Introduction
Family Cyprinidae is crucial in the fishery industry in Asian countries. Recently, worldwide reports indicate the presence of alloherpesviruses such as cyprinid herpesvirus-2 (CyHV-2), -3 (CyHV-3, commonly known as koi herpesvirus; KHV), and poxvirus including carp edema virus (CEV) (Ahmadivand et al., 2020; Badhusha et al., 2022; Kafi et al., 2022). CyHV-2 and KHV were first reported in the Japanese goldfish (Carassius auratus) in 1995 (Jung & Miyazaki, 1995), and common carp (Cyprinus carpio) in Israel and USA in 1998 (Perelberg et al., 2003), respectively. Moreover, CEV initially reported in Japan in 1974 (Murakami, 1976), has become a global concern as it spreads through subclinical infections that evade disease screening, and the World Organization for Animal Health (WOAH) designated it as an emerging virus (WOAH, 2021). Although different viruses can cause the disease, the clinical signs observed in infected fish are similar in terms of having gill necrosis and deposits of thick mucus under the gill covers being the most prominent (Bergmann et al., 2006; Granzow et al., 2014; Zhu et al., 2019). It is noteworthy that even though these viruses were identified decades ago, they continue to spread worldwide (Bergmann et al., 2020; Ouyang et al., 2023).
In Korea, KHV has been identified as an endemic virus in koi carp (Gomez et al., 2011). Recently, two other viruses, CyHV-2 and CEV, have also been reported in Korea (Jung et al., 2022; Kim et al., 2018). To minimize the risk of disease outbreaks in cyprinids, it is essential to investigate the epidemiological tracking of these viruses through genetic relatedness analysis. This study aimed to investigate alloherpesviruses (CyHV-2 and -3) and poxvirus (CEV) in domestic and imported cyprinids from 2021 to 2022. The molecular epidemiology approach was utilized to investigate the origin of identified viruses through sequencing and phylogenetic analysis. Additionally, virus isolation and challenge tests were performed to address the pathogenicity of identified viruses.
Materials and Methods
Between 2021 and 2022, a total of 19 cyprinid groups comprising 375 individual samples were collected from local markets and aquaculture farms (Table 1). The collected groups included three domestic groups containing 30 common carp, 200 koi carp, and 40 goldfish, and two imported groups consisting of 76 koi carp, and 29 goldfish. The fish were transported to the laboratory alive, and fish were observed for external symptoms. Subsequently, the gill tissue of each fish was collected for virus detection and analysis.
Polymerase chain reaction (PCR) was conducted for CyHV-2, KHV, and CEV detection. Genomic DNA was extracted from gill tissue (50 mg) using the yesG Cell Tissue mini kit (Genesgen, Busan, Korea) according to the manufacturer’s protocol. The extracted DNA was used for PCR analyses. PCR was conducted with a total volume of 20 µL containing 10 µL taq polymerase (Prime Taq Premix, Daejeon, Korea), 1 µL of DNA template, 7 µL of DEPC treated water, and 1 µL each of primer sets. The primer information and the PCR conditions for detecting each virus are described in Table 2.
