Introduction
Ethiopia is endowed with numerous lakes and rivers, that are believed to have a promising capacity for fisheries resources. It has several water bodies including ponds, reservoirs, and wetlands. Wood & Talling (1988) estimated the surface area of major lakes and reservoirs to be 7,334 km2 and the length of rivers is 7,185 km. In general, there are nine main river basins (Blue Nile or Abay, Tekeze, Baro-Akobo, Omo-Ghibe, Afar/Denakil, Awash, Ogaden, Wabi-Shebelle, and Genale- Dawa) in Ethiopia and many of which are transboundary. The total mean annual flow from all those nine river basins was estimated to be 122 billion m3.
Foods are among the foremost vital exogenous factors that are essential for the property of each living organism throughout its lifetime and feeding may be a continuous method to derive energy for their future activities. The key factors that deeply influence the distribution, growth, reproduction, migration rate and behavior are mostly dependent on the provision of preferred prey organisms. The study of the food and feeding habits of fish is the subject of continuous analysis as a result it constitutes the premise for the event of a successful fisheries management program (Ikpi et al., 2012). Different studies that are done on the Blue Nile River approved that, cyprinid fishes were the dominant family over other fish families of the river (Awoke & Kefale, 2015; Beletew, 2007).
The decrement in diversity and abundance of large cyprinid fishes in this largest Ethiopian river (Labeobarbus intermedius, Labeobarbus nedgia and Labeo forskalii) have also been seen from time to time. L. nedgia is endemic to Lake Tana and its tributaries whereas, L. intermedius and L. forskalii are not endemic to Lake Tana. According to Getahun (2010) and Natugonza et al. (2023), L. nedgia and L. forskalii were under the least threatened species of International Union for Conservation of Nature (IUCN) Red Lists. However, as the Blue Nile River is the major river basin of Ethiopia with very important habitats of the endemic cyprinid fish fauna of the country, knowledge on the diets and feeding habits of those fish species of the river has not been systematically studied and explored. The necessity of conducting this study was to fill gaps in such biologically essential scientific information for the management and conservation of those dominant fish families of the river.
