Introduction
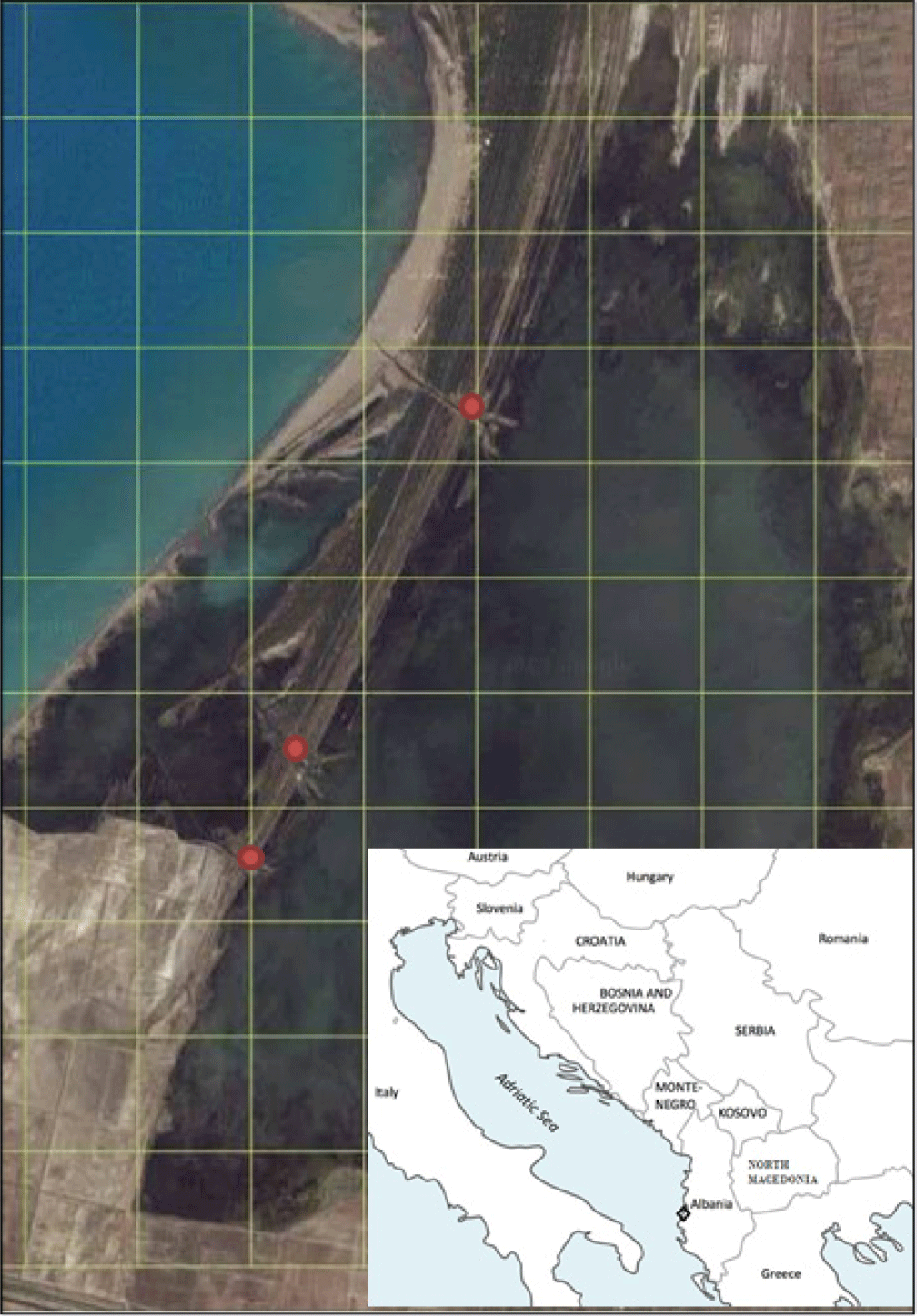
The transitional water ecosystems are dynamic one with high ecological value, as they not only play an important role in nutrient recycling, but also serve as essential habitats for many aquatic organisms, especially fish (Elliot & Hemingway, 2002; Sapounidis & Koutrakis, 2021). Further on different studies are attempting to define the effects of different factors including climatic change and the subsequent responses of various marine ecosystems (Jackson et al., 2001; Kim et al., 2022). At the global scale coastal lagoons and coastal shelf waters at intermediate latitudes are considered to be among the most productive ecosystems in the world (Haimovici & Cardoso, 2016; Pérez-Ruzafa & Marcos, 2012). These relatively isolated ecosystems might help to understand the high productivity of costal lagoons and their importance as shelter for fisheries resources (Cataudella et al., 2015), while coastal waters enriched by continental runoff and diverse oceanographic processes are responsible for nearly 10% of world fisheries landings (Pauly & Christensen, 1995). The fish community has been considered as a key biological component in conservation management and monitoring of the transitional water bodies by the Water Framework Directive (WFD; European Council, 2000). Further on, there has been developed numerous fish-based indices for the assessment of the ecological status of transitional water bodies (Borja et al., 2004; Breine et al., 2007; Coates et al., 2007). These approaches rely on structural aspects of fish assemblages (e.g., species richness and taxonomical composition and presence with different habitats, etc.), which are developed to indicate the fish quality elements with transitional bodies in the WFD (European Council, 2000). Along with this approach there are also under use other attributes as functional properties of fish assemblages (e.g., nursery function, trophic integrity) to assess the functioning of transitional water bodies ecosystems, following the recommendation provided by Elliott & Quintino (2007). It is worth to mention that use of fish with these contexts provides also opportunity to consider the natural variability of these systems which compounds the problem of distinguishing natural from human-induced stress (the estuarine quality paradox; Elliott & Quintino, 2007). The general purpose of this survey is the situation analysis of fishery in Karavasta lagoon with its associated problematic issues (Fig. 1).