| Target virus | Target gene | Primer | Sequence (5′ to 3′) | Length | Condition | Object | Reference |
|---|---|---|---|---|---|---|---|
| KHV | Thymidine kinase | TK-F | GGGTTACCTGTACGAG | 409 | 94°C, 5 min, 40 cycles (94°C, 1 min, 55°C, 1 min, 72°C, 1 min), 72°C, 10 min | Detection | Bercovier et al., 2005 |
| TK-R | CACCCAGTAGAT TATGC | ||||||
| Enlarged TK gene-F | AACGCGGGCCAGCTGAACAT | 1,001 | 94°C, 5 min, 40 cycles (94°C, 1 min, 58°C, 1 min, 72°C, 1 min), 72°C, 10 min | Sequencing | Kurita et al., 2009 | ||
| Enlarged TK gene-R | TGTGTGTATCCCAATAAACG | ||||||
| SphI-5 | Gray sph-F | GACACCACATCTGCAAGGAG | 292 | 94°C, 30 s, 40 cycles (94°C, 30 sec, 63°C, 30 sec, 72°C, 30 sec), 72°C, 7 min | Detection | Gray et al., 2002 | |
| Gray sph-R | GACACATGTTACAATGGTCGC | ||||||
| KpnI/SacI fragment | KHV-86f | GACGCCGGAGACCTTGTG | 78 | 50°C, 2 min, 95°C, 10 min, 40 cycles (95°C, 15 sec, 60°C, 60 sec) | Detection and quantification | Gilad et al., 2004 | |
| KHV-163r | CGGGTTCTTATTTTTGTCCTTGTT | ||||||
| KHV-109p | CTTCCTCTGCTCGGCGAGCACG | ||||||
| CEV | Partial 4a | CEV For B | ATGGAGTATCCAAAGTACTTAG | 528 | 94°C, 3 min, 40 cycles (95°C, 30 sec, 55°C, 30 sec, 72°C, 30 sec), 72°C, 10 min | Detection and sequencing | Matras et al., 2017 |
| CEV Rev J | CTCTTCACTATTGTGACTTTG | ||||||
| CyHV-2 | Helicase | CyHV-2 HelF | GGACTTGCGAAGAGTTTGATTTCTAC | 366 | 94°C, 5 min, 35 cycles (94°C, 1 min, 60°C, 1 min, 72°C, 1 min), 72°C, 10 min | Detection and sequencing | Waltzek et al., 2009 |
| CyHV-2 HelR | CCATAGTCACCATCGTCTCATC | ||||||
| Marker A | oPVP382 | CCACTTAGAGTAACCACTTAGAG | 475 | 94°C, 8 min, 35 cycles (94°C, 30 sec, 58°C, 30 sec, 72°C, 30 sec), 72°C, 10 min | Sequencing | Boitard et al., 2016 | |
| oPVP383 | GCGTTGACTCATTTGCGGTTTG |
To determine the genetic characteristics of the cyprinid virus identified by PCR, we performed sequencing and phylogeny analysis based on the enlarged thymidine kinase (TK) gene of KHV, p4a gene (putative major core protein) of CEV, and marker A gene of CyHV-2, respectively. PCR was conducted as described in Table 2. PCR amplicons were sequenced using an ABI3730XL DNA Analyzer (Applied Biosystems, Waltham, MA, USA), and the nucleotide homology was confirmed using the National Center for Biotechnology Information (NCBI) website with Basic Local Alignment Search Tool (BLAST) program (http://www.ncbi.nlm.nih.gov/BLAST). The phylogenetic tree was constructed by the maximum likelihood method with 1,000 bootstrap values using MEGA software (ver.11.0.10, MEGA International, Paris, France).
To isolate the PCR-detected virus, the common carp brain (CCB) and koi carp fin (KF-1) cells were used. A total of 20 mg of PCR-positive gill samples were homogenized in 1 mL of phosphate-buffered saline solution and then centrifuged at 15,000×g for 10 min at 4°C. The resulting supernatant was collected and diluted in 5 mL of Eagle’s minimal essential medium (MEM; Gibco, New York, NY, USA) supplemented with 2% fetal bovine serum (FBS) and Leibovitz’s L-15 medium (L-15; Gibco) supplemented with 2% FBS, respectively. And then, diluted homogenates were filtered through 0.45 µm syringe filter. The filtered homogenate was used as an inoculum (MEM- and L-15 inoculum). The inoculum was inoculated to CCB cells (MEM-inoculum) and KF-1 cells (L-15 inoculum) that formed confluency of approximately 90% at 25-cm2 tissue culture flask (SPL, Pocheon, Korea) followed by incubation at 23°C. After the appearance of a cytopathic effect or after 14 days post-inoculation, the supernatant was collected and DNA was extracted. The DNA was used for the PCR template for confirmation of virus isolation. The primer sets and the PCR conditions are listed in Table 2.
