Introduction
Vitamin C is a water‐soluble micronutrient essential for normal physiological functions, and immune and stress responses, thereby being integral to growth rate and survival of aquaculture fish (Dawood & Koshio, 2018; Le et al., 2021). Vitamin C plays multiple roles in fish such as collagen synthesis, iron absorption, immune response, wound healing, and proximate body composition of the fish (Darias et al., 2011; Dawood & Koshio, 2018; Padayatty & Levine, 2001). However, fish have to acquire vitamin C from food as they cannot synthesize it (Dawood & Koshio, 2018; Fracalossi et al., 2001). Fish that do not acquire enough vitamin C show various symptoms of disorders such as malformations, immunodepression, reduced growth, increased susceptibility to diseases, and increased mortality (Dawood & Koshio, 2018; Le et al., 2021).
The red drum (Sciaenops ocellatus) has been a valuable seafood and recreational fishery resource throughout its natural range for over 20 years. This species has become a popular farmed species in the United States and Asia, particularly in Vietnam due to its high tolerance to the environmental condition, large size, high meat yield, nutritious meat, and high market price (Yen et al., 2021). The nutritional requirements of S. ocellatus have been studied extensively in recent years, particularly the protein levels (Watson et al., 2020), lipid (González-Félix et al., 2018); vitamin E (Peng & Gatlin, 2009); inorganic and organic dietary copper (Chen et al., 2020). A previous study has found that a minimum vitamin C level of 15 ± 3 mg/kg in their diet is required for the growth and normal activities of red drum juveniles with an initial body weight (BW) of ~3.6 g (Aguirre, 1999). However, the optimal vitamin C levels for growth and fish health are currently unknown. Therefore, this study aimed to assess the general effects of vitamin C on the growth, body composition, and biochemical parameters of red drum juveniles.
Materials and Methods
The red drum (S. ocellatus) is not on the list of endangered, precious, and rare species of wild plants and animals, in the National Regulations for the Use of Animals in Research in Vietnam: The Law of Animal Husbandry of Vietnam, 2018 and The Government Decree 32/2006/ND-CP. Therefore, ethical approval requirements for conducting experiments with this species are not required in Vietnam. However, the authors have implemented their best practice following the guidelines of using animals in research based on EU directive 2010/63.
Red drum (S. ocellatus) juveniles were bred at Cam Ranh Centre for Tropical Marine Research and Aquaculture, Institute of Aquaculture, Nha Trang University, Vietnam. Before the experiment, we acclimatized fish juveniles (size: BW = 1.82 ± 0.40 g and total length = 5.75 ± 0.41 cm) to laboratory conditions for 14 days, which was similar to our previous study (Le et al., 2021). Ten juveniles were assigned to a rectangular fiberglass tank (length × weight × height = 0.8 × 0.5 × 0.5 m, a total of 21 tanks filled with pre-filtered 200 L seawater). During the acclimation period, fish were fed a commercial diet (~5% BW; INVE, Wachirabarami, Thailand), two times a day at around 08:00 and 17:00. The rearing conditions such as temperature, salinity and pH were kept at 29 ± 0.5°C, 29 ± 3.1 ppt and 7.6–7.8, respectively. Ammonia was lower than 0.05 mg/L, and dissolved oxygen was maintained above 6.0 mg/L by continuous aerations in each tank.
The basal diet (crude protein: 55%, lipid: 9%) was formulated according to the marine finfish feed requirement and feed content illustrated in Table 1. Ascorbic acid (vitamin C) powder (Acid Ascorbic Vitamin C 99%-E300 DSM, Shandong Luwei Pharmacy, Shandong, China) was added to the base diet in different amounts to obtain 7 experimental treatments: control (no vitamin C supplement to the artificial feed), 100, 200, 400, 600, 800 and 1,000 mg vitamin C equivalent/kg diet (3 replicates each treatment). We used reverse-phase high-performance liquid chromatography to analyze the ascorbic acid levels. Table 1 shows detailed information about the experimental diets after the addition of vitamin C. The experimental diets were kept cold in a refrigerator at 4°C. During the experimental period, the rearing system, water conditions and feeding levels were kept consistent with their values during the acclimation period. Tanks were cleaned every two weeks and each time was concurrent with when fish were removed and weighed. The feeding trial lasted for 10 weeks.
