Introduction
The exploitation of fisheries resources starts from a basic human need to obtain animal protein sources. Squid is one of the protein sources from the ocean, and nearly all body parts are edible. Since 1950, capture production of cephalopods has continued to grow (Doubleday et al., 2016; Hunsicker et al., 2010), with total commercial annual catches between 3.5 and 4.9 million tons in 2008–2017 (FAO, 2019), almost 4.6 times higher than that of the 1950s. Cephalopods on average support approximately 15% and 20% of marine fishery landings and landed values, respectively (FAO, 2019; Hunsicker et al., 2010). This group has unique life history characteristics, including rapid growth, short lifespan, and semelparous reproductive strategy, giving them both sensitivity and resilience to anthropogenic exploitation and oceanographic variability (Rodhouse et al., 2014). The species within the family Ommastrephidae support approximately 33.8% of the global cephalopod’s landings (FAO, 2019). This group is recognized as voracious and adaptable predators of a broad range of prey including small crustaceans and fishes at early life stages and shift to micronekton, larger fishes, and cephalopods (including cannibalism) as they grow (Alegre et al., 2014; Nigmatullin et al., 2001). Despite its economic importance, the offshore oceanic squid resources’ exploitation rate is relatively low (Worms, 1983). The flying squids (Ommastrephidae; Oegopsid) cover about 65% of the world’s commercial cephalopods (Brunetti, 1990; Roper et al., 1984). In 2002, total world catch of Ommastrephidae squids was about 1,914,182 tons at the same percent value (FAO, 2003). The flying squids Sthenoteuthis oualaniensis (Lesson) and Ommastrephes bratamii are the oceanic species of this family that are distributed from the Indo-Pacific to the Indian Ocean. According to Voss (1973), the potential of the purpleback flying squids in the Central Eastern Pacific is at least 100,000 metric tons. This species is caught commercially in the eastern and southern East China Sea, from Taiwan to Okinawa by hook and line with light at night (Okutani & Tung, 1978; Tung, 1981; Yoshikawa, 1978). The deep-sea squids caught by traditional fishermen of Manado Bay, North Sulawesi, in the Sulawesi Sea have been identified as a dwarf form of S. oualaniensis (Pratasik et al., 2022). These species are highly migratory and undertake diel vertical migrations of several hundred meters and seasonal migrations between the shelf and open ocean (Gilly et al., 2006; Stewart et al., 2013) so that they can act as important linkages between both neritic and oceanic food webs (Arkhipkin, 2013).
There are numerous studies on bait types to find the highest catch, from fish and shrimp flesh, live bait, and artificial bait. Fish and squids were observed to be attracted to squid jigging vessels due to the phototaxis (Rao, 1996). It is related to their behavior to avoid predators or enhance feeding efficiency (Solomon & Ahmed, 2016), and their response depends upon species, ontogenic development, light source characteristics, intensity, color, and wavelength (Mallawa et al., 1991). Therefore, fishermen catch squid using light that illuminates in water as well as jigs to attract the squids to aggregate and bite the jigs (Asokan & Krishnan, 2021). It relies on the artificial bait of shrimp-like siliconized jig fishing (Altinagac, 2006; Aydin & İlkyaz, 2021; Paighambari et al., 2012; Reza et al., 2019; Ulaᶊ & Aydin, 2011). Other studies on hand-line fishing are also done using different colors of shrimp-shaped jigs (Altinagac, 2006; Aydin & İlkyaz, 2021; Paighambari et al., 2012; Ulaᶊ & Aydin, 2011). Squid fishing in North Sulawesi is done by traditional fishermen using 5 to 7 M-boat and artificial bait either shrimp-like bait or other bait types (Fig. 1).