For the challenge test, only KHV was used for experimental infection. Before the challenge test, healthy koi carp (mean body length, 6.74 ± 0.91 cm; mean body weight, 4.92 ± 0.96 g) were obtained from an aquafarm in Seosan and confirmed free of cyprinid viruses (CyHV-2, KHV, and CEV) by PCR analysis described above. The fish were acclimatized for 10 days at 23°C in a 100 L tank. Koi carp were intraperitoneally injected with 100 µL of cultured KHV (ScKc-2105-K) at a concentration of 102.6 TCID50/fish (n = 50). As a negative control, MEM medium alone was intraperitoneally injected into 50 koi carp. After the viral challenge, the fish were maintained at 23°C in 50 L tanks with 50% water exchange daily and were fed twice a day. Mortality was observed over 14 days, and the gill tissue was collected from all dead and surviving fish. The total DNA was extracted using the yesG Cell Tissue mini kit. The KHV genome copy was measured by real-time PCR (Table 2). All animal experiments were performed according to the Animal Research and Ethics Committees of Pukyong National University guidelines (Permission No. PKNUIACUC-2021-23).
Results and Discussion
Herein, we investigated CyHV-2, KHV, and CEV in 19 different cyprinid groups. CyHV-2 was detected only in goldfish imported from Thailand (Table 1), and three groups of these goldfish (ThGf-2101A, ThGf-2101B, and ThGf-2107) were identified as harboring CyHV-2 without any specific external symptoms. The detection rates for CyHV-2 in these groups were 25.0% (2/8), 37.5% (3/8), and 92.3% (12/13), respectively. In a previous study (Boitard et al., 2016), it was suggested that the marker A region containing tandem repetitive sequences could be used for epidemiological source tracking of CyHV-2. Notably, three CyHV-2 isolates (ThGf-2107-Cy, ThGf-2101A-Cy, and ThGf-2101B-Cy) identified from goldfish reveal diverse repetitive sequence sizes (Table 3), despite being grouped within the same clades as isolates from various geographical regions, including China, France, Poland, and Israel (Fig. 1). Interestingly, while ThGf-2101A-Cy and ThGf-2101B-Cy were closely related to Chinese isolates (SY, SY-C1, and 1301) with 33 bp repeating sequences, ThGf-2107-Cy showed a perfect match with isolates from France (FR), China (YZ-01, CCNDF-TB2015), and Poland (RSD-PL). Recently, CyHV-2 was detected by helicase gene-specific PCR assay in both imported and domestically cultured goldfish in Korea (Song et al., 2018). Although the genetic relatedness with previously reported CyHV-2 could not be analyzed due to the lack of maker A gene sequences, the plausibility of the international trade of live goldfish contributing to the introduction of CyHV-2 in Korea could not be ruled out.
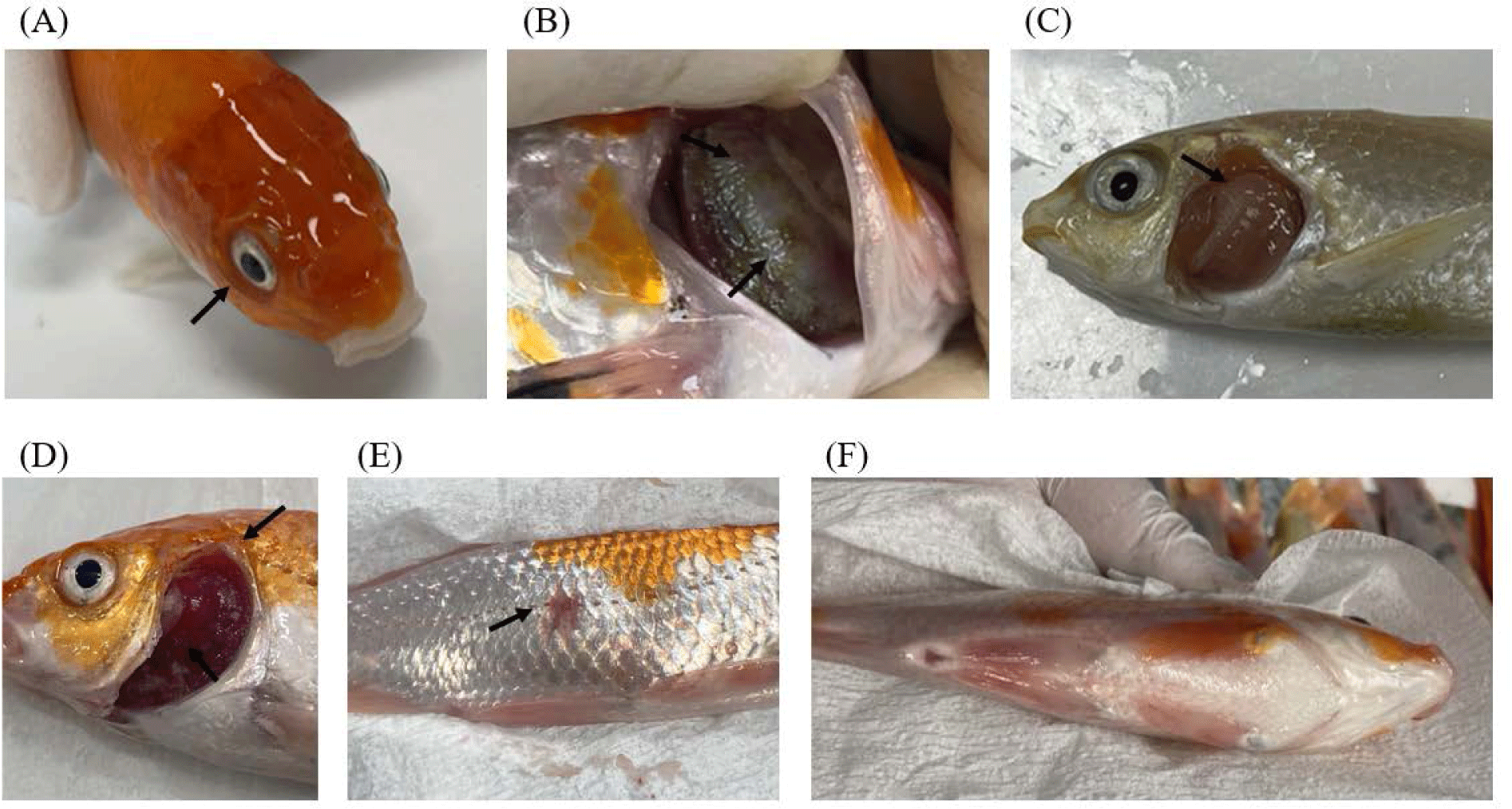
Two groups of domestic koi carp (ScKc-2105 and GhKc-2207) were found to be co-infected with KHV and CEV, with different detection rates between groups (Table 1). In the ScKc-2105 group, KHV was detected more frequently than CEV (KHV-TK, 96.7%; KHV-Gs, 50.0%; CEV, 6.7%). While in the GhKc-2207 group, CEV was detected more frequently than KHV (KHV-TK, not detected; KHV-Gs, 13.3%; CEV, 83.3%). After transportation to the laboratory, the ScKc-2105 group suffered from high mortality rates (approximately 80% after three days) with typical symptoms of KHV infection including enophthalmos, gill discoloration, necrosis, and abnormal swimming (Fig. 2A–2C). In contrast, the GhKc-2207 group survived after transportation but exhibited small ulcers, redness, and gill necrosis, which are general clinical signs of CEV infection (Fig. 2D–2F). Recently, there have been reports of mass mortality events caused by coinfection of KHV/CEV globally (Adamek et al., 2019; Kim et al., 2020; Toffan et al., 2020). In this study, we also found cases of KHV/CEV coinfection in two different forms. The ScKc-2105 group was identified as KHV dominant KHV/CEV coinfection, while in the GhKc-2207 group, CEV was dominant. While each virus exhibited unique external symptoms, such as the presence of ulcers in CEV infection, the clinical manifestation of gill pathology was strikingly similar. Furthermore, there is an increasing trend in cases of coinfection of KHV and CEV (Adamek et al., 2019; Kim et al., 2020; Toffan et al., 2020; Tolo et al., 2021; Zrnčić et al., 2020). It is essential to continue investigating to avoid overlooking either KHV or CEV. Additionally, further research is necessary to determine the relationship between pathogenicity and coinfection.