The main purpose of this survey is the situation analysis of fishery in Karavasta lagoon with its associated problematic issues. So, the paper is providing a general description of fishing activities; international framework on fishery of Albania, law and government’s policy on fishery; relevant regulation of fishery that are employed within lagoon ecosystem; description of operational modes of fish traps in three channels by Fishery Management Organization (FMO); stakeholder and problem analyses for a sustainable fishery and finally it recommends possible activities to be implemented with aim enhancement of fishery status. These analyses aimed at to reveal ways how to enable the current actors and how fisheries can be improved to maximize both conservation and income generation for the FMO and end users.
Presently the coastal and lagoonal environments present high levels of complexity, diverse habitats and support a high level of biodiversity (Munari et al., 2010), while in the Mediterranean region they are characterized by shallowness, relative isolation from the open sea due to coastal barriers, presence of inlets or channels for exchanges with the sea, and presence of boundaries with strong physical and ecological gradients (McLusky & Elliott, 2007; Tagliapietra & Volpi Ghirardini, 2006). All these features are fitting with location, functioning and ecological characterizations of the Karavasta lagoon. Further to this due to specific climatic location and beside its size Karavasta lagoon present a considerable variability in its environmental conditions (e.g., size, hydrological connectivity, geomorphology, salinity range, siltation processes, etc.), which may affect fish assemblages’ structure and functioning. In order to find the best drivers that likely are associated with type-specific fish assemblage reference conditions, the spatial variability of both the environmental features and the fish assemblages need to be further investigated.
The Karavasta lagoon is the largest surface (4,100 ha) of this wetland complex; it is 15.4 km long and 4.1 km wide. The maximum depth is 1.3 m with an average depth of 0.7 m (Fig. 1). The lagoon is divided from the sea by Divjaka pine forest and Godulla lagoon. Agricultural land borders the lagoon. The coastal morphology of Karavasta lagoon was highly dynamic during the last 135 years because of the changes which occurred to the Shkumbini and Semani river deltas, due to sediment transport at the lowland areas (Munari et al., 2010). Karavasta is connected to the sea via three channels (Brew, 2003). The northern inlet, at present is completely blocked and disconnected from the sea. The central and southern inlets communicate with the Godulla lagoon, which is connected to the sea by two other shallow channels. The creation of the drainage canals of Terbufi and Myzeqe in the 1980s, together with associated irrigation and drainage schemes, has isolated Karavasta from a significant part of its former drainage basin area. Early in its formation, which goes back to about 1860, the Karavasta lagoon covered double the area it now occupies.
The commercial fish species Karavasta lagoon complex is typical of Mediterranean lagoons with two groups of species: the migratory and sedentary one (Bourquard, 1985; Crivelli et al., 1996). The top commercials include: European eel (Anguilla anguilla), species of Family Mugilidae (Mugil cephalus, Liza ramada, Liza salienes and Chelon labrosus), Seabream (Sparusaurata), Seabass (Dincentrarchus labrax), etc. Following discussions with old local fisherman, the two more commercial species sandsmelt (Atherina boyeri) and lagoon crab (Carcinus aestuaris), were part of catch but poorly marketed. They were part of the market before year 1970. Later on (1970–1985) these species were caught for the purpose of animal feeding. The migratory species are representatives of Sparidae, Mugilidae, Moronidae, Soleidae, Anguillidae and Belonidae, while the sedentary one by Gobidae, Cyprinodontidae, Atherinidae and Sygnathidae.