Materials and Methods
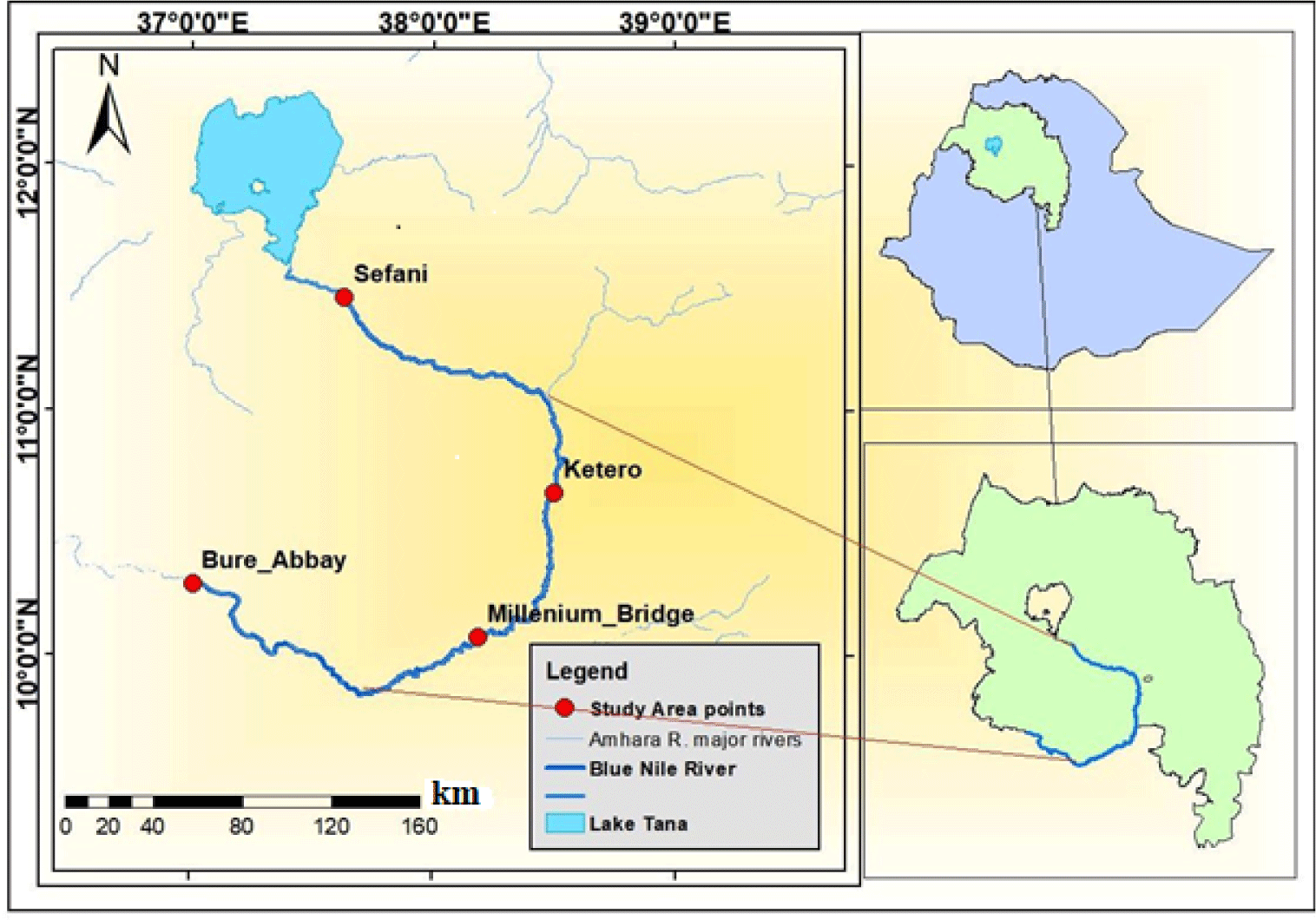
The main source of the Blue Nile River is the outflow from Lake Tana (Rzóska, 1976). The Blue Nile River flows down the eastern outskirts of Bahir Dar Town at the southern end of the Lake. The river flows down approximately 35 km in a southeast direction where it forms the famous Tiss-Isat Fall and drops into a gorge having a depth of about 45 m (Dile et al., 2013). The river flows down with deeper gorges for approximately 800 km to reach the Ethio-Sudan border, approximately 600–700 km beyond it joins with the White Nile River (Swain, 1997). Blue Nile River lies in the west of Ethiopia between 7° 45´ and 12° 45´ N, and 34° 05´and 39° 45´ E.
Four sampling sites (Sefani, Ketero, Millennium Bridge, and Bure-Abay) were selected by considering accessibility, nature and velocity of the flowing river, substrate type of the sediments, and suitability for setting gillnets, previous experience of traditional fishing and availability of fishes. The specific sampling sites were fixed by using GPS. The distances among sampling sites were 169 km from Sefani to Ketero, 109 km from Ketero to Millennium Bridge, 193 km from Millennium Bridge to Bure-Abay and 471 km from Sefani to Bure-Abay. The main features, coordinates and elevation of the sampling sites are described below (Table 1 and Fig. 1).
This study has been conducted from November 2016 to March 2017 during the post-rainy and dry seasons, respectively in the upper Blue Nile River, Ethiopia. The whole gut contents of specimens of the large cyprinid fishes of the river from all selected sampling sites were collected during both seasons.
Fishing gears used for sampling fish specimens were multifilament gillnets of different stretched mesh sizes (6, 8, 10, 12, and 14 cm) with a length of 25 m and a width of 1.5 m.
Monofilament gillnets with stretched mesh sizes of 6, 8 and 10 cm were also used to sample fish in all sampling sites of the river. Monofilaments were set for about two hours in the daytime for all sampling sites from 1:00 pm to 3:00 pm. The total length and total weight of each captured specimen were measured immediately after capture using a measuring board and a balance to the nearest 0.1 cm and 0.1 g, respectively. The all-food contents of all non-empty gut samples were taken with a bottle, information was written and preserved using a 5% formalin solution for further investigation.