On day 70 (the termination of the experiment), we collected a subset of 3 individuals/tank (9 individuals per treatment). We determined growth, body indices, hematological parameters including white blood cells (WBC), red blood cells (RBC), hematocrit, haemoglobin and proximate composition. Fish were first anesthetized with monophenyl ether glycol solution at a concentration of 150–200 ppm. Subsequently, we collected the blood samples from caudal veins using a 1-mL syringe. Lysozyme activity and hematological parameters in the blood samples were determined (see more details in Appendix 1). Afterwards, the BW was determined using a laboratory scale within a range 0–200 g and an accuracy of 0.01 g. We determined growth parameters including the specific growth rate (SGR, %/day) based on the natural logarithm (Ln) of final weight (LnWt) and initial weight (LnWi) during the experimental period (t, day) as 100 × (LnWt – LnWi) / t. To better understand the relationship between SGR and feed utilization, feed conversation ratio (FCR) was determined by dividing the amount of feed consumed (g, dry weight) by the increase in fish weight (g, wet weight). The protein efficiency ratio (PER) was based on the weight gain (WG, g) per protein intake (g). Protein productive value (PPV) was calculated as the retained protein (g) of the consumed protein (g): PPV = (final protein content – initial protein content) × (protein consumed)–1.
In the next steps, the fish liver and visceral organs were collected. BW (g), liver (LW, g) and visceral weights (VW, g) were determined. Similarly, we determined hepatosomatic index (HSI, %) = LW / BW × 100, and the viscerosomatic index (VSI, %) = VW / BW × 100. The vitamin C concentrations in the liver and the body were measured (Appendix 1). Finally, the fish body was used to measure the proximate composition (Appendix 1).