Materials and Methods
Along with field survey conducte din the period of 2020–2021, the local landings statistics were recovered from archival data reports of the Ministry of Agriculture and Rural Development (2019), through its Department for Fishery and Aquaculture Policy within Fishery and Aquaculture Service Directorate for the periods of 1937–1940 until 2015–2020.
The analysis of the fishery historical changes, life history of fishes and selected species was based on the available information of: (1) individual species fish productivity and share within stock distribution; (2) fish catch in tones following periods and species contribution in all fishing grounds of Karavasta lagoon; (3) larger sizes and maximum ages in commercial catches; (4) share of European eel (A. anaguilla) for the period of 2012–2020; (5) spawning type and seasons and the order of magnitude of the annual oscillations; and (6) life-history changes, as reduction in the recruitments, grounds, changes in catches and share in the stock.
Assessment criteria depended on available data of total landing of the stocks of each of the main species being mainly focused on commercial ones (mainly European eel, Mugilidae and Sparidae), including those in other landing locations on the southeastern part of the lagoon, catch per unit effort (CPUE), and the exploitation rate. CPUE and biological data of the main species landed by commercial fishing in Karavasta lagoon were obtained from archival data of its Department for Fishery and Aquaculture Policy within Fishery and Aquaculture Service Directorate for the periods of 1937–1940 until 2015–2020.
PAST 3 software was used to elaborate the data obtained. To check if the fish communities of the samples are homogeneous, a clustering method was applied. Principal component analyses (PCA) and multi-dimensional scaling was performed to determine the relative placement of the samples in a two-axis ordination plot according to their fish fauna and physical & chemical parameters.
Results and Discussion
The commercial fish species Karavasta lagoon complex is typical of Mediterranean lagoons with two groups of species: the migratory and sedentary one (Crivelli et al., 1996). The top commercials include: European eel (A. anguilla), species of Family Mugilidae (M. cephalus, L. ramada, L. salienes and C. labrosus), Seabream (Sparusaurata), Seabass (D. labrax), etc. (Fig. 2). Following discussions with old local fisherman, the two more commercial species sandsmelt (A. boyeri) and lagoon crab (C. aestuaris), were part of catch but poorly marketed. They were part of the market before year 1970 (personal communication with old local