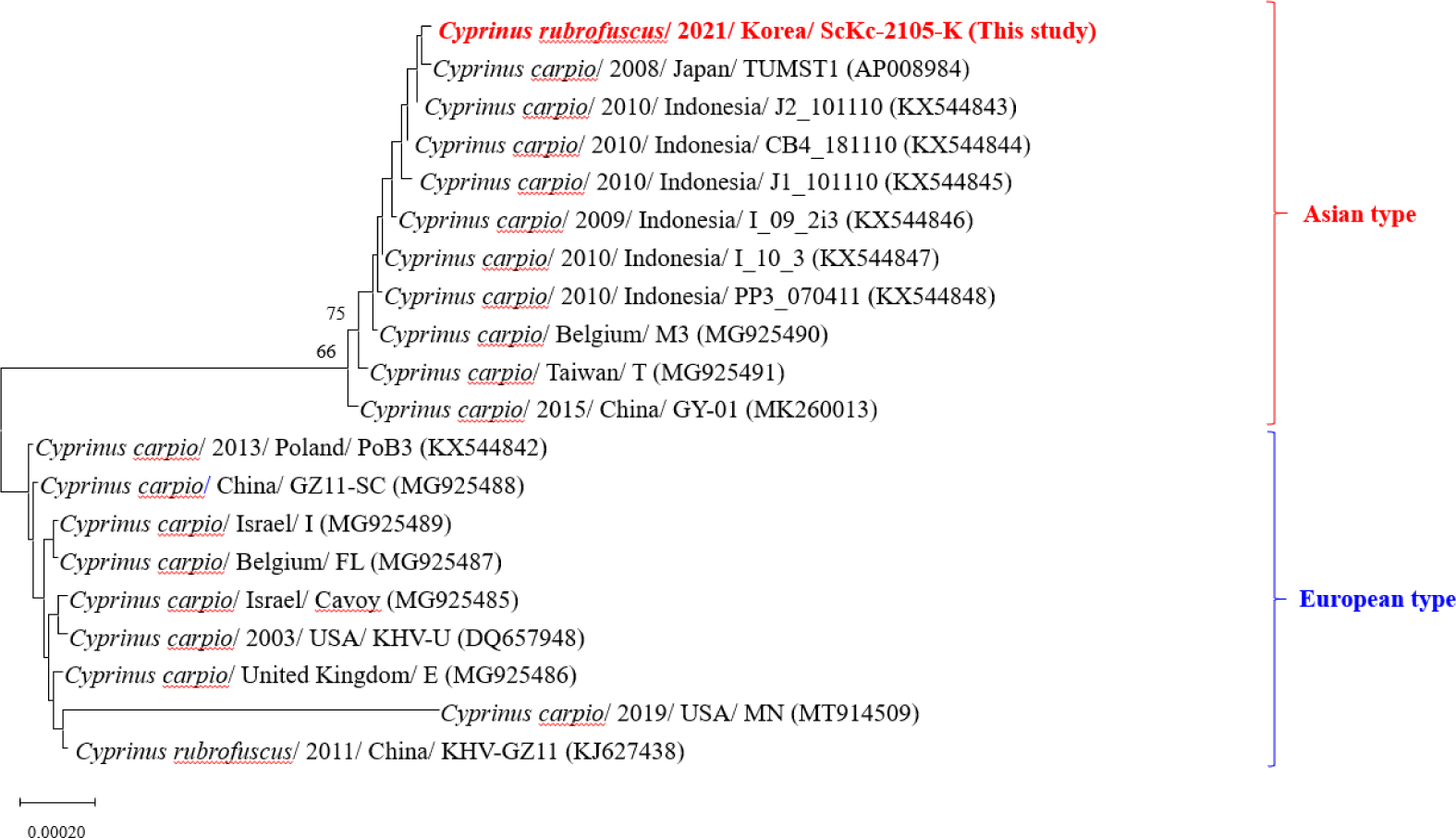
Based on the phylogeny of the enlarged TK gene, KHVs were classified into Asian and European types (Kurita et al., 2009). In this study, partial sequences of the TK genes from two KHVs (ScKc-2105-K and GhKc-2207-K) showed 100% homology with previously reported Korean isolate (GenBank accession number MN545485). However, the PCR amplicon of the enlarged TK gene was only generated from the ScKc-2105-K, which grouped with Asian type and showed close relatedness to isolates from Japan, Indonesia, Belgium, Taiwan, and China (Fig. 3).