The preserved contents of each gut were transferred into a graduated plastic cylinder, volumes were measured, and data were recorded on the laboratory result datasheet. Then, the contents of each gut were transferred into the Petri dishes for microscopic identification, identification of larger food items was performed visually, whereas stereomicroscope (LEICA S4E, 10X/23, Leica, Wetzlar, Germany) and compound microscopes (OPTIKA E-PLAN, 10X/0.25, Optika, Ponteranica, Italy) were used to identify microscopic food categories. The relative importance of food categories was determined using the frequency of occurrence and volumetric methods of analysis. In the frequency of occurrence, the number of stomach samples containing one or more of a given food item was expressed as a percentage of all nonempty stomachs examined (Bagenal, 1978). The proportion of the large cyprinid fishes that feed on certain food items was estimated by this method. In volumetric analysis, food items were sorted into different taxonomic categories, and the water displaced by a group of items in each category was measured in a partially filled graduated cylinder (Bowen, 1983). The volume of water displaced by each category of food items was expressed as a percentage of the total volume of the stomach contents (Bowen, 1983). The diet component with the highest volume was given 16 points and every other component was awarded 16, 8, 4, 2, 1, and 0 points depending on the volume relative to the component with the highest volume (Hynes, 1950). Finally, the length and weight relationships of fishes of the upper Blue Nile River system were estimated separately by using the power function equation as in Bagenal & Tesch (1978).
Data were described using descriptive statistics (mean, SD and percentage). A Chi-square test was used to compare the frequency of occurrence and volumetric contributions of the different food categories during the post-rainy and dry seasons at a 95% confidence level. All needed analysis and calculations were done by using Microsoft Excel 2007.
Results
From the total number of 401 collected samples, 279 (about 70%) were found with non-empty guts; while the remaining 122 (30.4%) were with empty guts. Food and feeding habit results using the frequency of occurrence and volumetric analysis in the present study showed that, the presence of phytoplankton, insects, mud, detritus, macrophytes, macrophyte seed, sand grains, nematodes, fish scale, gastropods, zooplankton, and some unidentified food items as the dietary composition of large cyprinid fishes (Table 2 and Fig. 2).
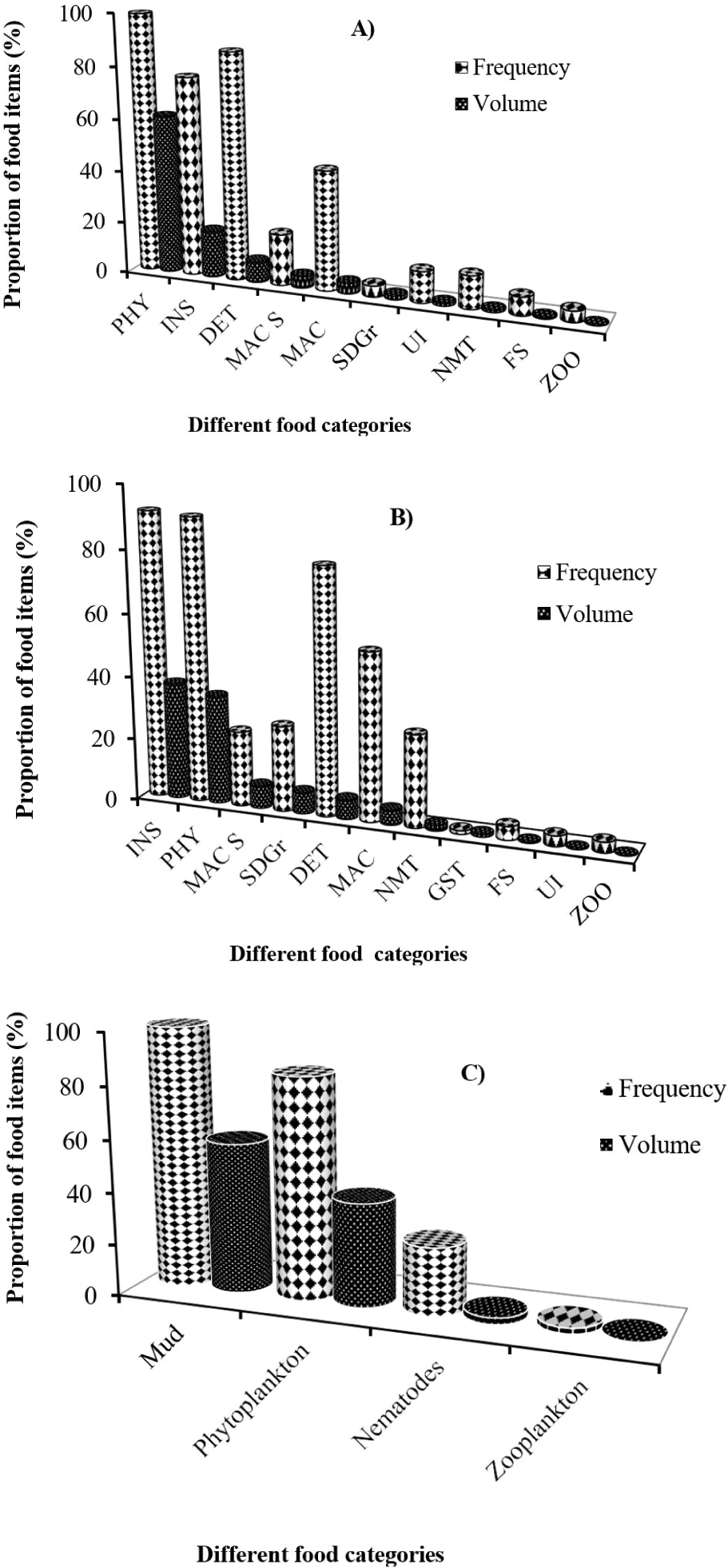
