Introduction
Tropical marine aquaculture farmed fish species are rearing close to their thermal temperature optimum. These species are highly vulnerable to large and abrupt temperature changes (i.e., marine heatwaves [MHWs]) as the temperature increase during a MHW can exceed their upper thermal optimum (Tewksbury et al., 2008). Extreme temperatures from MHWs may result in increased mortality and reduced growth rate of tropical farmed fish (Le et al., 2020, 2021). In tropical countries, aquaculture plays an important role in coastal communities (Basset et al., 2013). For example, finfish aquaculture production in Southeast Asian countries provides more than 20% of global fish production (FAO, 2022). In the next three decades, up to 40% of aquaculture species, particularly farmed finfish may not be suitable for aquaculture in tropical regions (Oyinlola et al., 2020). This may threaten the income and food security of coastal communities.
There has been an increasingly awareness of how the color of aquaculture systems (e.g., tank colour) may affect feeding, growth, thereby size disparity and cannibalism and improve hatchery production efficiency and profitability (McLean, 2021). Light intensity in the rearing systems may be affected by the colour. For example, colour may affect visibility of fish larvae and juveniles to prey items (Dehmelt et al., 2021; Ma et al., 2015). Tank colours have been known to affect key behaviours e.g., feeding (Bera et al., 2019; El-Sayed & El-Ghobashy, 2011; Wang et al., 2016), and phygiological parameters e.g., enzyme activities and digestion (Rungruangsak-Torrissen et al., 2006; Santisathitkul et al., 2020). The effect of tank colour on fish growth is species-specific. Milkfish larvae (Chanos chanos) showed the best performance in yellow tanks (Bera et al., 2019), whereas black or dark red is the suitable tank colour for the larvae of white bass (Morone chrysops), striped bass (Morone saxatilis) (Denson & Smith, 1996) and Golden pompano (Trachinotus ovatus) (Ma et al., 2015), pangas catfish (Pangasius pangasius), Eurasian perch (Perca fluviatilis, L.) (Tamazouzt et al., 2000). Contrarily, larvae of Asian catfish magur (Clarius magur) prefer white tanks (Ferosekhan et al., 2020) and tambaqui (Colossoma macropomum) larvae prefer light green tanks (Pedreira & Sipaúba-Tavares, 2001). Finally, the survival and growth performance, key parameters of fish production may be affected by tank colours (Bera et al., 2019; Ferosekhan et al., 2020).
The golden trevally (Gnathanodon speciosus) is a commercially valuable species in Vietnam. They distribute widely in the tropical and subtropical waters in Indo-Pacific and Atlantic Oceans. The golden trevally is a new farmed fish in Vietnam. Artificial seed production of this species is successful in captivity in the hatcheries. This species growth fast and has a high market value. To the best of our knowledge, no reports on the impact of tank colours combined with elevated temperature on golden trevally are available. This is a highly relevant topic given the increasing frequency, intensity and severity of extreme temperatures from MHW events in the last decade with huge socioeconomic impacts (Smith et al., 2021). Therefore, the objective of this study was to evaluate the effect of tank color on the quality of golden trevally juveniles such as growth, survival rate, salinity shock and deformation rate reared at elevated temperatures.
Materials and Methods
Based on the National Regulations for the Use of Animals in Research in Vietnam, golden trevally (G. speciosus) is not listed in two groups IB (endangered and critically endangered species) and IIB (threatened and rare species) (Decree32/, 2006/ND-CP, 2006). Therefore, this study does not require a permit or ethical approval. However, the authors have implemented their best practice of using animals in research.
