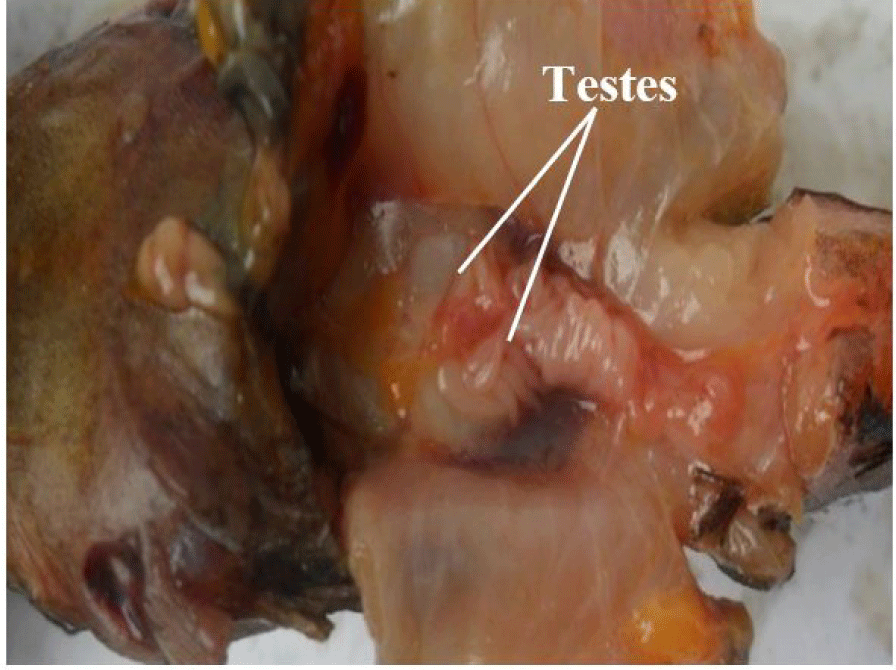
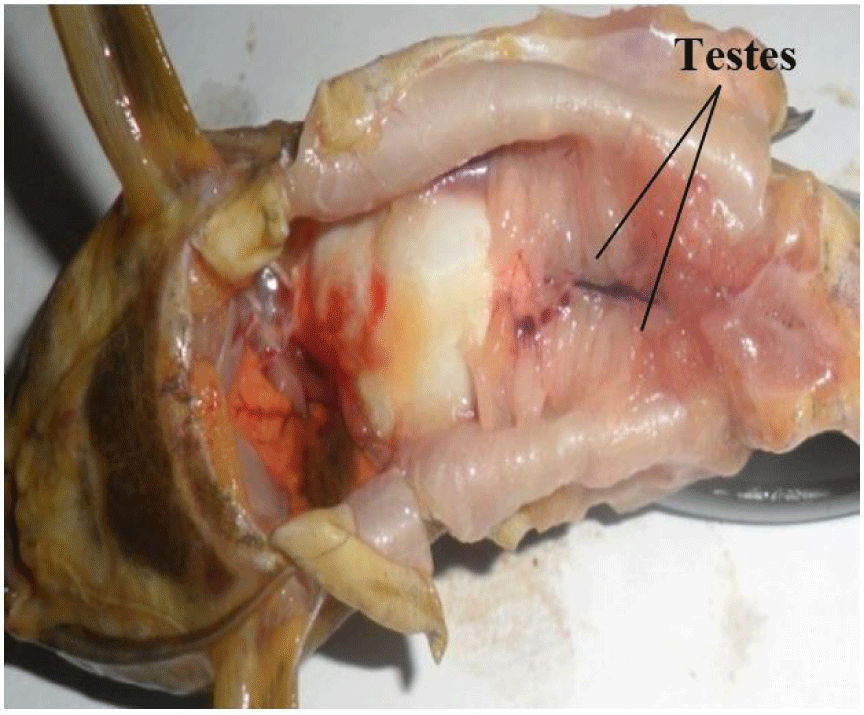
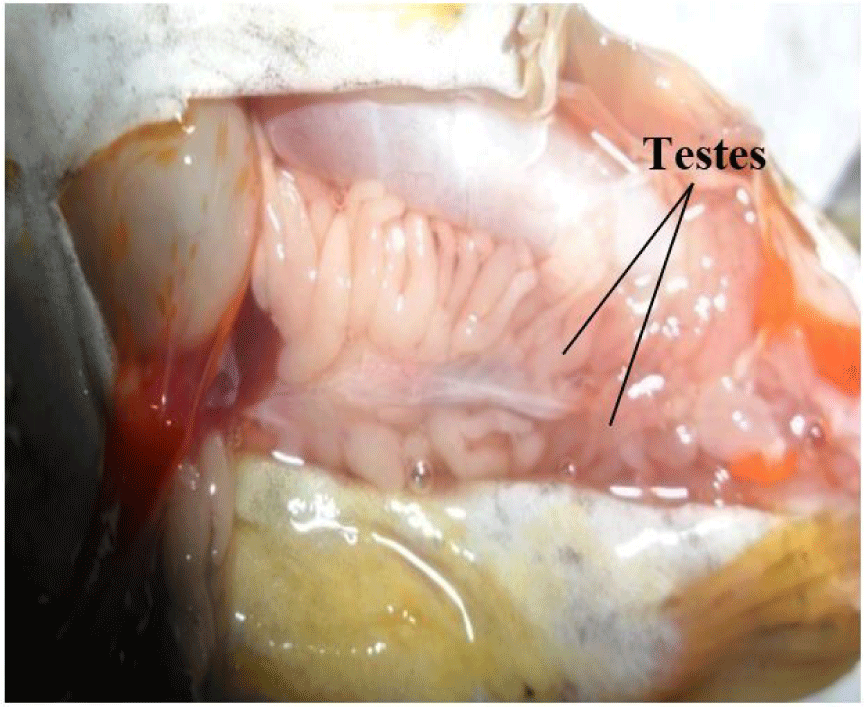
It consists of a pair of lobulate, enlongated, and partially fused (posteriorly) testes (Fig. 3). They are attached by mesenteries to the dorsal abdominal wall and situated