Specifically, L. intermedius frequently ingested phytoplankton, insects, and detritus, and these food items constituted bulk (87.5%) of the consumed foods by volume (Table 2A and Fig. 2A). The remaining food items such as macrophyte seed, macrophytes, sand grains, nematodes, fish scale and zooplankton accounted for 11.5% of the volume of the total food consumed. Phytoplankton were the most important food items occurring in 99.3% of the guts and contributing 61.3% of total volume whereas, insects and detritus had second and intermediate contributions with 18.1% and 8.1% volume of the total foods in the diet of this species in the Blue Nile River. Among the identified phytoplankton and insect groups, green algae represented by Microspora quadrata and Chironomid larvae followed by Ephemeroptera were important food items, respectively (Table 2A). However, insects were the most significant food items in the diet of L. nedgia followed by phytoplankton and macrophyte seed occurring in 91.3% of guts and accounting for 37.4% volume of total food items (Table 2B and Fig. 2B). Chironomid larvae were important food items followed by Ephemeroptera from the identified insect taxa. Whereas, blue-green algae represented by Microcysts frequently ingested by L. nedgia among the identified phytoplankton groups. The detailed contributions by other minor food items such as sand grains, detritus, macrophytes, nematodes, gastropods, fish scales, and zooplankton can be seen in Table 2B.
In the same manner, L. forskalii populations of the river mainly feed on the mud with 57.7% total volume contributions. Phytoplankton was the second important food item occurring in 84.4% of guts and contributing 39.8% volumetrically. From the identified phytoplankton groups, blue-green algae, which were represented by Microcyst were important food items (Table 2C). The contributions of nematodes and zooplankton were relatively low (Table 2C and Fig. 2C).
Results of the current study showed that phytoplankton, insects, mud, detritus, and macrophyte seed were vital food items and the presence of different prey selection behavior among the species as seen above.
The frequency of occurrence and volumetric contributions of the different food items consumed by large cyprinid fishes varied significantly (Chi-square [X2] test, p < 0.05) with seasons (Table 3). Insects were the most important food categories during the post-rainy in the diet of L. intermedius volumetrically. However, the contribution of insects declined during the dry months by volume. Except for Chironomid larvae and Ephemeroptera, all others insect groups were not more important during the dry season (Table 3A). While phytoplankton contribution was the highest during the dry season. Only green algae represented by M.quadrata spp. was important during both seasons volumetrically from the identified phytoplankton groups in the diet of the same species. Except for some unidentified foods, the remaining food categories were more important during the dry and post-rainy seasons both in the frequency of occurrence and volumetric contributions (Table 3A).