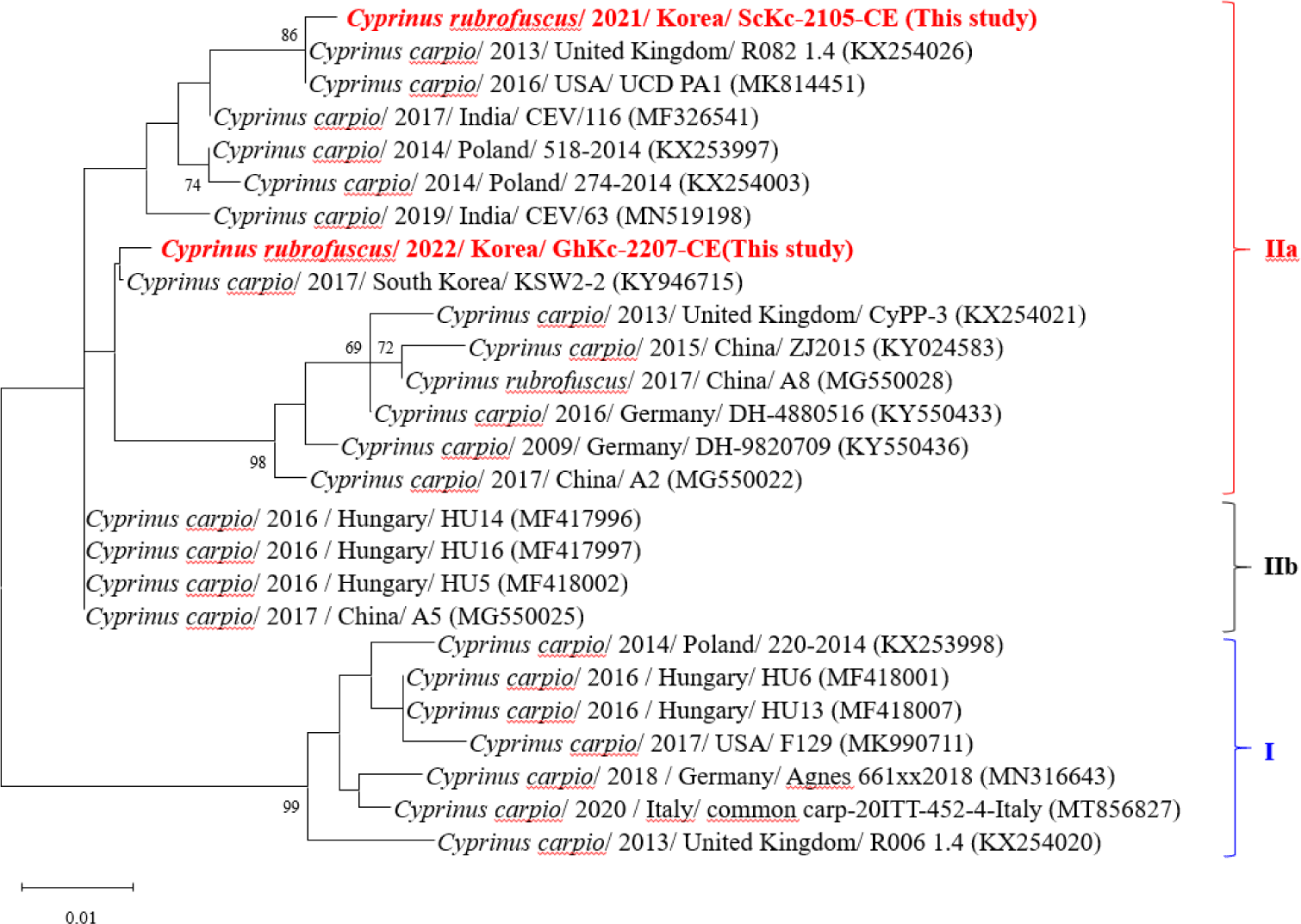
For the CEV, Adamek et al. (2017) has shown that CEV can be classified into three genotypes based on the phylogeny of the p4a gene: I, IIa, and IIb. Two CEVs from koi carp (ScKc-2105-CE and GhKc-2207-CE) were identified as belonging to the IIa genotype (Fig. 4). ScKc-2015-CE was found to be closely related to isolates from the United Kingdom, USA, India, and Poland. On the other hand, GhKc-2207-CE was found to be closely related to isolates from South Korea, the United Kingdom, China, and Germany. The detection of the IIa genotype in the CEVs along with their genetic proximity to isolates from various countries offers significant insights into the worldwide prevalence and variability of this virus. Moreover, this elucidates the evolutionary relationships and genetic heterogeneity of different CEVs, as well as deciphering the infection origins and transmission.