The experiments were conducted at the Cam Ranh Centre for Tropical Marine Research and Aquaculture, Institute of Aquaculture, Nha Trang University using round fiberglass reinforced plastic (FRP) tanks (diameter, 0.7 m; height, 0.8 m; water capacity, 0.1 m3) with conical bottom (manufactured by Institute for Ship Research and Development, Nha Trang University). The inner surface of the tanks was painted with Composite Epoxy paint from Chokwan Vina Company Ltd. based on JOTUN RAL standard colour chart (red code: RAL 3000-flame red; blue code: RAL 5012-ligh blue; yellow code: RAL 1003-signal yellow; grey code: RAL 7040-window grey; white code: RAL 1003-signal white (http://sonjotun.vn/bang-mau-ral-voi-2013-mau-son-co-ban/250/1298.html). Clean filtered seawater treated with 20 ppm chlorine was filled into the rearing tanks 12 h before stocking the juvenile. Dissolved oxygen (5–6 mg/L) was maintained by a single air stone placed at the bottom of each tank. The salinity (30 PSU) and pH (7.89–8.05) were maintained during the experimental period. Triplicate sets of golden trevally juveniles were reared in white, blue, grey, yellow and red tanks combined with three temperatures (30°C, 32°C, and 34°C) for 4 weeks. The total experimental units were 45 tanks. The temperature was controlled by heaters (HZ-Q5, 500W, Ou Gecali, Shanghai, China). Continuous illumination of 1,200–1,600 lux (24/0 h light/darkness) was applied (Alejos & Serrano, 2018; Mapunda et al. 2021) using natural light during the day and neon light bulbs at night. Nine thousand golden trevally juveniles with an average initial weight of 0.12 ± 0.04 g and initial length of 17.24 ± 1.65 mm were procured from a local farm. After two days of acclimation, the fish were randomly distributed into 45 FRP tanks (200 individuals per tank). During the acclimation and throughout a 30-day experimental period, the fish were fed to satiation twice daily at around 8:00 and 17:00 with a commercial diet (Inve NRD G8-12, Inve, Wachirabarami, Thailand), containing 55% protein, 10% fat and 8% moisture. Uneaten feed and feces were siphoned daily before morning feed.
We determined the growth parameters of G. speciosus juveniles including the increase in body weight (BW, g), total length (TL, cm), the specific growth rate (SGR) in length and weight.
After the experimental period, 10 juveniles from each tank were randomly collected and shocked at three different salinities (0, 5 or 10 ppt) at the temperature of 30°C as osmotic stress only. The survival rate was observed and calculated after 5, 10 or 15 minutes.
To determine the skeletal deformities, we stained the fish bone at the termination of the feeding experiment following protocols detailed by Darias et al. (2010). Fish samples were first fixed in a formalin solution until staining skeletons. Before staining, fish juveniles were transferred to distilled. Subsequently, fish were stained with alcian blue water for 24 h. We used 100% ethanol and 1%KOH solution to neutralize the residual acid in the larvae tissues, then rehydrated fish by submerging them in ethanol solutions (95%, 70%, 40%, 15%) for 15 min each. Subsequently, we used 3% H2O2 and 1%KOH solutions to remove black pigmentations in fish samples and then stained them with Alizarin red solution for 20 h. G. speciosus juveniles were washed with distilled water and subsequently with 1%KOH solution to eliminate the staining background before incubating in 40% glycerol + 60% of 1%KOH in 2 h and 70% glycerol + 30% of 1%KOH in 6 h. The stained juveniles were preserved in 100% glycerol. The morphology and abnormalities of juvenile skeletons were observed, identified and imaged using a stereomicroscope (Amscope SM-2T-EB, AmScope, Irvine, CA, USA) with a camera (a resolution of 10 MP).
All data were visualized as mean ± SD. Statistical procedures were performed using SPSS software (SPSS 22.0, Armonk, NY, USA). Assumptions for two-way analysis of variances were checked with the Kolmogorov-Smirnov and Levene’s tests, respectively. Duncan post hoc tests (p < 0.05), were used to determine significant differences between the experimental groups.