alongside the swim bladder. They are connected posteriorly to the sperm duct which opens to the exterior via the genital pore. They are smooth, with finger-like projections arising from its central axis. The paired testes may be of equal or varied lengths and sizes, but this follows no particular pattern (it may be the left or right testes).
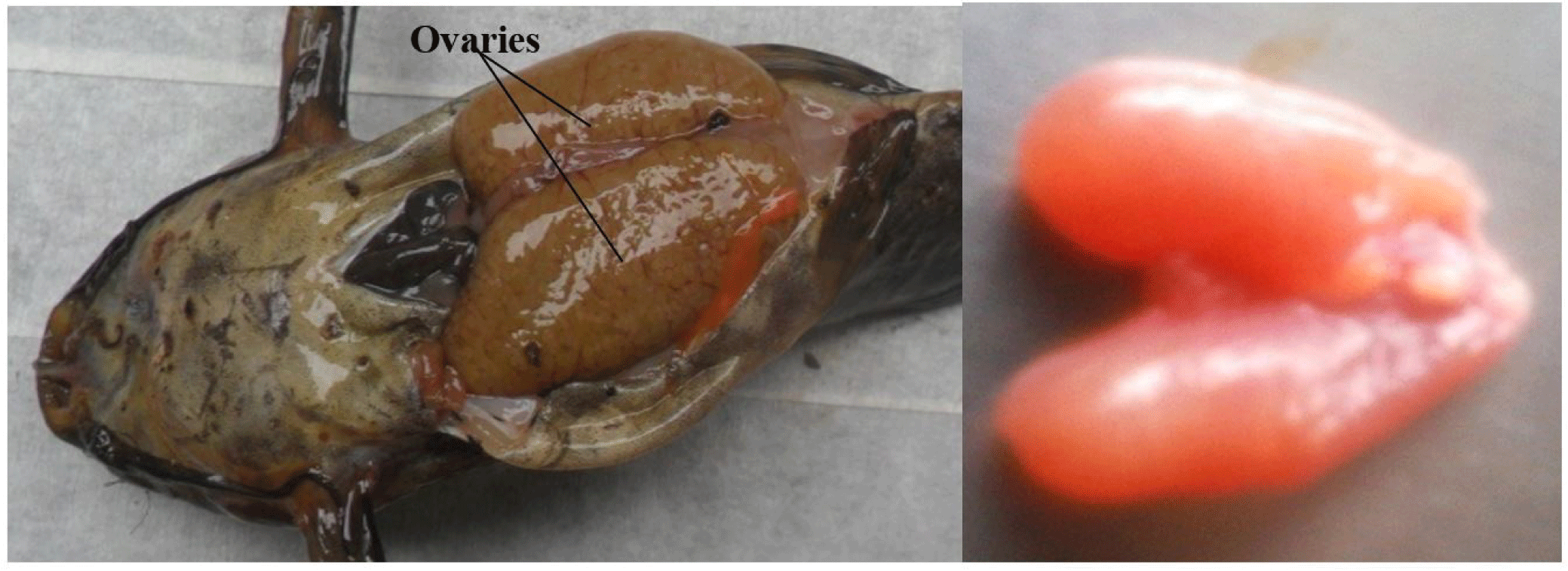
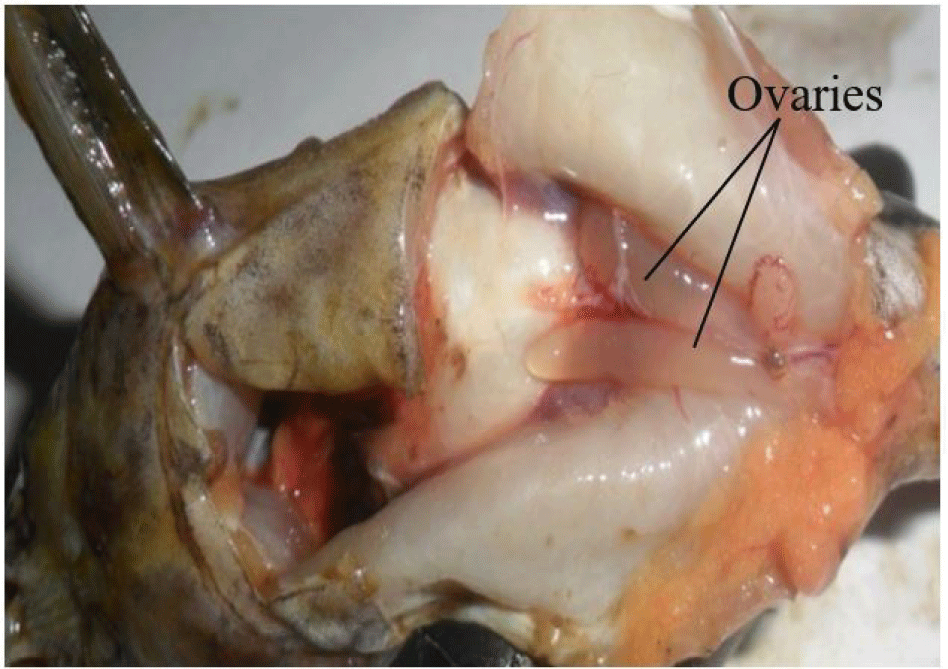
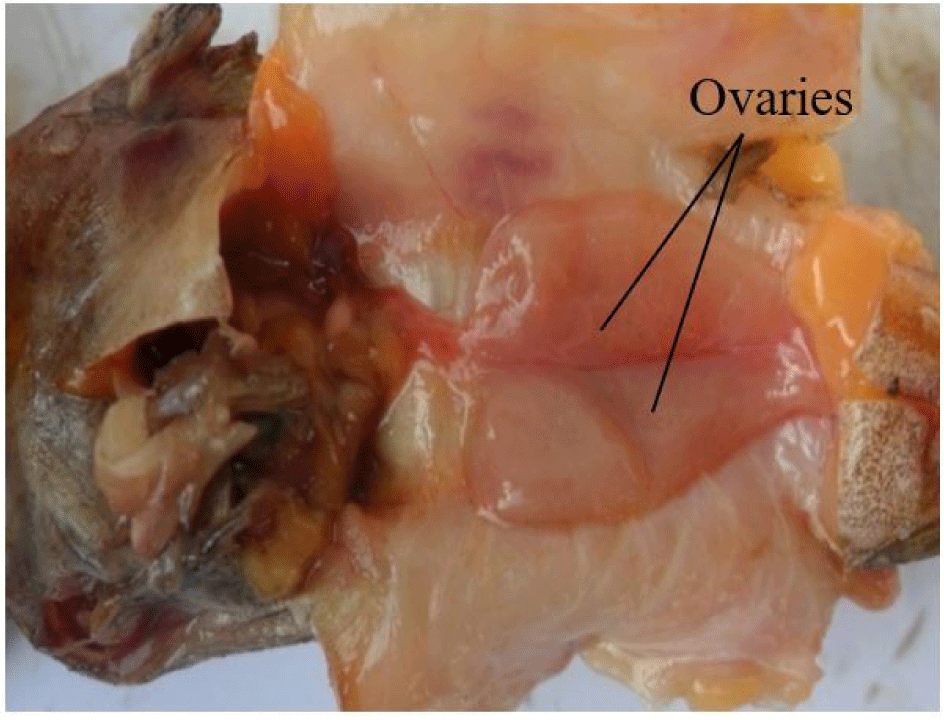
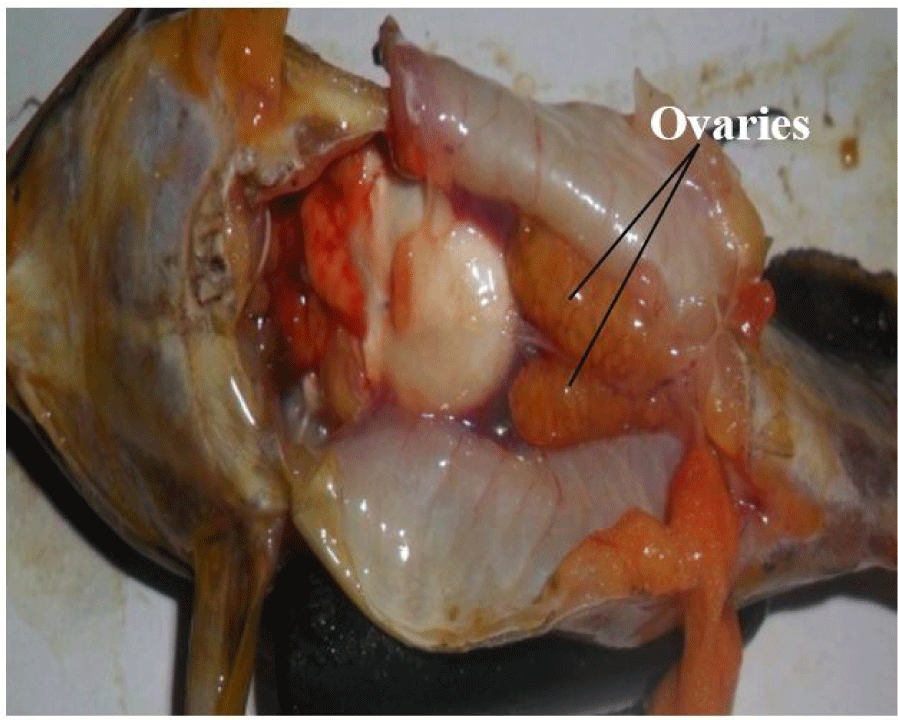
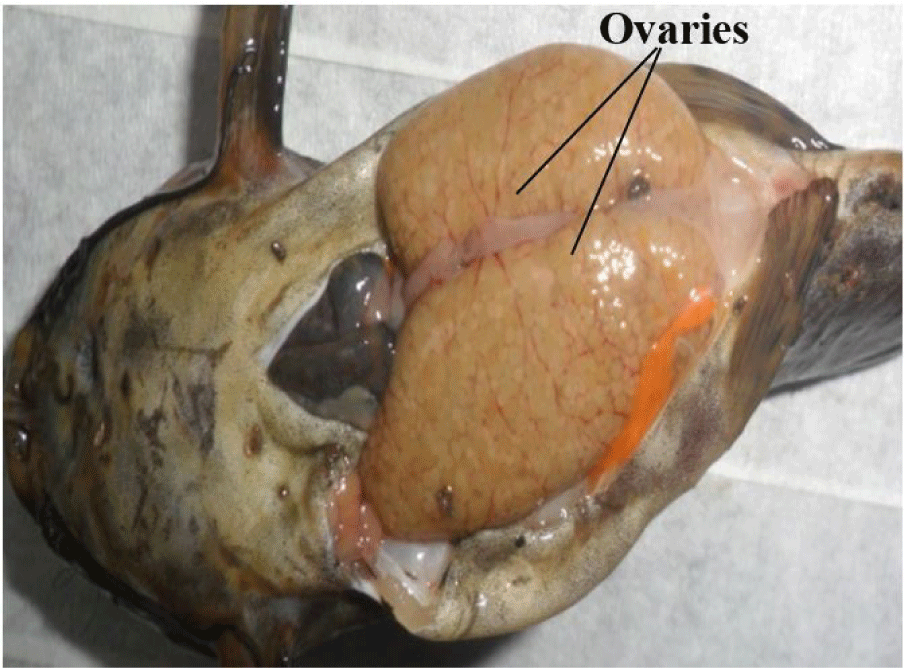
It consists of a pair of sac-like, cystovarian and partially fused ovaries (Fig. 4). They are attached and suspended by mesenteries to the dorsal abdominal wall and situated alongside the swim bladder. They may be of equal or varied length and size, taking no particular defined order (left or right ovary may be larger or longer).