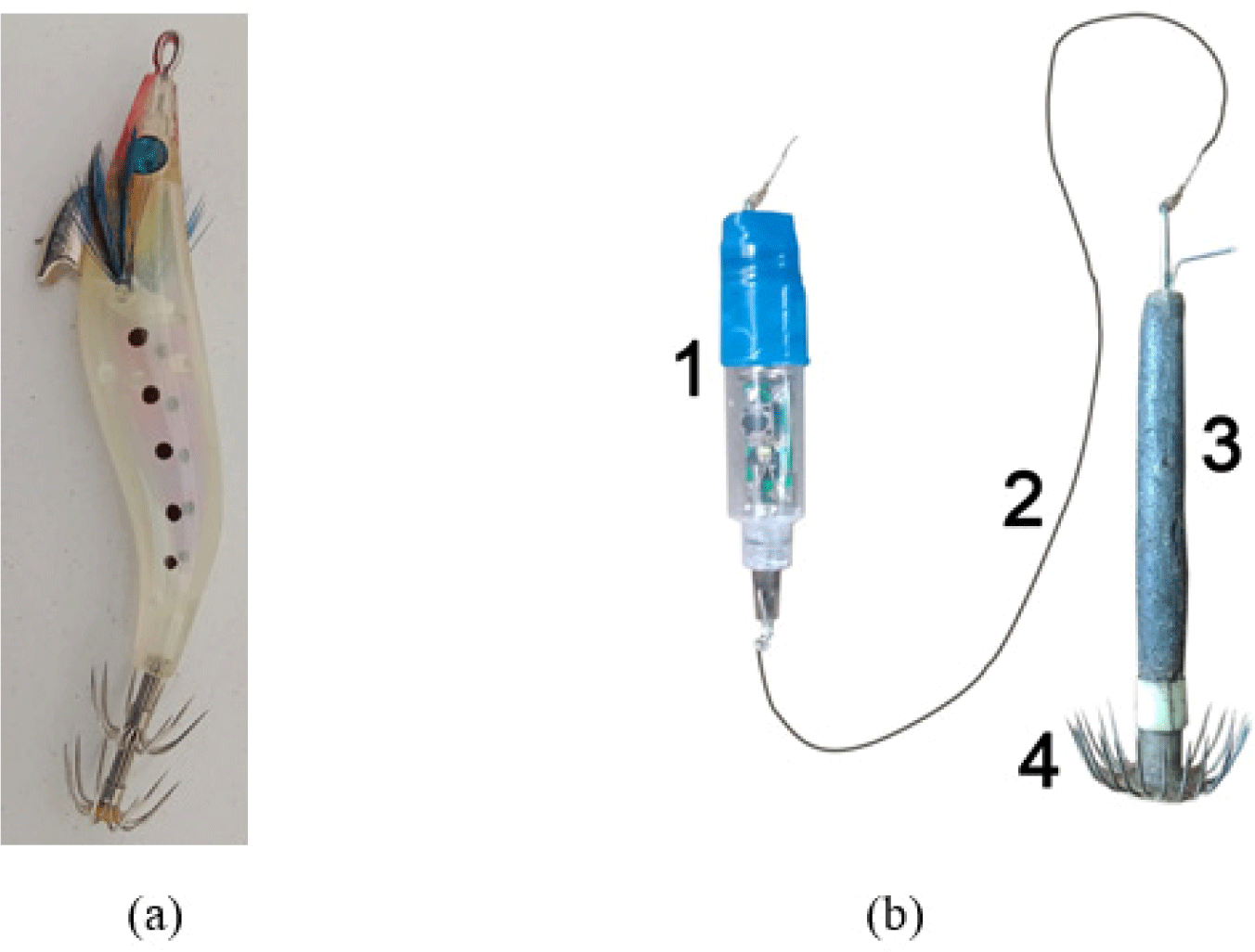
For deep-sea squid S. oualaniensis fishing, the fishermen use a mini-battery-supported flashlight artificial bait sold in the fishing stores. The flashlight artificial bait contains several different alternately blinking light colors to get the squid to bite. This study modifies the light colors to find the best modification of light color against the catches.
Materials and Methods
This study was carried out from June to July 2020. Traditional fishermen catch deep-sea squids S. oualaniensis in the Sulawesi Sea, North Sulawesi, at night (Fig. 2). The flashlight bait is facilitated with a mini-battery to be able to produce several different light colors to attract the squid. The flashlight was connected by a one-meter line to the hook working also as a lead. In fishing operations, the lead was coated with fish flesh as bait.