Results
In all colours, survival was generally decreased following the increase of temperature from 30°C to 34°C (main effect of temperature, p < 0.05; Table 1 and Fig. 1). The effects of tank colours on survival following the order: survival in yellow = red > white = grey = blue colour of tank (main effect of tank colour, p < 0.05; Table 1 and Fig. 1), but this color effect was independent on temperatures (tank colour × temperature, p > 0.05).
| Effect | df1, df2 | F-value | p-value |
|---|---|---|---|
| Tank color | 4, 30 | 5.95 | 0.002 |
| Temperature | 2, 30 | 6.857 | 0.004 |
| Tank color × temperature | 8, 30 | 0.705 | 0.685 |
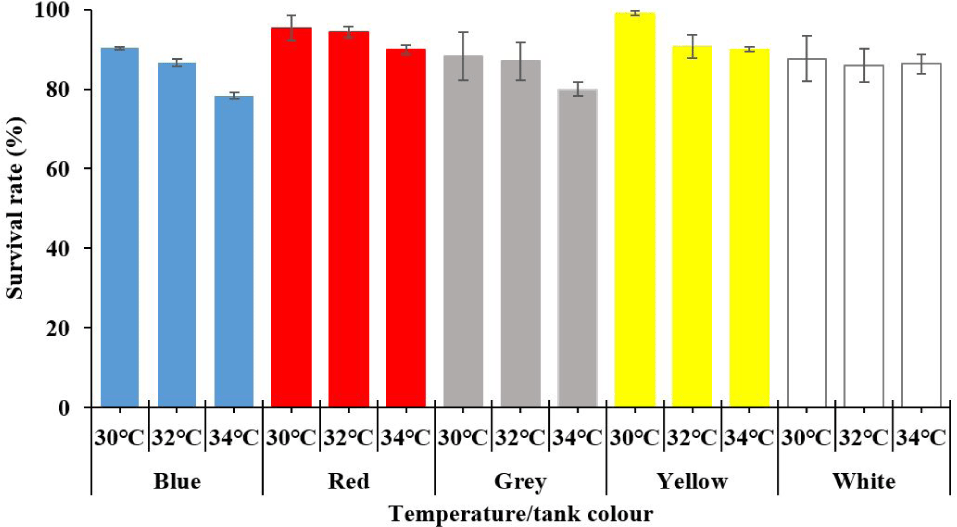
All growth parameters including final length and weight, increased length and weight, SGRs, and FCR of the golden trevally juveniles were significantly decreased by increasing temperatures from 30°C to 34°C (main effect of temperature, p < 0.05; Table 2). Temperature effects on the growth performance of the golden trevally juveniles were similar in five tank colours (Table 2). These parameters were not significantly different by five tank colors (p > 0.05; Table 2).
At a salinity of 10 ppt, the survival rate of the golden trevally juvenile was 100% in all treatments (p > 0.05; Table 3). However, the significant differences between treatments were retained at the 5 ppt and 0 ppt of salinity shock (p < 0.05; Table 3). In both salinities of 0 and 5 ppt, survival was reduced in juveniles reared under elevated temperatures (p < 0.05; Table 3). The colour tank strongly affected the survival of the golden trevally juveniles in salinity shock at 0 ppt or 5 ppt (p < 0.05; Table 3). At a salinity of 0 ppt, the survival rate of the golden trevally juveniles was the highest in red tanks and seemed to be lowest in blue tanks. However, the combined effect of tank colours and elevated temperatures was not significantly affected the survival rate of fish juveniles for salinity shock at 0 ppt or 5 ppt (p > 0.05; Table 3).