We used one-way analyses of variance for analyzing the data, and followed by using Duncan’ multiple-range test to compare differences between dietary groups. The results are described as the mean ± SEM, and the significance level was set at p < 0.05. The optimum vitamin C concentrations were estimated by using broken‐line polynomial regression analysis. All statistical analyses were performed by using SPSS 22.0 (SPSS, Armonk, NY, USA).
Results
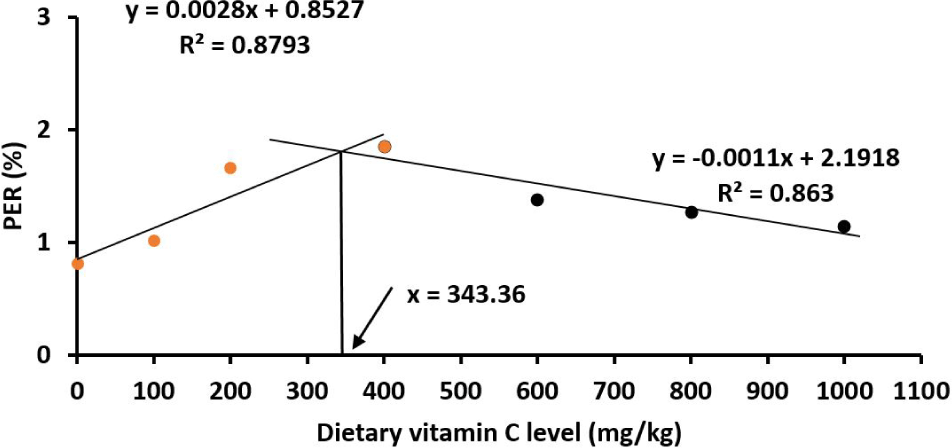
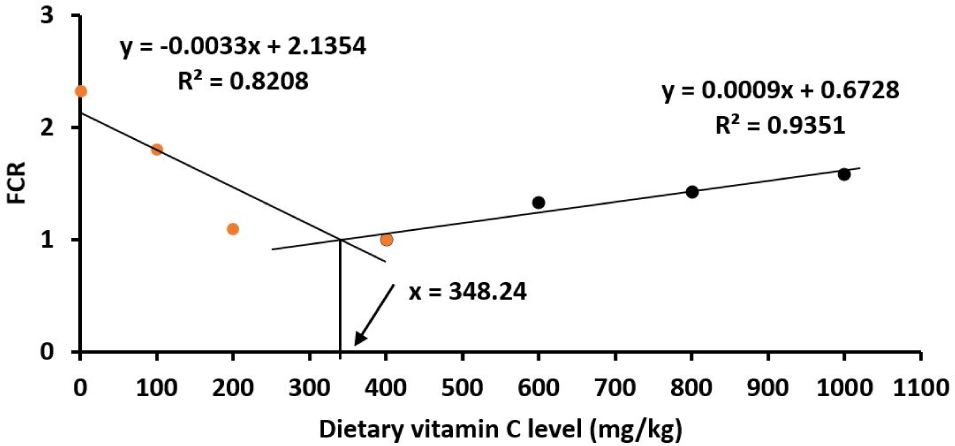
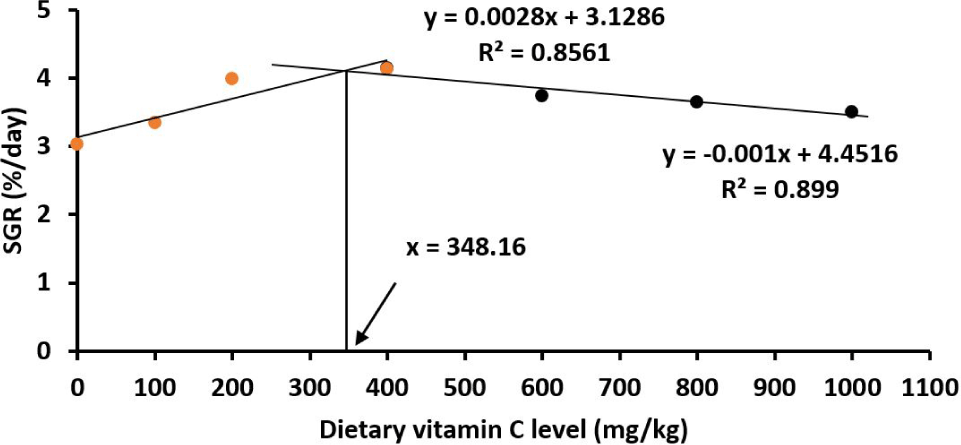
All fish survived to the end of the experiment. All the measured parameters including SGR, FCR, PER, PPV, HSI and VSI red drum juveniles were significantly affected by vitamin C (p < 0.05; Table 2). Juveniles showed an increase in WG and SGR when increasing the vitamin C levels in the diets from the basal (23.2 mg vitamin C/kg diet) to reach the highest growth at 400 mg vitamin C/kg diet, then the growth rate was steadily reduced when the vitamin C levels increased further to 600–1,000 mg/kg (p < 0.05). The patterns of PER and PPV resemble the similar pattern of the growth parameters (Table 2). Similarly, the FCR of fish juveniles was lowest at the vitamin C level of 400 mg/kg (Table 2). It was estimated by the broken‐line polynomial regression analysis that the optimal vitamin C levels were 345.88 mg/kg for WG (Fig. 1); 343.36 mg/kg for PER (Fig. 2); 348.24 mg/kg for FCR (Fig. 3) and 348.16 mg/kg SGR (Fig. 4). HSI and VSI values were not significantly different among diets (p > 0.05; Table 2).