fisherman). Later on (1970–1985) these species were caught for the purpose of animal feeding. The resident species are numerically prevalent with the two species A. boyeri and Aphanius fasciatus showing the highest abundances in these types of ecosystems. Other present species are A. anguilla, Syngnathus spp., Gobius bucchichi, Belone belone, D. labrax, Sparus aurata, etc. (Crivelli et al., 1996).
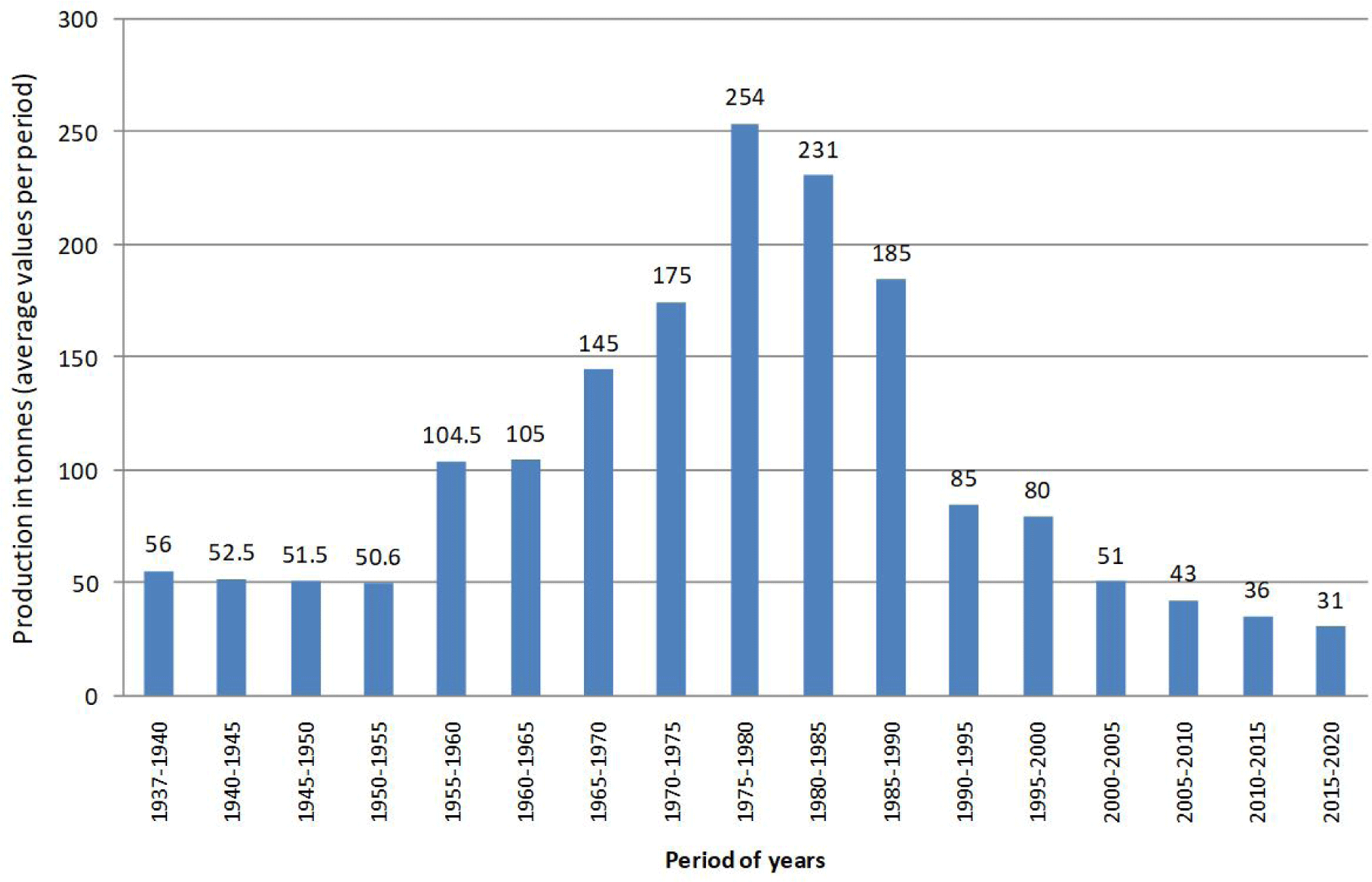
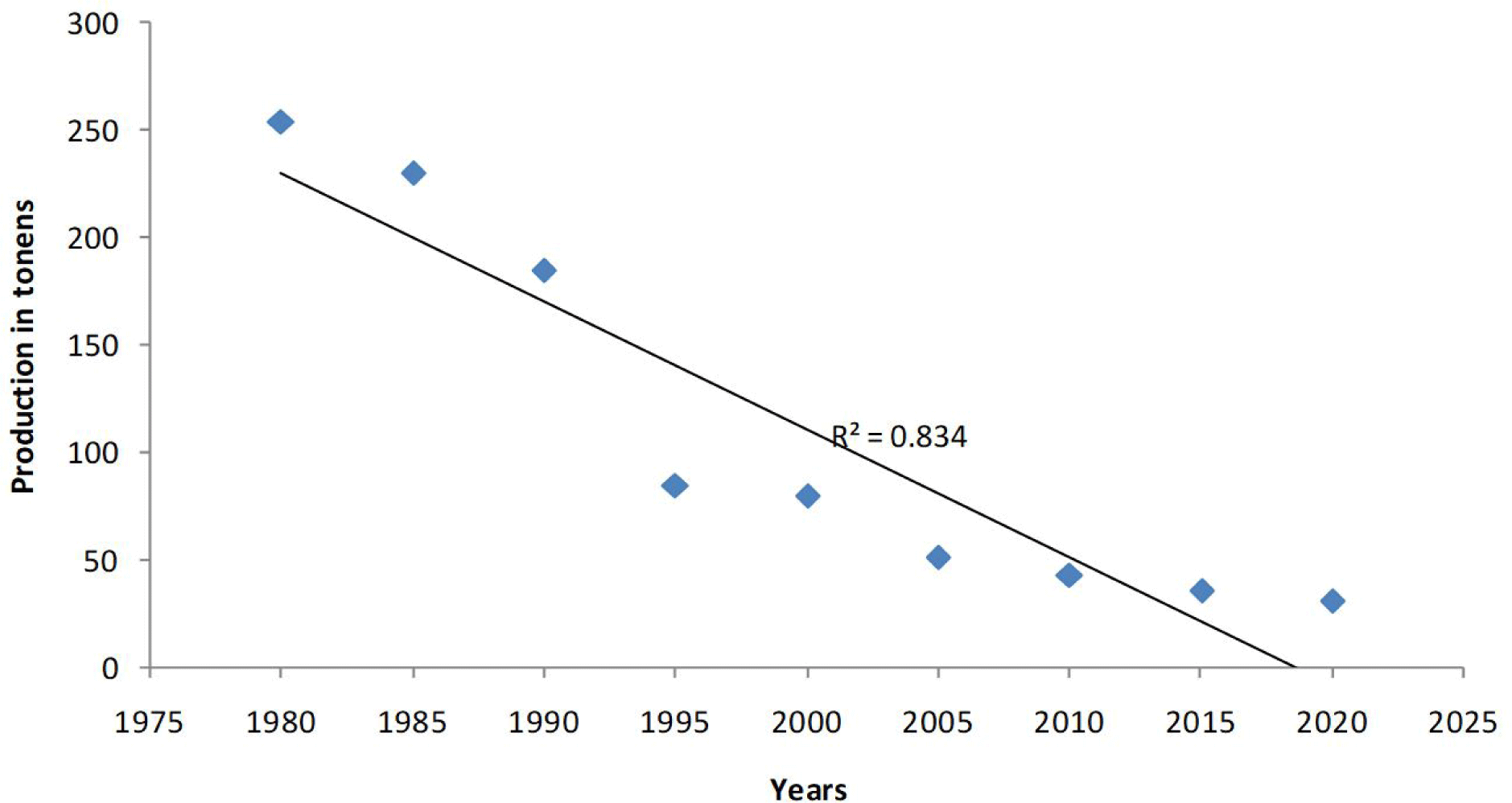
Following the Fig. 3, it is clear that in the 1970–1990 a significant increase of fish catch it linked to inclusion in fish catch of Sandsmelt and lagoon crab from one side, and from the other side to an increased fishing effort linked with increased number of fisherman and improved fishing tool, devices, operational fishing in communicating channels. So, the fish catch is oscillating from 56 tones (1937–1940)-175 tones (1970–1975)-254 tones (1975–1980)-185 tones (1985–1990) and 31 tones at the current period.
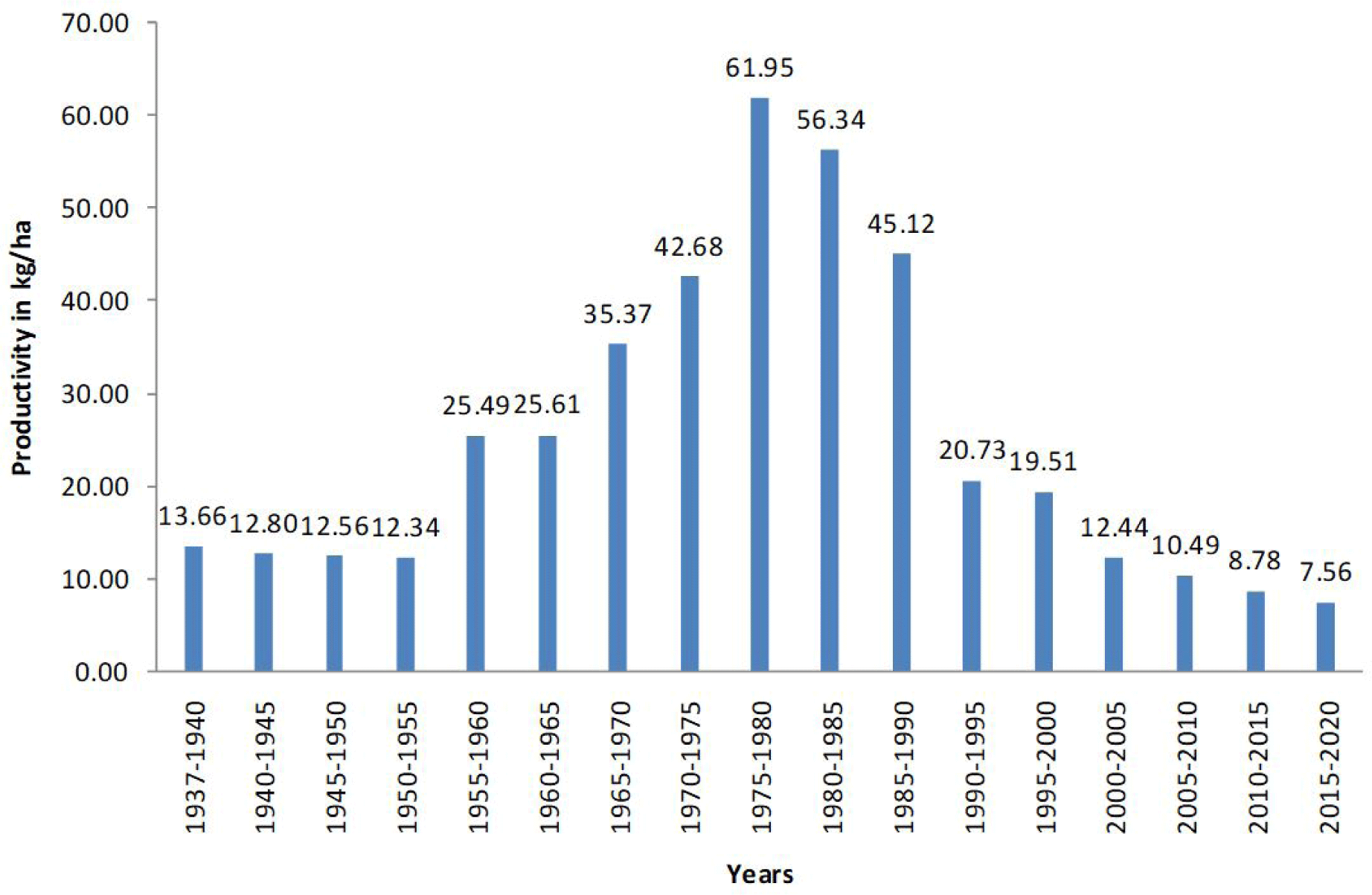
The trend of productivity of lagoon ecosystem in terms to kg/ha is presented in Fig. 4, where the maximum value of 61.95 kg/ha is recorded in period of 1975–80, while the lower value of 31 kg/ha in year 2020 although as mentioned above the fish data has to be critically considered (Crivelli et al., 1996).