In the same manner, insects and phytoplankton were the essential dietary ingredients both in the frequency of occurrence and volumetric contributions in the foods of L. nedgia through the post-rainy and dry seasons. Except for Plecoptera, all groups of insects were more important during the post-rainy months in volumetric contributions. Ephemeroptera was more important followed by Chironomid larvae during the dry season in the diet of this species volumetrically. Among the identified phytoplankton groups, the contribution of blue-green algae represented by Microcysts spp. was highest during the dry months in both frequency and volumetric contributions. Except for the frequency of occurrence contributions of the fish scale, all of the remaining food categories were important during both seasons in the diet of L. nedgia (Table 3B).
Similarly, the volumetric contribution of the mud was highest during the post-rainy season in the diet of L. forskalii species. However, mud contribution declined during the dry season. Whereas, the contributions of phytoplankton increased during the dry season both in the frequency of occurrence and volumetric contributions. There were no contributions of green algae and diatom groups of phytoplankton during the post-rainy months in the diet of the same species. Except for Aphanizomenon spp., all the identified blue-green algae were more important during the dry months volumetrically. The contributions of the remaining food categories such as nematodes and zooplankton can be seen in Table 3C.
Results of the current study showed that, in the post-rainy season, insects, phytoplankton, as well as mud were the vital food items volumetrically and phytoplankton was the most important food categories both in the frequency of occurrence and volumetric contributions during the dry season Table 3.
The percentages mean volume contributions of different food items in the diet of different size classes of large cyprinid fishes are presented in Fig. 3. In the smallest size class (< 20.0 cm FL) of L. intermedius, the mean volume of the diet was dominated by phytoplankton (57.3%) and detritus (20.2%) (Fig. 3A). The contribution of other food items such as macrophytes (11.7%), insects (9.8%), and sand grains (1%) was relatively low. In the size class, 20–30 cm FL of the same species, phytoplankton (45.5%), insects (13.0%), and macrophyte seed (12.7%) were important food items. In this size class, the contribution of macrophytes (10.0%), detritus (8.6%), sand grains (8.6%), unidentified food items (0.8%), nematodes (0.5%), and fish scale (0.1%) were relatively low. In the size class, 30–40 cm FL, the contributions of phytoplankton (54.9%), and insects (25.9%) were increased while the contributions of macrophyte seed (9.7%), macrophytes (5.7%), and detritus (1.7%) were relatively declined. The contributions of unidentified foods and fish scales are relatively very low. In the largest size class (> 40.0 cm FL) of L. intermedius, the contribution of phytoplankton (85.4%) was increased. The contribution of other food items such as insects, macrophytes and nematodes was relatively low (Fig. 3A).