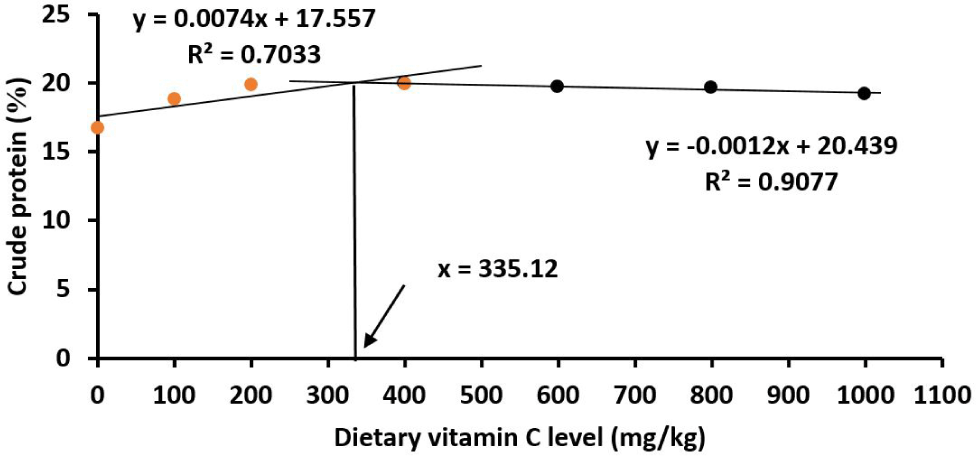
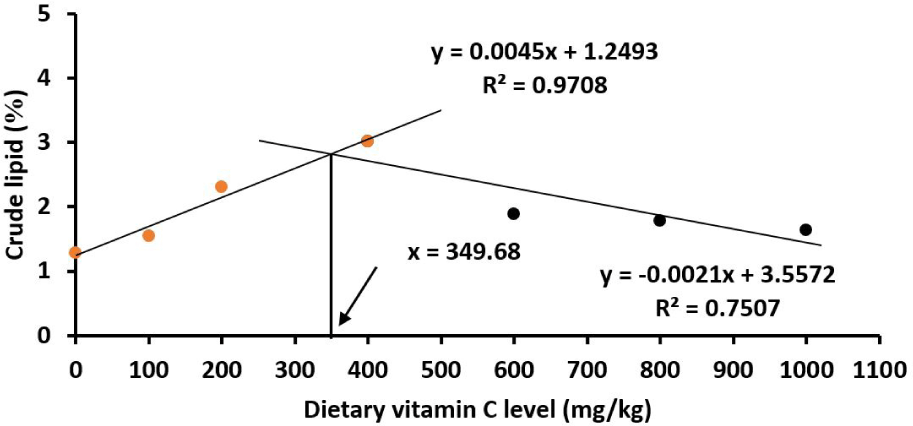
The data showed that the crude protein and lipid contents in the fish body were highest at the vitamin C level of 400 mg/kg diet (p < 0.05). Based on the broken‐line polynomial regression analysis, the optimal vitamin C levels were 335.12 mg/kg for crude protein (Fig. 5) and 349.68 mg/kg for lipid (Fig. 6). The moisture and ash contents were not affected by vitamin C in the diet as indicated by non-statistically significant differences of these two parameters in different vitamin C treatments (p > 0.05; Table 3).
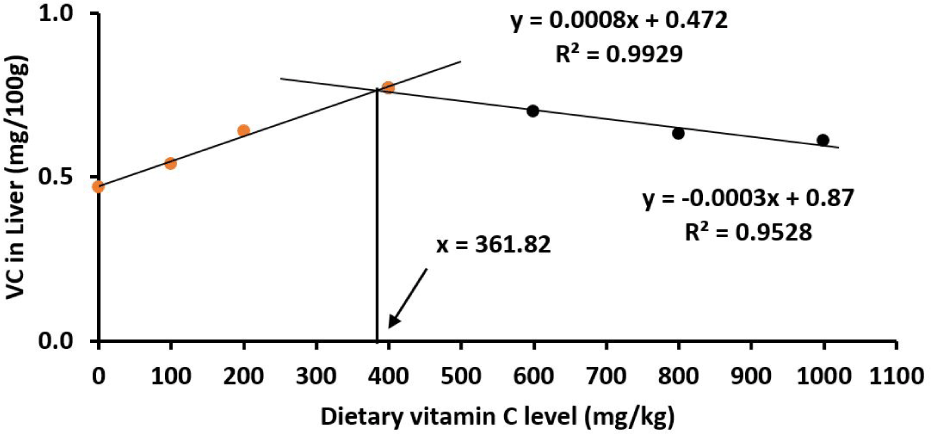
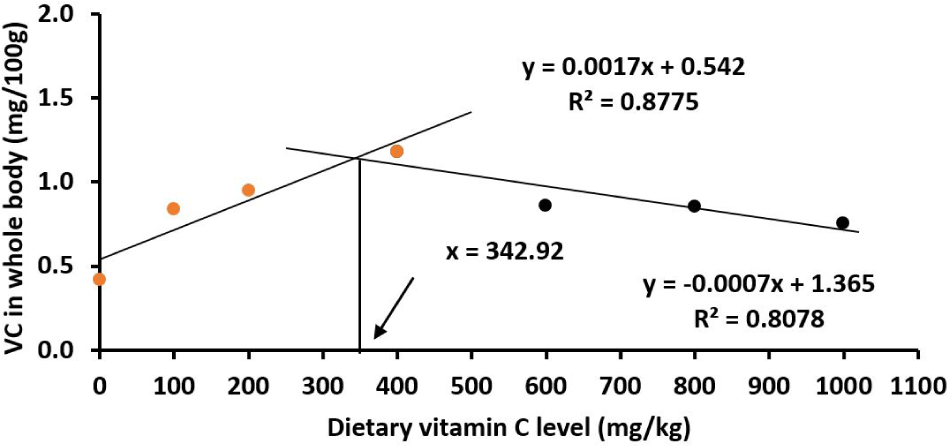
Similarly, juveniles had the highest body and liver vitamin C levels at 400 mg/kg vitamin C diet (p < 0.05; Table 4) with the estimated optimal vitamin C levels being 361.82 mg/kg in the liver (Fig. 7) and 342.92 mg/kg in the body (Fig. 8).