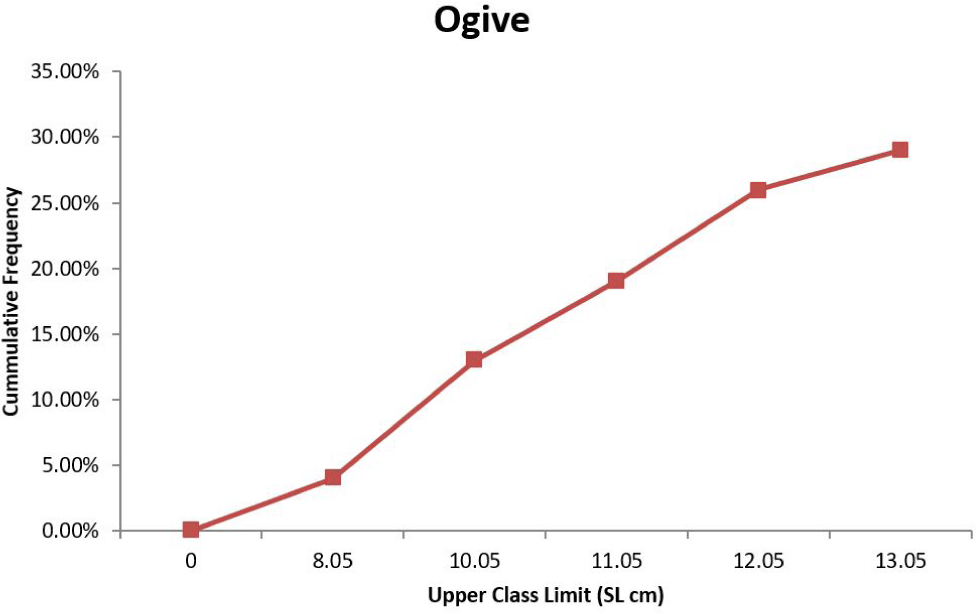
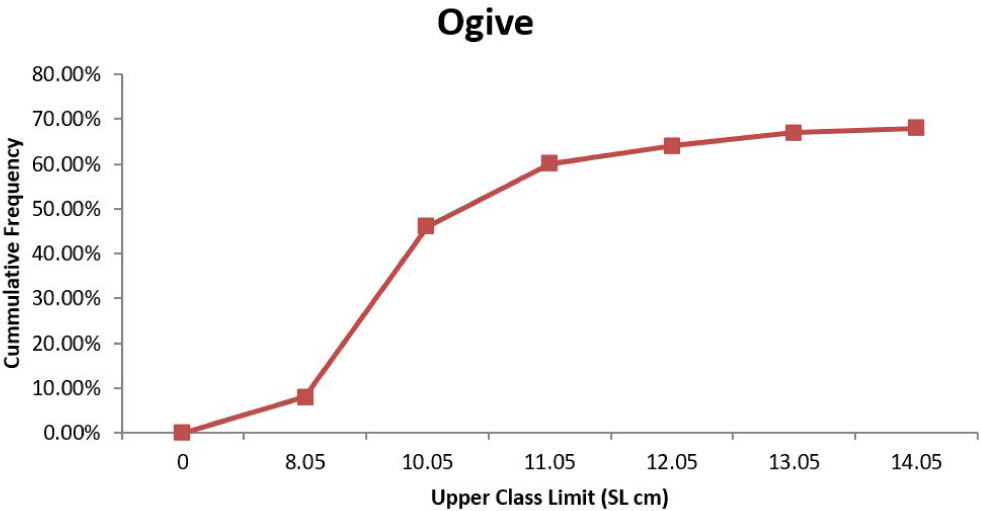
Table 1 shows the size and weight class for the sexed fishes used to ascertain size at sexual maturity. The proportion of mature individuals increased between 6.70 and 13.00 cm SL for both males and female (Table 1). The smallest size at which mature specimens were observed for males was 6.70 cm and 7.20 cm for females. The first maturity sizes observed for S. Schall were (L50 = 10.05 cm) for males and (L50 = 9.05 cm) for females (Figs. 5 and 6).