Refereeing to individual fish species, at the current circumstances the European eel (A. anguilla), represents among the main species within catch in the last decade comprising from 19.35% of entire catch in year 2020, while the maximum share of 26% is recorded during 2016.
What are the main issues? From one side the nature conservation policy and actions are needed, while from the other side the end-users right also needs to be respected. This hasten been achieved so far. The ecological effects of agriculture expansion and consequences of the hydrodynamic regime are clearly reflected at the current status of the ecosystem (Crivelli et al., 1996; Munari et al., 2010). Following the pressures (Grillas & Shumka, 2015) and the ecological condition of Karavasta, an intensification of aquaculture activities must be considered with caution, since the lagoon seems at significant risk of serious hypereutrophication (Munari et al., 2010). Presently the situation is aggravated by the limited water exchange with the marine environment due to the irregular dredging of the communication channels. The reason for such phenomenon is the position of Karavasta lagoon, i.e. it is located at the junction of the sea and the lagoon.
It is expected that the population density in the wider area of lagoon of Karavasta is going to be increased at the year 2031 at 62,500 inhabitants (Municipality of Divjaka, 2017). This is leading to similar development to other Mediterranean coastal zones where the conflict between the human exploitation of water resources and the ecological needs of aquatic ecosystems is an issue (Thompson & Flower, 2009). After the year ’90, entire Albanian coastal and transitional water ecosystems including coastal lagoons were becoming subject of serious concerns due to multi-faced human intervention associated with biota changes. Further on the direct connection to terrestrial ecosystems and lack of maintenance of hydrological connectivity leads towards affection lagoon functioning.
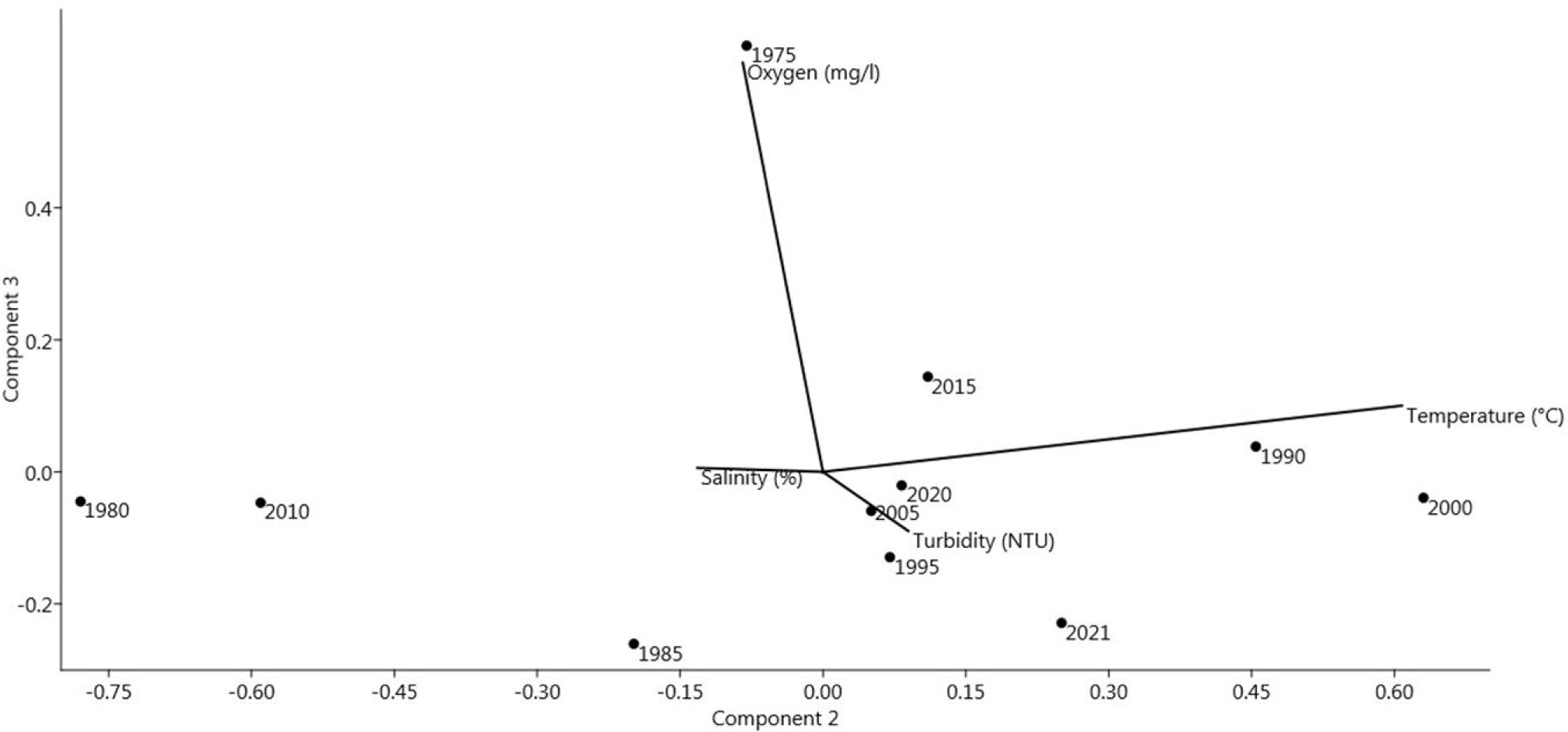
In the last three decades the agriculture, the dominant sector of local economy was becoming more intensive, with of land couple of times during the year associated with increased use of nutrients, chemicals and fertilizers (Munari et al., 2010). The intensification of urbanization with a shift of many activities to the coastal zones, as well as the increase of interventions