This experiment modified the standard commercial flashlight bait sold in the fishing shop to produce different light colors: red-green, blue, green, and red. These different light colors were used in 10 fishing trips. Twelve skillful fishermen were used in this experiment in which they were divided into 4 groups of 3 people in 4 separate traditional boats (7 m long) to operate each light color in the same fishing ground. The common commercial flashlight bait was also used as a control treatment. Each line used only one jig and all jig-fishing activities were carried out at the same time. The fishing line was lowered down to the depth range of 20–25 M in the deep sea of Sulawesi Sea waters and jigged. This fishing depth is consistent with the dispersal range peak of S. oualaniensis (Jerep & Roper, 2010).
The use of the red-colored bait was eventually terminated because it was always cut off and lost. The experiment utilized only commercial bait, red-green bait, blue bait, and green bait as treatment with 3 replications represented by 3 local skillful fishermen for each bait light color. Data collections were squid catches. The catch data were analyzed with one-way analysis of variance facilitated by statistical software for comparisons. The difference between treatments was then tested using Tukey’s honestly significant difference (HSD) procedure.
Results
This study caught a total of 30,687 squids S. oualaniensis during the fishing experiment in the Sulawesi Sea, North Sulawesi. Different light color applications highly significantly influenced the number of squid catches (p < 0.001). Analysis of variance demonstrates that both trip and bait light color influence the squid catches (Table 1).
Tukey’s HSD test revealed that S.oualaniensis differently responded to the bait’s light colors. All treatment applications gave a significantly different number of catches. Comparisons between treatments showed that all bait light color modifications gave a higher number of catches than the commercial one (Table 2).
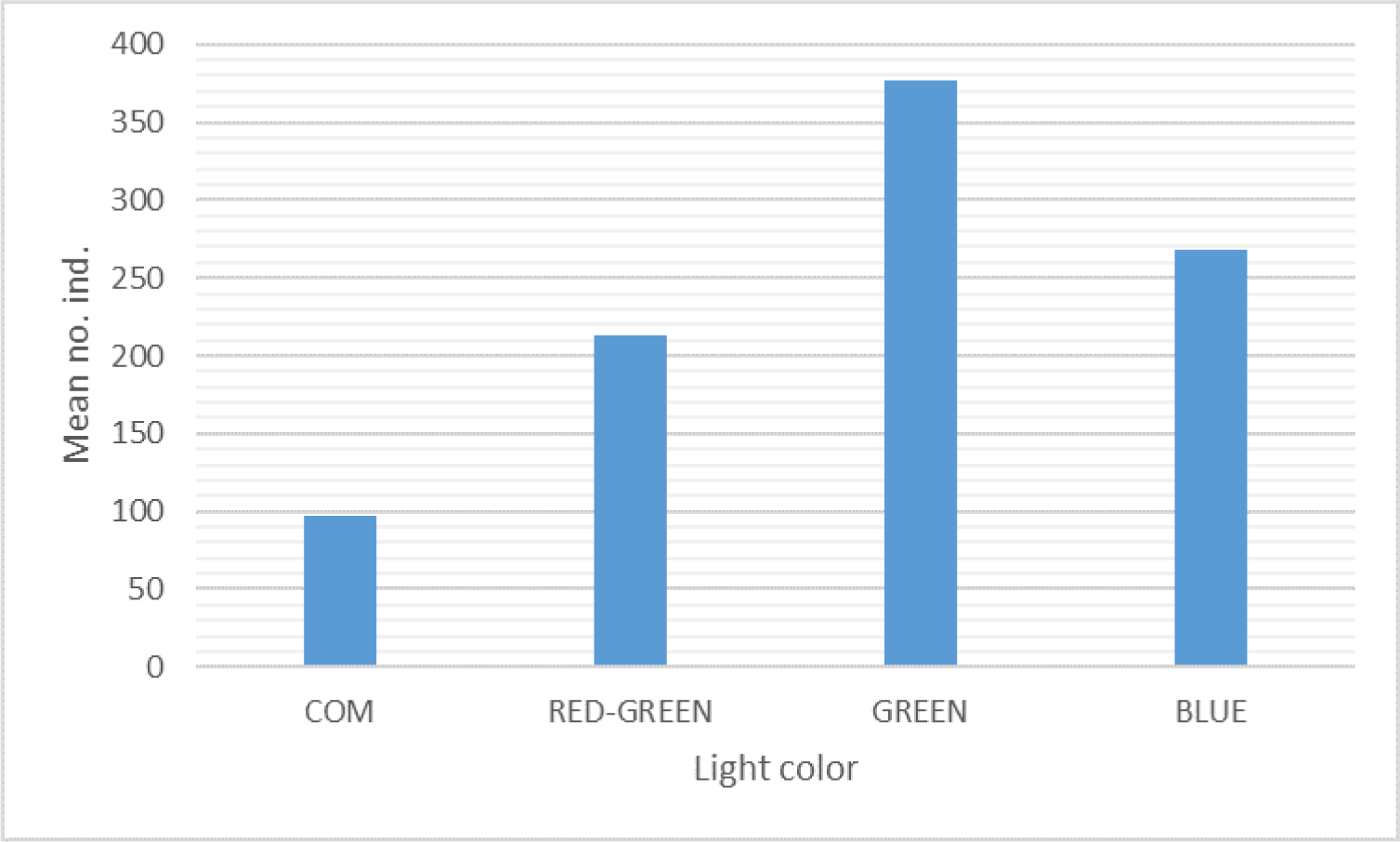
The significantly different squid catches are also indicated by the mean number of squid catches (Fig. 3). The green-lighted jig yielded the highest mean squid catches, 377.37 (39.46%), followed by blue light color, 268.3 (28.06%), then red-green, 213.43 (22.32%), and the lowest catches in the commercial artificial bait, 97.13 (10.16%) (Table 2 and Fig. 3). Multiple comparisons between treatment applications yielded 6 pairs of comparisons and indicated that all treatment flashlight jig colors yielded significantly different squid catches, in which single light color also gives the squid a higher response to taking the lure (Table 2).
Discussion