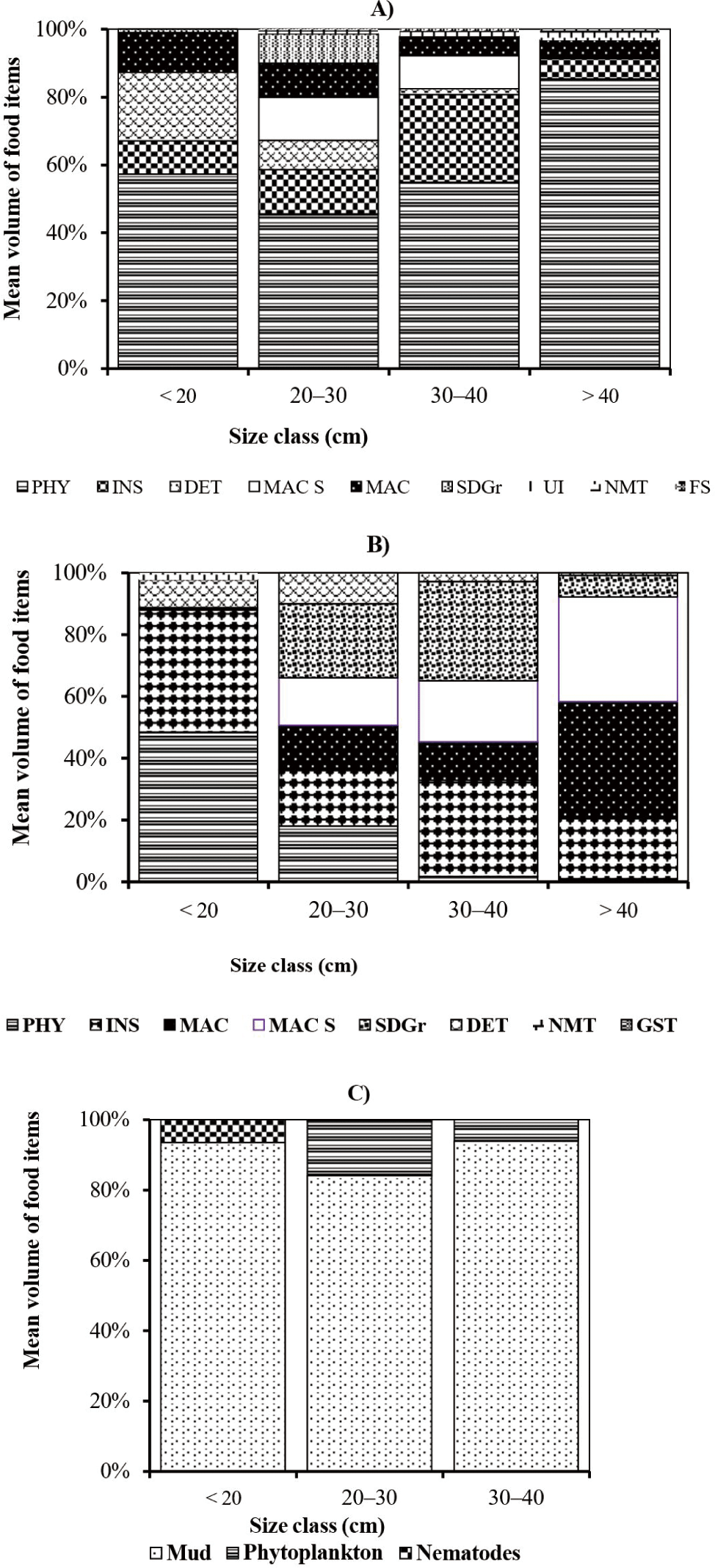
In the same manner, the smallest size class (< 20.0 cm FL) of L. nedgia, mostly ingested phytoplankton (48.4%) and insects (39.7%) of the mean volume of the diet (Fig. 3B). The contribution of detritus (9.0%), nematodes (2.2%) and macrophytes (0.7%) were relatively low. In the size class of 20–30 cm FL of the same species, mean volume contributions of the diet were dominated by sand grains (24.0%), insects (18.3%), and phytoplankton (18 %) (Fig. 3B). The contributions of the remaining food categories such as macrophyte seed (15.3%), macrophytes (14.4%), and detritus (10%) were relatively low (Fig. 3B). In the size class, 30–40 cm FL of L. nedgia, the contribution of sand grains (32.1%) and insects (31.1%) were the significant food items (Fig. 3B). In his size class, the contributions of macrophytes (12.5%) and phytoplankton (1.7%) were declined, while, the contribution of macrophyte seed was increased. The contribution of nematodes (2.8%) was relatively very low. In the size class, > 40.0 cm FL of L. nedgia, the contribution of macrophytes (38%) and macrophyte seed was increased, while the contributions of insects (20%) and sand grains (7%) were diminished. The percent mean volume contributions of other food items such as phytoplankton and gastropods were relatively low (Fig. 3B). In the size class, < 20.0 cm FL of L. forskalii, mud contributed 93.5% of percent mean volume and nematodes was 6.5% (Fig. 3C). In the size class of 20–30 cm, FL of similar species, mud (84.1%) was decreased. In this size class, phytoplankton contributed 15.5% and there was no contribution of nematodes. In the size class of 30–40 cm of these fish species, mud (94%) increased, while phytoplankton (6%) contribution was declined.