The deformation rate increased significantly by increasing temperature from 30°C to 34°C (main effect of elevated temperature, p < 0.05; Table 4 and Fig. 2). The deformation also increased was highest in white, followed by grey, blue, yellow, and lowest in red tanks (main effect of tank color, p < 0.05; Table 4 and Fig. 2), particularly under higher temperatures (interaction of temperature and colours, p < 0.05; Table 4 and Fig. 2).
| Effect | df1, df2 | F-value | p-value |
|---|---|---|---|
| Tank color | 4, 30 | 229.25 | < 0.001 |
| Temperature | 2, 30 | 68.375 | < 0.001 |
| Tank color × temperature | 8, 30 | 3.681 | 0.004 |
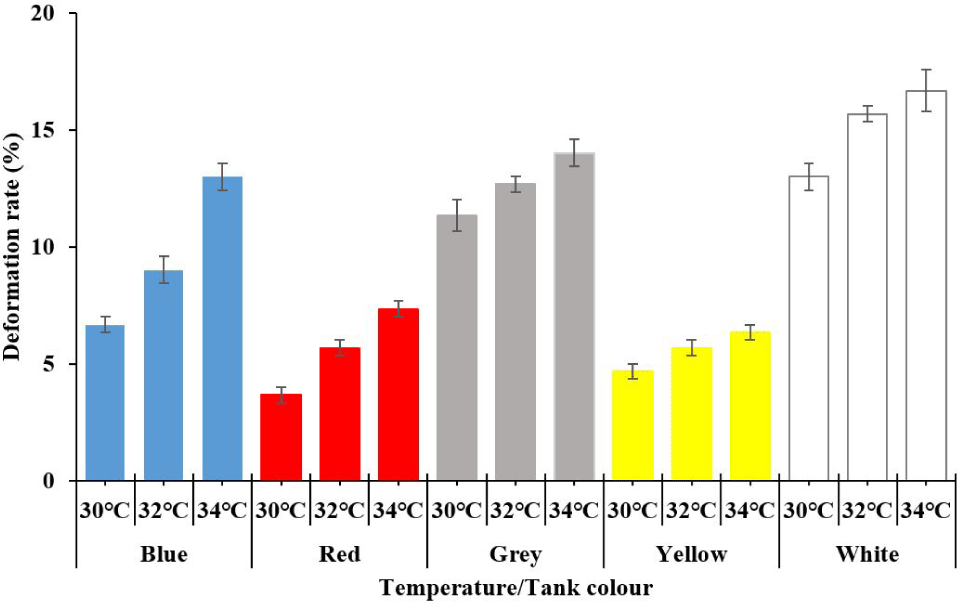
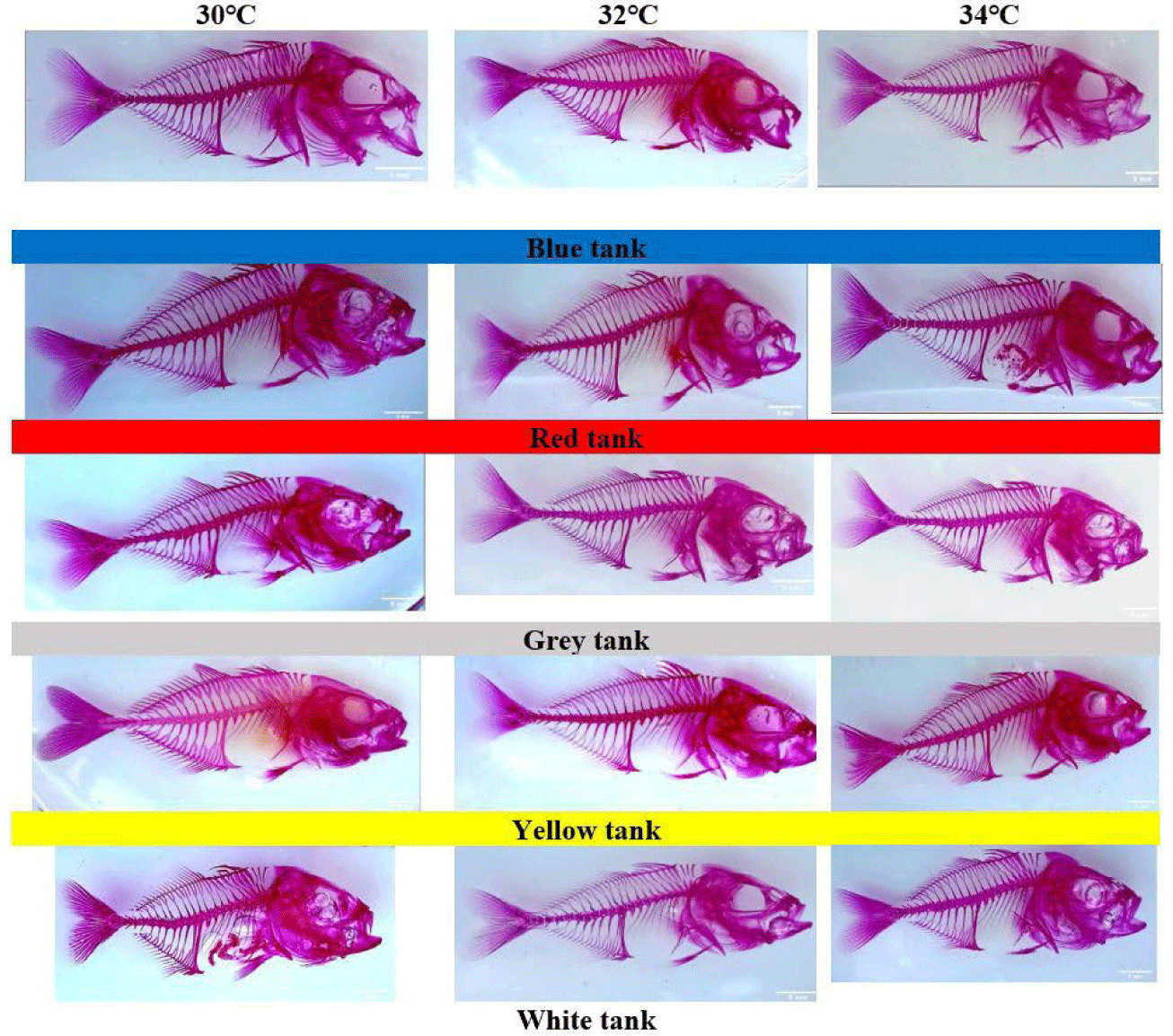
The skeletal staining showed that the golden trevally juveniles reared at the white color tank and grey color tank to receive the deformity at the vertebrate (Fig. 3). However, the golden trevally juveniles reared at the blue, yellow or red color tank were not seen any deformity at the vertebrate (Fig. 3).
Discussion
This is the first study investigating how the colour of rearing systems may alleviate the heat stress on the golden trevally, a new aquaculture fish species in Vietnam. In general, elevated temperatures resulted in lower survival, reduced growth rate and fish health as indicated by the capacity to survive in a salinity shock, with applications in larvae and juvenile rearing of this species, and tropical finfish species in general.
There has been increasing concern about the thermal tolerance of aquaculture fish species to heat stress with increasing frequency and severity of MHWs under ongoing climate change. For example, 8 of the 10 most severe recorded events have taken place in the past decade (Smith et al., 2021). Extreme temperatures can cause several physiological impairments or dysfunctions such as mitochondrial dysfunction, muscular and heart failures (Ern et al., 2023), a reduced efficiency of oxygen uptake and delivery could also contribute to fish mortality (Pörtner & Farrell, 2008; Pörtner et al., 2017). In tropical aquaculture fish such as cobia showed increased mortality at 32°C (Le et al., 2020; Nguyen et al., 2019). In agreement, the survival of the golden trevally juveniles was also reduced, particularly at 34°C, which may be explained by similar physiological dysfunctions as above.