aimed at implementing tourism-related facilities and agriculture practices have a strong impact on the lagoons’ ecosystem. For example, agriculture activities in the watershed often imply agricultural wastes and nutrient input into the lagoons as well as wastewater that may be contaminated with pesticides and contaminants, especially in those areas with intensive agriculture or industrial activities. Frequently, lagoons have even been considered as dumping areas for urban and industrial wastes (Grillas & Shumka, 2015), the near catchment of the lagoons is contrasted and partly reflected in the quality of sediment. The eastern catchment of Karavasta lagoons is occupied by agriculture and urbanization. Several drainage channels bring water from this area in the eastern part of the Karavasta lagoon resulting in high loads of organic matter and nitrogenic sediment. Further on following Grillas & Shumka (2015) the southern side of the lagoon host only very small villages and agricultural field are limited by soil salinity. Although drainage channel reaches the south of Karavasta lagoon, it should not bring significant pollution. The PCA analyses (Fig. 5) shows that productivity is also related to water temperature and salinity, beside that they do not show rapid changes over long time periods. This is a need of further long-term survey, while recent studies and projections for the similar ecosystems shows that climate change together with the peculiarities of mixing water will undoubtedly alter hydrological regime of this lagoons and biota components including primary productivity and sequencing food chain within ecosystem (Bertolini et al., 2021; Jakimavičius et al., 2018). This study shows that in the Karavasta lagoon in the latest decade, there have been years where high water temperatures were recorded for long periods over the summer while winters had colder values than other years (1990, 2000, 2004) as well as a cluster of years when extremely high summer water temperatures were observed (2017, 2019, 2021).
The increased population and urbanization have been associated with addition waste water flushing into lagoon ecosystem. Severe and widespread environmental problems experienced in many other coastal areas of Albania are associated with an excess of nutrients. Further on at the Mediterranean scale the eutrophication is a common phenomenon observed in many other coastal lagoons.