Jigging is an essential fishing method to exploit squids selectively and avoid over-exploitation to conserve resources and energy (Asokan & Krishnan, 2021). It helps to adjust operational depth according to the concentration depth of squids. They are attracted to lights and fast-moving bait or any bait-like object. A typical jig consists of a shrimp or stalk-like body made of flexible plastic with one to three hooks or more sharp barbless steel hooks at the end. Other jigs are facilitated with the mini battery-supported light blinking.
Squids are known as color-blinded animals, but the degree of contrast is important for squid behavior to attack the jig (Flores et al., 1978). The use of a flashlight jig, in fact, gave a stronger degree of contrast in the water column at night fishing than the use of light above the water and could give a stronger stimulus to the squid to attack the fish flesh bait connected to blinking light. The flashlight jig also has a higher degree of contrast than the shrimp-like siliconized jig so that the squid more sensitively responds to the flashlight jig color in the water column. The flashlight acts as a squid-aggregating device, while the squid feeds on the fish’s flesh, then caught by the hook. The flashlight jig could help the purpleback flying squid get the bait. All squids were hooked on the arms, indicating that the squids are feeding on the fish’s flesh coated on the lead. On the other hand, cephalopods (squid, cuttlefish, and octopus) are well known as voracious predators of many preys, such as fish and crustaceans, or even have cannibalism behavior, so the contrast moving objects in the water column could indicate the presence of moving prey.
Furthermore, the present study revealed that the modified light colors of the artificial bait caught a higher number of deep-sea squid S. oualaniensis in the Sulawesi Sea than the common commercial artificial bait sold in the fishing store with a combination of several different colors. There was also a significantly different effect of all light color modifications on the squid catches with the highest catch in the green light. The low attacking preference of the purpleback flying squid to the multiple light colors could result from the squid’s perception of the blinking multiple colors of the flashlight bait as the aposematic coloration of the prey, in which the animal shows the unpalatability or toxicity through warning coloration. This defense mechanism is widely discussed by Endler (1978), Kang et al. (2015), Mappes et al. (2005), Mochida et al. (2015), and Toledo & Haddad (2009). Aposematism is commonly found across the animal kingdom as a defense mechanism, and it could either be chemicals, such as toxins, harmful secretions, and venoms, or physical defense, such as spines, bites, and stings (Mappes et al., 2005).