An overall of 130 specimens of S. schall were sexed. Of this number 54 males and 76 females were identified, which gave a sex ratio of 1:1.41. In the month of April (1:0.36), males significantly (p < 0.01) outnumbered females, while in August (1:2.09) females occurred in significantly higher proportions (p < 0.01) than males. Almost equal proportions of males and females were observed in July. The overall sex ratio (1:1.41) of the population when tested statistically showed no significant difference (χ2 = 3.723, df = 1, p > 0.05) from the anticipated 1:1 male to female ratio (Table 2).
Table 3 show the testes and ovaries classification based on the macroscopic appearance of the fresh gonads, using the maturation scheme by Nikolsky & Birkett (1963).
| Maturity stage | Male | Female |
|---|---|---|
| I. IMMATURE | Testes are small, elongated with smooth, tiny finger-like projections of almost equal sizes arising from the central axis. They are whitish in colour and occupies ¼ the VC length. GSI ranged from 0.35 to 1.24 (mean = 0.64) (Fig. 7). | Ovaries are small, smooth, oval, translucent and pale white in colour with no visible occytes. Occupies ¼ the VC length. GSI varied from 0.27 to 1.44 with a mean of 0.60 (Fig. 10). |
| II. RESTING | Testes are small, whitish and occuping ¼ the VC length. GSI varied from 0.40 to 3.51 with a mean of 1.34 (Fig. 8). | Ovaries increases in size, creamy-yellow, with no visible oocytes. Occupies about ½ the VC length. GSI ranged from 1.13 to 12.32 (mean = 6.34) (Fig. 11). |
| III. RIPENING | Testes increases in size, with finger-like projections becoming more conspicuous and larger as they progress to the anterior end. They are creamy-white in colour and occupies ½ the VC length. GSI ranged from 0.71 to 1.43 (mean = 1.03) (Fig. 9). | Ovaries increases in size, creamy-yellow, with visible oocytes. Occupies about ½ the VC length. GSI range from 1.13 to 12.32 (mean 6.34) (Fig. 12). |
| IV. RIPE | Not represented. | Ovaries are enlarged, yellowish in colour with large number of ova distinctly visible. Occupies ¾ or the entire VC length. GSI ranged from 5.21 to 20.19 (mean = 12.35) (Fig. 13). |
| V. SPAWNING | Not represented. | Not represented. |
| VI. SPENT | Not represented. | Not represented. |
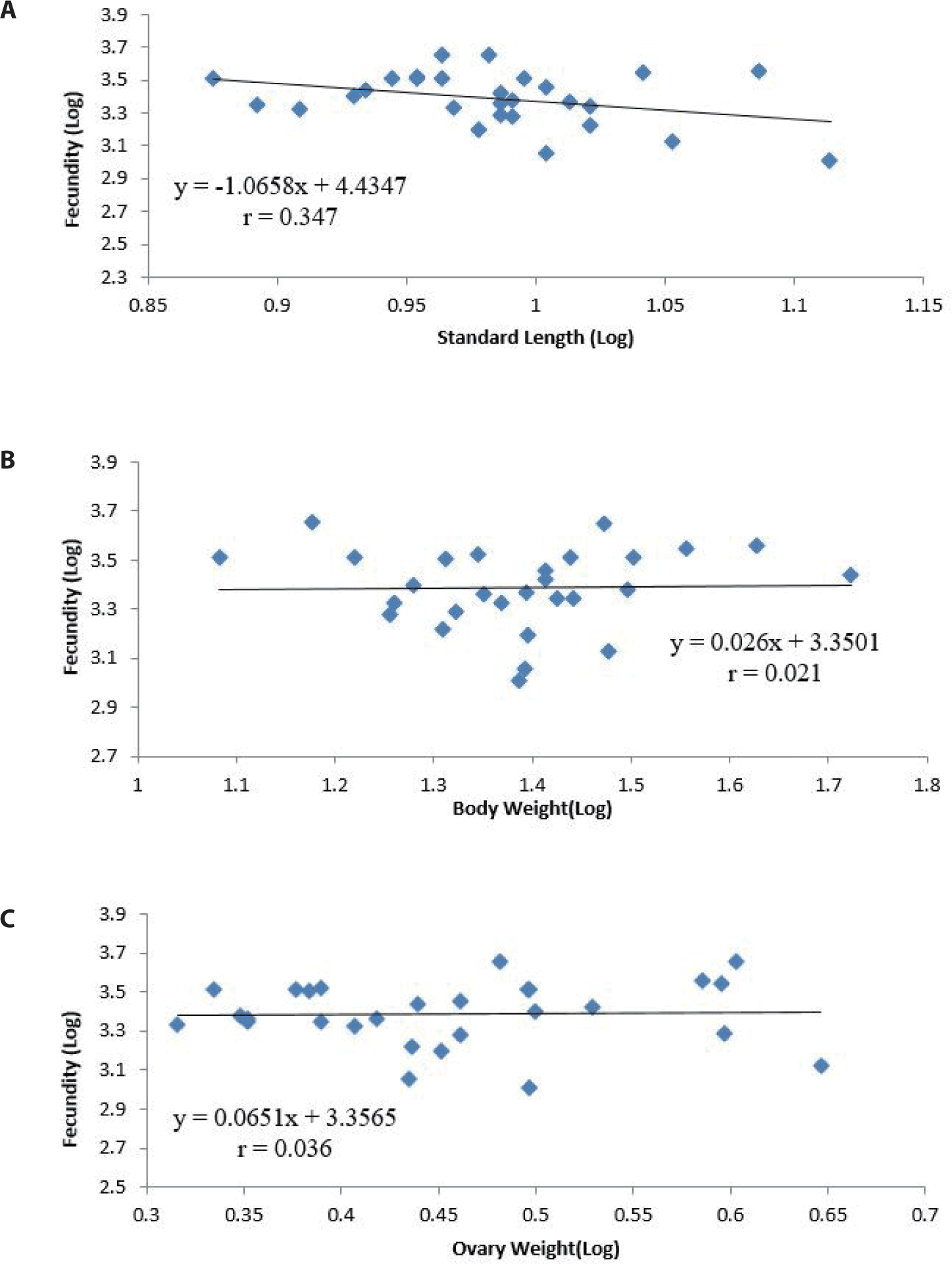
For the twenty-eight (28) ripe females observed, fecundity varied from 1,020 to 4,520 eggs with a mean of 2,480.54 ± 970.24 and was determined in fish of total lengths of 9.20 to 19.00 cm, standard lengths of 7.50 to 13.00 cm, body weights of 12.11 to 52.78 g and ovary weights of 2.07 to 4.43 g. Eggs were observed to be oval, yellowish and of almost uniform size. Regression analysis of fecundity–standard length, fecundity–ovary weight and fecundity–body weight showed a positive relationship with correlation coefficients (r) of 0.347, 0.021 and 0.036 respectively with no significant correlation and regression (p > 0.05).