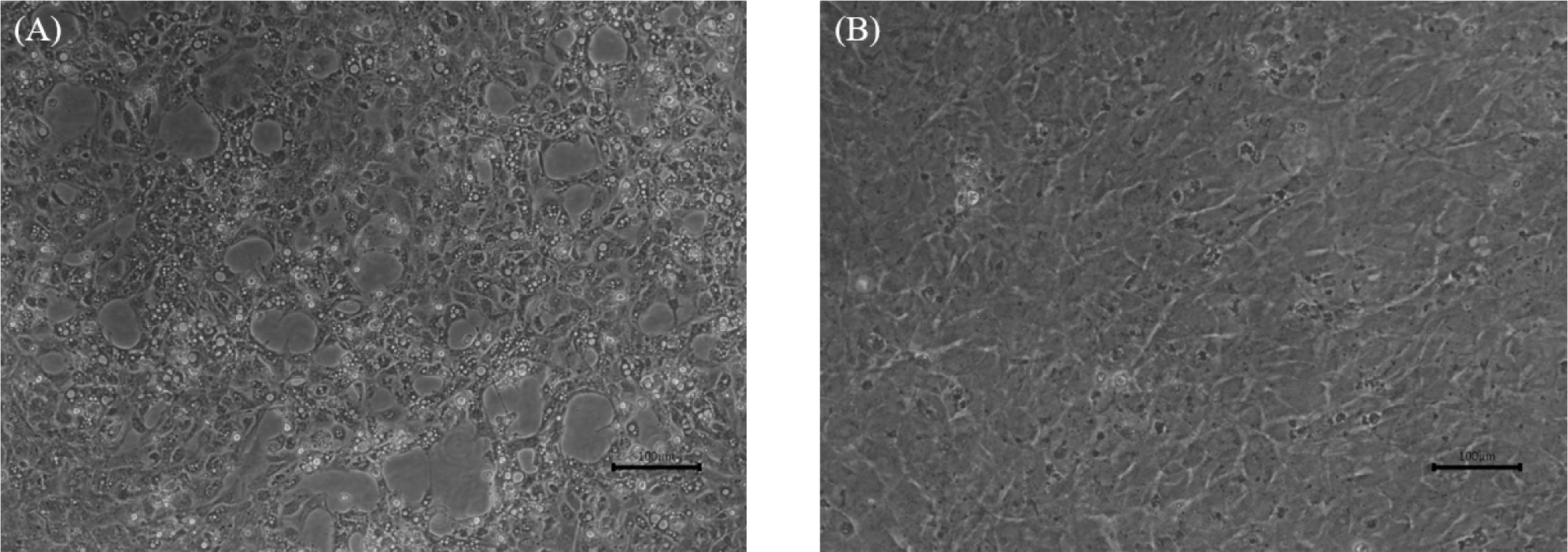
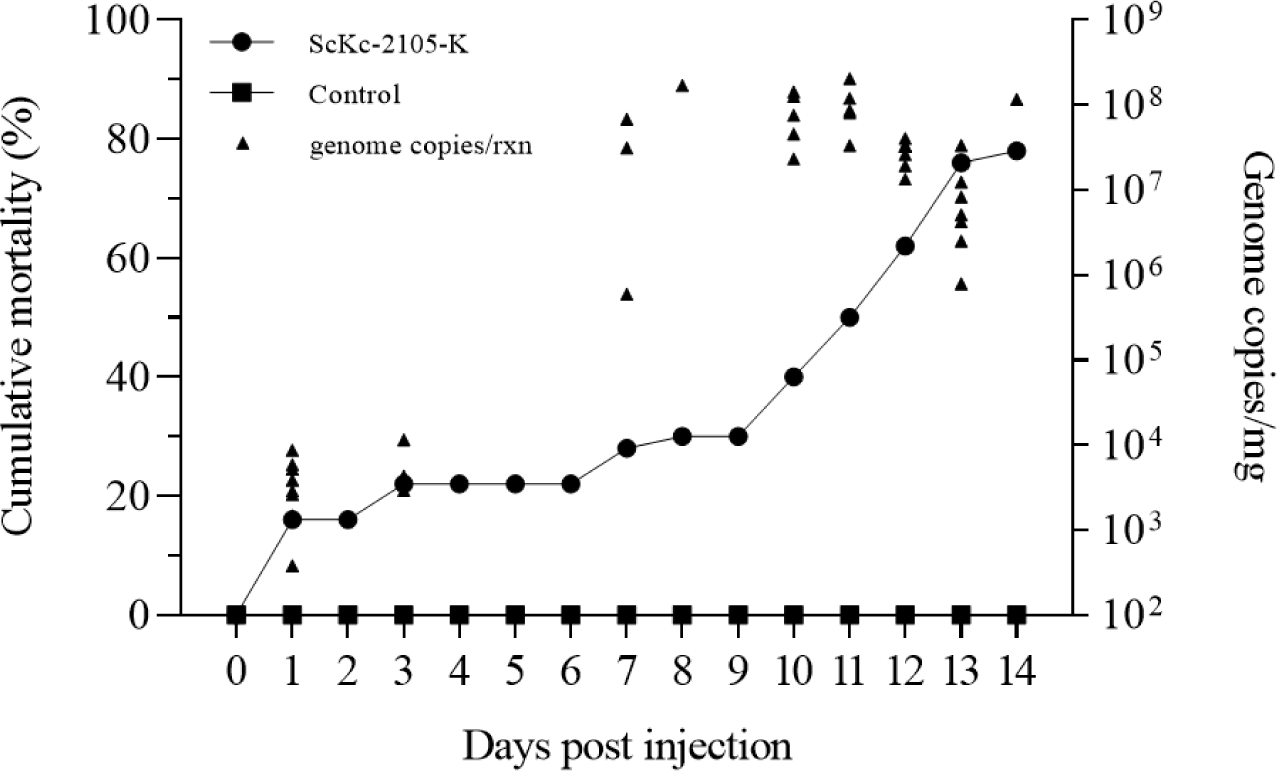
Although two cell lines from cyprinids were used, only KHV (ScKc-2105-K) was successfully isolated from CCB cells (Fig. 5), while CyHV-2 and CEV were not isolated in either CCB or KF-1. In a previous study, it was found that CyHV-2 could be isolated from KF-1 cells with only two passages, but not from epithelioma papulosum cyprinid and fathead minnow cells (Wang et al., 2012). Despite several attempts, no highly permissive cells for CEV isolation were identified until recently. Therefore, we conducted challenge tests only with cultured KHV (ScKc-2105-K), as it was the only virus successfully isolated in CCB cells. The observation of cumulative mortality of 78.0% (39/50) in koi carp injected with cultured ScKc-2105-K at a concentration of 102.6 TCID50/fish (corresponding to 9.20 × 107 viral genome copies/fish) within 14 days post-injection (dpi), while no mortality was observed in the control group (Fig. 6), indicating that the ScKc-2105-K is highly pathogenic in koi carp. A similar result was reported by Miyazaki et al. (2008) in common carp, where a concentration of 102.3 TCID50/fish resulted in 80.0% cumulative mortality. The dead koi carp exhibited typical symptoms of KHVD, such as enophthalmos and gill discoloration (data not shown). The viral genome copy number in dead koi carp was below 104 copies per reaction (average: 9.14 × 102 viral genome copies/rxn; Fig. 6) between 1 and 9 dpi. However, after 9 dpi, the viral genome copies increased to over 107 copies per reaction (average: 1.13 × 107 viral genome copies/rxn). The difference in viral genome copy number between the early and late stages of infection in koi carp may be due to either insufficient viral replication or differences in immune resistance during the early stages of infection.
This study aimed to investigate the presence of alloher-pesviruses (CyHV-2 and KHV) and poxvirus (CEV) in cyprinids. CyHV-2 was only detected in imported goldfish from Thailand, which suggests that the transmission of viral disease in cyprinids may be facilitated through international trade. Notably, the detection of two distinct forms of KHV/CEV coinfection in domestic koi carp indicates the complex nature of viral interactions in fish populations and the need for further research to understand the underlying mechanisms involved. Furthermore, these findings emphasize the importance of ongoing surveillance and monitoring of fish viruses to prevent and manage outbreaks.