In general, during the study period, the contributions of plant-origin foods, namely phytoplankton, macrophyte seed, macrophytes, detritus, and mud were increased with the sizes of large cyprinid fishes. Whereas, the contributions of animal-origin foods such as insects, nematodes, fish scales, zooplanktons and gastropods decreased as the size of these fishes increased (Fig. 3).
Discussion
Based on the frequency of occurrence and volumetric analysis, results of the current study clearly showed that the foods of large cyprinid fish species (L. intermedius, L. nedgia and L. forskalii) were constituted of diversified food items; phytoplankton, insects, macrophytes, mud, detritus, sand grains, macrophyte seed, nematodes, fish scale, gastropods, zooplanktons and some of the unidentified foods. This is virtually similar to reports for other fish species Heterotis niloticus and Labeo coubie from Lower Rive Benue (Solomon et al., 2017), from Lake Hawassa by Admassu & Dadebo (1997) and Lake Tana by Nagelkerke & Sibbing (1996) for Barbus species. The stomachs of cyprinid fishes comprise different types of microalgae, detritus, plant seeds, crustaceans, and the larval and adult forms of insects, which implies the trends of diversified feeding habits (Felley & Felley, 1987).
L. intermedius mainly fed on phytoplankton, insects, and detritus by appointing the bulk of the consumed foods volumetrically. The remaining food items such as macrophyte seeds, macrophytes, and others were relatively rare. The same results were reported from Lake Ahozon (South Benin) (Gbaguidi et al., 2016) for Sarotherodon galilaeus. Contrasting the present study, Engdaw et al. (2013) reported the detrital feeding habits of similar fish species from Lake Koka (Ethiopia) and gastropods were reported as a major food item of the same fish species from Lake Tana by (de Graaf et al., 2008) and from Lake Hawassa by (Desta et al., 2006). Food and feeding studies of big barbs from some other Ethiopian inland waters revealed them to be omnivorous and the fish can change its diet depending on the availability of foods, and seasonal and spatial differences (Admassu & Dadebo, 1997). The reason why L. intermedius mainly ingested phytoplankton in the current study might be due to the highest diversity of algae during the dry months and its nature of adaptive capacity to the environmental oscillations. There was no study on the specific feeding habits of the same species in riverine habitats of Africa including Ethiopia to compare the present results.
Insects were the most important food items followed by phytoplankton and macrophyte seed in the diet of L. nedgia which occur in 91.3% of guts and represented 37.4% of the total volume of the consumed prey items. Phytoplankton and macrophyte seed contributed 41.9% of the total volume. Whereas, the contributions of the remaining food items such as sand grains, detritus, macrophytes, nematodes, gastropods, fish scale, and zooplankton were relatively low. This is comparable to the results reported from Lake Tana by de Graaf et al. (2008) for the same species. The values of the remaining food items such as sand grains, gastropods, nematodes, zooplankton and fish scales were relatively low. Information is scarce on the feeding behavior of this species in riverine and lake habitats to make comparisons, except for the above-mentioned. Nevertheless, mud was a vital food item in the foods of L. forskalii with 57.7% total volume contributions. Phytoplankton was the second important food item occurring in 84.4% of guts and 39.8% volumetrically. Solomon et al. (2017) found the same results for L. coubie in the Lower River Benue. There was no comparative information on the feeding natures of L. forskalii in both African major rivers and lakes.