This finding is in agreement with Altinagac (2006) and Paighambari et al. (2012) that the green bait color is more efficient in squid jig fishing even though it does not have a significantly different effect from the use of red color in Turkish waters (Altinagac, 2006) and the blue color (Paighambari et al., 2012) on the catch rate of purpleback flying squids in Iranian waters of the Oman Sea. The sensitivity of fish and some of their food animals to blue and green colors is higher because of the long wavelengths that make them penetrate deeper into the water column (Solomon & Ahmed, 2016). The use of dark green jig color is also shown by the traditional fishermen, particularly in North Minahasa, North Sulawesi, as a potential bait color for demersal fish jig fishing (field obs.). Nevertheless, Ulaᶊ & Aydin (2011) found that the red jig is the most efficient in squid Loligo vulgaris Lamarck (1798) fishing on the Middle Eastern Coast of Aegean Sea, Turkey. All those findings were obtained using the shrimp-like siliconized bait. The local fishermen of North Sulawesi commonly use the shrimp-like siliconized jig to fish shallow water squids Sepioteuthis lessoniana. A different finding is shown by Arnupapboon et al. (2008) that the squid moves to white and blue more often than green, while the red color seemed not to attract the squids.
This fishing experiment reconfirms the previous finding concerning the most efficient bait color and shows that the use of single bait light color yielded higher catches than that of multiple colors (p < 0.05). This study did not use the red light color as a treatment, since the red-lighted bait was always taken and cut off. Therefore, we had to use a wireline to the bait to know what causes the loss and found that the red light color was taken by the cutlassfish Trichiurus sp. The difference in squid’s preference for jig color could result from environmental conditions with locality, such as predator-prey interactions that may alter the feeding behavior on-site and species. The presence of a higher level of the predator, such as cutlassfish Trichiurus sp., particularly in Sulawesi waters which is also attracted to the red-light jig has diminished the chance of the deep-sea squids S. oualaniensis to take the red jig or the squid S. oualaniensis is vulnerable to predation risk for feeding on the red-light jig.
According to Asokan & Krishnan (2021), the efficiency of squid jigging is influenced by jig structure, jigging motion, light intensity, sea state, and sea surface temperature (Cabanellas-Reboredo et al., 2012; Roberts & Sauer, 1994; Yu et al., 2015), wind speed, moon phase, and atmospheric pressure (Cabanellas-Reboredo et al., 2012), sea surface height anomaly (Yu et al., 2015), turbidity (Roberts & Sauer, 1994), chlorophyll (Hurst et al., 2012), salinity (Yu et al., 2015), and large scales climate predictors, such as the Southern Oscillation Index and the North Atlantic Oscillation (Morales-Bojórquez et al., 2001; Pierce et al., 2006; Roberts & Sauer, 1994), etc. These factors will influence the catches, recruitment, migration (Koopman et al., 2018), and distribution of the squids. During squid jigging with lights, the quality of light (e.g., wavelength), the quantity of light (e.g., power), and the arrangement of fishing lights affect the squid’s attraction. These factors create underwater irradiance levels and distribution influenced by the optical characteristics of seawater, and it influences squid behavior during fishing (Arakawa et al., 1998; Yamashita et al., 2012). According to Cabanellas-Reboredo et al. (2012), environmental variables, such as sea surface temperature, atmospheric pressure, and moon cycle can also influence squid catches. This experiment focused only on the effect of different jig light colors on the squid bites since the fishing was conducted in a single lunar cycle with different tide conditions. The jiggers took advantage of wind or current direction to position their boats in certain areas to avoid being drifted too far out of the mainland due to the use of the small boat (approximately 5–7 M long).
These findings showed that all light color modifications of the multiple flashlight-squid baits have contributed to the artificial squid flashlight bait development concerning the squid fishing effectivity. Light colors also influenced the feeding behavior of S. oualaniensis, and the single color gave the squid a higher response to getting the lure than the multiple colors. The highest squid catch was recorded in the green light color and the lowest was in the commercial artificial bait. Therefore, the present study has contributed to developing the mini-battery-supported artificial bait for effective exploitation to maximize offshore squid production and fisheries development so that the use of offshore squid resources could be increased. This information is also useful for traditional fishermen to increase their personal income through deep-sea squid fishing. Nevertheless, more studies on squid feeding behavior and other influencing environmental factors are needed for future squid population sustainability.