Similar to the survival, all growth parameters of the golden trevally juveniles were reduced under elevated temperatures, particularly at 34°C which was similar to the temperature effects on other tropical fish species such as cobia Rachycentron canadum (Le et al., 2020; Nguyen et al., 2019; Sun & Chen, 2014; Sun et al., 2006), or spinefoot rabbitfish Siganus rivulatus (Saoud et al., 2008). The reduced growth rate may be a result of lower food conversion efficiency coupled with a higher energy expenditure on basal maintenance (Killen et al., 2016). Indeed, we found a higher FCR at 32°C and 34°C. Rapid upregulation of energetically costly heat shock proteins was observed in tropical fish species e.g., in barramundi following exposure to heat stress (Newton et al., 2012) as a key physiological mechanism to deal with heat stress.
There were some small differences in the survival of the golden trevally juveniles reared at different tank colours; lowest in white tanks and highest yellow and red tanks. Indeed, a previous study has shown that fish reared in red tanks had a lower level of cortisol and higher levels of heat shock protein (HSP) than those in other tanks (Morshedi et al., 2022). HSPs have been well known for their role in coping with general stress in organisms (Morshedi et al., 2022). Interestingly, tank colours could not change the lethal effect of temperatures on the golden trevally juveniles. Several previous studies have found effects of tank colours on the growth rate of marine fish such as milkfish (C. chanos, Bera et al., 2019), barramundi (Lates calcarifer, Morshedi et al., 2022). The yellow color may increase the visibility of milkfish larvae to food items, thereby increasing feeding and growth rate (Bera et al., 2019). The increased growth rate of barramundi reared in red tanks may relate to their feeding behaviours in nature where barramundi prey upon benthic macroinvertebrates and small mid-water pelagic fish (Morshedi et al., 2022). However, we did not find an effect of tank colours on any effect on the growth parameters of the golden trevally juveniles, which may be explained by a similar food conversion efficiency. Indeed, FCRs of the folden trevally juveniles was not affected by rearing tank colours. While we did not assess the feeding rate or feeding behaviours of this species, it was likely that all five tank colours did not create different contrasts between the feed and the background colours.
When challenged to a salinity shock, fish reared in white and blue tanks showed 3–5 times lower survival than those in red and yellow colours. A similar effect of tank colour on the skeletal deformity rate was also observed. Previous studies have suggested that marine fish are more vulnerable to short wavelengths (Villamizar et al., 2011), which may be more in blue, white, or grey tanks than in red and yellow. The low survival of challenged fish in these colours suggests lower fish health, which could be linked to a higher skeletal deformity rate.
We found a generally insignificant interaction of heat stress and tank colours on survival, growth rate, and the capacity to tolerate a salinity shock of the golden trevally juveniles, suggesting that tank colours could not mitigate the lethal heat stress from the MHWs. These result suggest that technical adjustments to keep rearing temperature not going beyond the thermal threshold of the the golden trevally juveniles is critically important to reduce the direct heat-induced mortality or growth rate. This is important in the context of increasing frequency and magnitude of MHWs under ongoing climate change (Frölicher et al., 2018). While tank colours could not mitigate visible effects of the heat stress, particularly during a heat wave, temperature-induced increased skeletal deformity rate was much more pronounced in blue tanks and lowest in yellow tanks. However, it would not be possible to detect with the naked eye of live fish juveniles. We would have seriously underestimated the effects of tank colours on the quality of the golden trevally juveniles if we did not assess the skeletal deformities. This is critical as different forms of skeletal deformity may result in long-term effects on survival and growth (Imsland et al., 2006).
Conclusion
In conclusion, elevated temperatures and blue and white rearing tanks resulted in reduced survival, growth and increased mortality of fish challenged with salinity stress, but there were no interactive effects of both factors on these key productive parameters of golden trevally juveniles. However, we found a “hidden interactive effect” of elevated temperature and tank colours on the skeletal deformities, which may affect fish survival and growth during the grow-out period. Blue and white colours are not recommended for rearing golden trevally juveniles, particularly during heat stress such as heatwave periods which are becoming more frequent and severe under ongoing climate change.