Discussion
The size and age at sexual maturity have been intensely related with growth, maximum size and longevity (Froese & Binohlan, 2000). The size at maturity of S. schall was 10.05 cm for males and 9.05 cm for females. When compared with the values of 16.0 and 15.0 cm for females and males of S. schall by Lalèyè et al. (2006), 28.2 and 29.4 cm by Mekkawy & Hassan (2011), and 26.4 and 23.1 cm for females and males respectively by Dadebo (2016) from studies in Benin, Egypt and Ethiopia, this showed a huge contrast. Environmental conditions have been known to induce phenotypic flexibility in fishes with changes in age and size at maturity (Wertheimer et al., 2004). Thus it can be inferred that size at first maturity varies within species and its bio-geographical zone (Sossoukpe et al., 2013).
The overall sex ratios observed for the fish population in this study, showed a balanced population; since it was not substantially different from the anticipated 1:1 ratio. An overall sex ratio of 1:1.41 observed for S. schall, is similar to reported sex ratios for same species of 1:1.1 (Lalèyè et al., 2006), 1:1.04 (Mekkawy & Hassan, 2011), 1:1 (Akombo et al., 2011), 1:0.9 (Oboh et al., 2013), 1:1.35 (Akombo et al., 2015) and 1:1.39 (Dadebo, 2016). While Ebochuo et al. (2019) recorded a ratio of 2:1 for Synodontis omias were males outnumbered females. In all of these investigations almost equal proportions of males and females were obtained. This is also same for some related species, S. eupterus (0.96:1), S. clarias (1:1) and S. nigrita (1:1.47) by Shinkafi & Daneji (2011), Akombo et al. (2011) and Olojo et al. (2012) respectively. But in other related species, S. nigrita (1:2.5), S. membranaceus (1:2), S. resupinatus (1:8) and S. nigrita (1:3) females significantly outnumbered males (Akombo et al., 2011; Lalèyè et al., 2006; Olele & Etim, 2011). Differences in sex ratios among same and related fish may be due to the types of fishing gear used during sampling, mortality and survival rate among species, migration of different sexes during feeding and spawning, age difference and sex present during sampling (Oboh et al., 2014). Also, adult sex ratios influences pairing behaviors which include male to female, male to male and female to female interfaces (Alonzo & Sheldon, 2010).