The distribution and spread of the Atlantic blue crab (Callinectes sapidus) are reported from marine, transitional and freshwater environments along the coasts of Adriatic Sea (Albania, Eastent Mediterranean sea). Increased abundance of the invasive blue crab was recorded for the period of 2011 to 2021 in the area of Karavasta lagoon. During the field survey of October 2020, a wide presence of a serpulidpolychaete (Ficopomatus enigmaticus) has been recorded in the large drainage channels in the wider project area due to drainage works. F. enigmaticus (Fauvel 1923), originally named Mercierella enigmatica, is a serpulid polychaete worm that builds and inhabits white calcareous tubes that vary from 1.85 to 2 mm in mouth diameter and 20 to 40 mm in length. Colonies were firstly identified for other Albanian costal area (Shumka et al., 2014).
Valonia aegagropila (Chlorophyta) is a species found in warm regions (subtropical to tropical latitudes) where it occupies lagoons and closed bays with low hydrodynamism (Grillas & Shumka, 2015). It is found in oligotrophic habitats between 1 and 7 meters depth and is more tolerant to high than low salinity (below 20 psu). V. aegagropila can double biomass in favorable conditions so with climate changes might lead to serious changes within ecology of ecosystem.
The climate variations can also be responsible for the invasion of unusual species in lagoon and jellyfish Aurelia aurita and Rhizostoma pulmo. In year 2015 presence of R. pulmo has beenrecorded in lagoon of Karavasta. They increased abundance may affect fishing activities or even have an effect on the fish commercial value (Molinero et al., 2009).
The breeding avifauna of Karavasta lagoon includes a small but remarkable colony of Dalmatian Pelicans (Pelecanus crispus) and likewise, the number of pairs of the Great and Pygmy cormorants (Phalacrocorax carbo, Microcarbo pygmeus), and other species is considerably high. Apart from its high ecological value, this important lagoon and its surroundings offer other key ecosystem services such as fisheries, recreation and bases for tourism and agriculture development. In the last three decades there are signs of eutrophication (Crivelli et al., 1996; Grillas & Shumka, 2015; Munari et al., 2010).
The communication between Karavasta lagoon and the sea tends is secured via Godulla lagoon with one opening to the sea and three communications between Godulla and Karavasta. It experiences and risks be closed by sedimentation resulting from coastal geomorphologic processes. The communication between the lagoons and the sea needs to be maintained artificially with high associated cost. The history of the construction of canals to maintain communication to the sea is summarized in Crivelli et al. (1996).
The maintenance of the communication to the sea is necessary to support the fish populations and thus the economy of fisheries and the populations of fish-eating birds notably pelicans. The improvement of the communication between the sea and the lagoons should improve this functions and services provided by the lagoons.
Following specific lagoon environmental particularities such as depth, limited connections with the sea, sediment dynamics, size, as well as water temperatures and productivity, they become very vulnerably systems that might be affected by global climate change and the rise of sea level (De Wit, 2011; Nicholls et al., 2007). Specific modeling approaches might be applied for defining the projections and impacts onto Karavasta lagoon ecosystems.
Fishery practices and communities of fishermen have been living off the lagoon for the old time. However, depending on how the water was managed, they have benefitted in very different ways, sometimes barely surviving and other times thriving. In case of Karavasta lagoon major parts of the problems that have affected the lagoon integrity throughout its history (particularly in the last several decades) have been man-made: agriculture development, modification to the environment and primarily to habitats original one, overfishing, pollution (of different type, waste water, solid waste, agricultural nutrient discharge, chemicals leakage, and lately, non-native species, global warming and destructive fishing practices). Exclusive fishing rights to one small party have also created great inequality among fishermen in the past. Management structures instability, frequent changes within stuff and managers, political interventions and un-sustainable short-term solution have led towards a degradation of fishery system and depletion of the resources. An active and adaptive management will be needed to maintain biodiversity under a changing climate particularly at the vulnerable ecosystems as coastal lagoons. In the study area, active management need to be oriented towards further improvement of the protection from human interference, while specific conservation need to include interventions in species and ecosystem processes that are stronger and more hands-on than today’s. Current agriculture and water use practices has to be oriented to low impute approaches and rational use of resources, while water protection from eutrophication activities has to be a priority. In every case biodiversity values must be actively considered in the face of climate change and in the context of competing uses for resources in land and sea. This requires an ongoing process to anticipate how ecosystems will respond to a changing climate while interacting with other environmental modifiers to change the dynamic interactions between species and therefore ecosystem functions with focus to resilience solution. In particular, globalization has altered the balance of goods and services produced by the lagoon and redistributed the economic benefits flowing from them, adversely affecting the livelihoods of the local fishing community. The fishermen need to introduce a new system of stock management and created alternative revenues, such as transformation of fish and new hospitality activities, to increase lowering margins. Increasing water temperatures demand daily monitoring and adjustments using sea water to diminish temperature, oxygen injections or pumping of asphyxiated water out.