In the current investigation, the diet composition of large cyprinid fishes showed some seasonal variation both in the frequency of occurrence and volumetric contributions; and slight differences among the species Table 3. The differences in the frequency of occurrence and volumetric contribution of different food categories in the diet of fishes during the study period may be due to the difference in sampling period and the productivity of the rivers that create a conducive environmental condition for the seasonal reproductive cycle of the prey groups. The fact that the diet of cyprinids exhibited seasonal variations might be attributed to the changes in its location in certain periods for feeding purposes. Alike to this, Engdaw et al. (2013) reported the presence of seasonal variation in the diet of other fish species Oreochromis niloticus from Lake Koka. The current study correspondingly indicated that, in the post-rainy season, insects, phytoplankton, and mud were more important diet categories volumetrically in the diet of L. intermedius, and L. nedgia while mud contributed the highest proportions in the diet of L. forskalii in the same season. However, phytoplankton was the vital food category both in the frequency of occurrence and volumetric contributions during the dry season Table 3. This might be due to the low flow rates of the river and the high succession ability of algae during this season. A study conducted on tropical rivers showed that temperature plays a much-lessened role and the greatest densities of phytoplankton coincide with low water flow rates in riverine habitats (Talling & Rzoska, 1967). Although insects represented by Chironomid larvae and Ephemeroptera were the most important food items during the post-rainy season in the diet of L. intermedius and L. nedgia. This might be due to the deposition of organic matter that occurs in the lower regions of the stream, facilitating the establishment and availability of insects in the substrate during this season. In addition to this, the presence of dense vegetation during the post-rainy season of the study might also contribute to the abundance of insects when compared to the dry season.
The importance of mud in the diet of L. forskalii in the present study might be due to the inflow of a high number of sediments and related organisms such as crustaceans, terrestrial insect larvae, and decomposed plant remains by local runoff to the river during the post-rainy season. The important inputs into the aquatic system due to runoff include small non-aquatic insect larvae creatures and organic matter from terrestrial sources. As the floods advance, invertebrates, especially ants and termites caught by the rising flood and incorporated into the aquatic system. There was also a continuous input of insects, seeds, leaves, pollen and other material from flooded forests and grasslands, which either enters by the drift in flowing waters or settles to the bottom where it is decayed by bacterial and fungal activity (Marxsen, 1980). According to Scheffer & Carpenter (2003), mud and detritus are bottom deposits that represent two rather different kinds of food items in the river. The detritus feeders rely on coarser decomposing plant material together with associated microorganisms and animal communities. These comprise a high proportion of species, particularly in headwater streams and forested habitats, where leaf fall accumulates in the slack of the pools sor is close to floating vegetation where litter is also abundant. The resulting coarse detritus tends to be a feature of low-order streams and it becomes finer with progress downstream until in the autumn it forms fine organic mud. Bayley (1983) also described that mud contains amino acids and other organic products of decay, which can be used as fish food in combination with the saprophytic bacterial and protozoan microorganisms in the riverine habitats. The diet composition of large cyprinid fishes during the study period showed that, the omnivorous feeding habits of L. intermedius and L. nedgia populations; and the detrivores feeding natures of the L. forskalii species. Similar to the present results, omnivorous feeding habits of L. intermedius and Cyprinus carpio have been reported by Dadebo et al. (2013), and Dadebo et al. (2015), respectively from Lake Koka. Unlike the current results, Lake Tana (de Graaf et al., 2008) reported the insectivorous feeding habits of L. nedgia. Variations in feeding habits might be due to habitat differences, availability of the prey items, and the ability of prey selection by fishes in a given environment. The piscivorous feeding behaviour had not been seen during the study period. While eight piscivores Labeobarbus species have been reported from Lake Tana by Nagelkerke (1997).
In the current study, the increment of plant-origin foods contribution with the sizes of the fishes might be the implications of special adaptive mechanisms of these fishes. The presence of well-developed pharyngeal teeth used for processing food prior to digestion and long digestive tracts that increase the gut passage time to improve the efficiency of plant material digestion are the two major anatomical or physiological adaptation mechanisms of cyprinids to continue their life in changes of a given environment (Persson & Eklov, 1995). Results of this study also revealed that the ontogenetic dietary shifts were not probably evident and the only slight variation in percent mean volume contributions of different food items in the diets of different size classes is observed. Dadebo (2000) also reported similar slight variation results for Clarias gariepinus from Lake Hawassa. As most cyprinid fishes are generalists described by omnivory, detrivory or insectivory in their feeding habits, the ontogenetic diet shift may not be expected. Therefore, the absence of valid ontogenetic diet shifts during the study period might be due to the lack of fingerling feeding data and combinations of factors explained above.