The presence of paired gonads is typical for most bony fishes. The possession of genital papilla in adult males is a distinguishing feature of most catfishes (Holden & Reed, 1972), with females generally bigger and weightier than males, with the largest gonads being the ripening and ripe in males and females respectively (Shinkafi & Daneji, 2011) which is evident of sexual dimorphism. This has similarly been reported for S. schall and S. nigrita in Oueme River (Lalèyè et al., 2006) and S. eupterus in River Rima (Shinkafi & Daneji, 2011). Maturity stages I, II, III and IV were observed for females and I, II and III for males. Spawning (stage V) and spent (stage VI) stages were not encountered during the course of study. Gonad development was observed to be closely associated with development and increase in visceral weight of the fish from the immature to mature stages. The absence of spawning and spent males and females may be due to seasonality, gear selectivity, time of sampling and human factors. Changes in water level may also change the course of fishing route and areas of coverage, since shallow areas become deeper and are avoided by fishermen.
For fecundity to be properly estimated attributes such as size at first sexual maturity (Hossain, 2010; Lambert, 2008), duration of spawning season, daily spawning behaviour and spawning fraction (Murua et al., 2003) are to be considered, but this was not the case for this study, as it was limited by female specimens being at various stages of ovarian-maturation, but other factors such as condition factor, GSI and ovary weight were considered. Absolute fecundity is the total number of eggs that are likely to be spawned in one spawning season (Kant et al., 2016) and ranged from 1,020 to 4,520 eggs (mean = 2,480 eggs). From the results of the study it was shown that fecundity was independent of standard length, ovary weight and body weight ovary, while information on the size at first sexual maturity for fecundity estimation for S. schall is almost none existent, thus its absence from this study. This indicates that an increase in the number of eggs in the ovaries does not necessitate a corresponding increase in the proportion of body length, body weight and gonad weight of the fish species (Hossain et al., 2012).
The fecundity of S. schall (1,020 to 4,520 eggs) in this study was observed to be much lower than those reported for same species; 7,910 to 64,450 eggs (Araoye, 2001), 1,841 to 15,076 eggs (Lalèyè et al., 2006), 1,440 to 102,600 eggs (Mekkawy & Hassan, 2011), 1,530 to 13,965 eggs (Oboh et al., 2013) and 983 to 3,797 eggs (Dadebo, 2016). It however, compares with that of S. nigrita (675 to 3,642 eggs) by Olele & Etim (2011). And was lower than the range of 11,014 to 16,903 eggs reported by Ebochuo et al. (2019) for S. omias. Fish fecundity is best estimated accurately using two morphometric variables taken together with less accuracy for length than weight variables (body weight and ovary weight) (Bhatt et al., 1977), with gonad weight being more accurate for estimating fish fecundity than the use of body length and weight (Hossain et al., 2012). But in reality this is not possible under field conditions for live specimens (Bhatt et al., 1977). The linear relationship between fecundity and standard length, fecundity and body weight as well as fecundity and ovary weight has previously been reported by various authors whom have worked on fish reproductive biology. Similarly the straight-line relationship reported for absolute fecundity with body parameters such as standard length, ovary weight and body weight were observed by the authors listed above. The changes in absolute fecundity recorded can be ascribed to changes in food availability, ecological conditions, density-dependent mechanisms, and fish size (Bagenal, 1978; Nikolsky, 1969; Sztramko & Teleki, 1977; Treasure, 1981).
Among members of the same species, variation in fecundity is an outcome of diverse adjustments to environmental surroundings like water temperature, changes in water level due to seasonality and pollution load (Witthames et al., 1995). Also within the same stock, variation in fecundity have been shown to vary annually, exhibit long-term change, proportionate to fish size and condition (Kjesbu et al., 1998; Murua et al., 2003; Rijnsdorp, 1991). Bagenal (1978) emphasized that there exists a wide fluctuations in fecundity among fishes of the same species, age and size. Females with the highest number of eggs did not have the highest standard lengths and body weights.
The presence of almost uniform sized eggs in S. shall is an indication that is a total spawner. This corroborates with previous studies of Lalèyè et al. (2006) and Oboh et al. (2013) for S. schall. This study has contributed to the existing data on the reproductive biology of S. shall in Nigeria. Also, findings from this study will serve as important fishery management tools in the conservation and utilization of this valuable freshwater fish species.