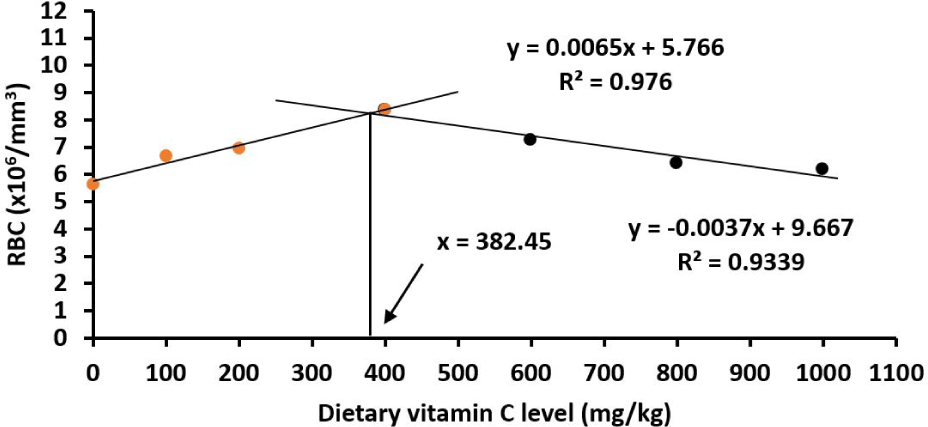
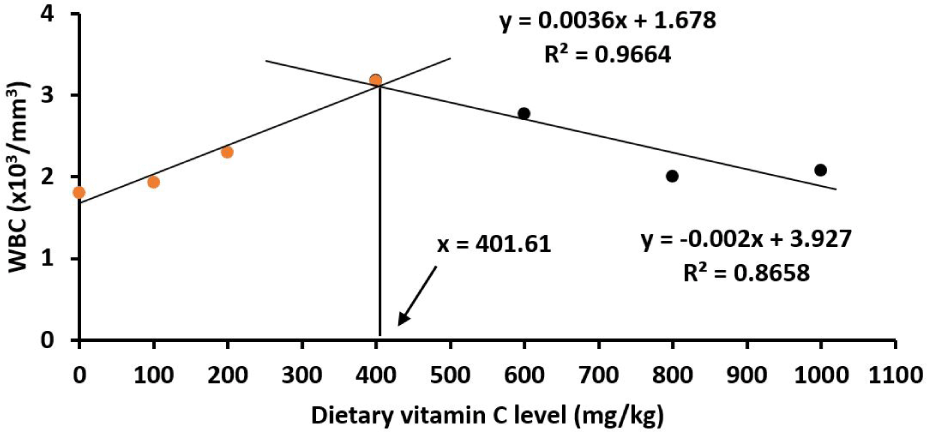
The numbers of serum RBC and WBC numbers were significantly lower in control fish juveniles than in all other treatments (p < 0.05). For the number of serum RBC, it decreased when increasing vitamin C levels from 400 to 1,000 mg/kg diet (p < 0.05). The number of serum WBC was lowest in juveniles fed on the basal diet and increased to the highest at 400 mg/kg (p < 0.05; Table 5), then decreased gradually from 400 to 1,000 mg/kg (p < 0.05; Table 5). The optimum vitamin C requirements for red drum juveniles were 382.45 and 401.61 mg/kg based on the number of serum RBC content (Fig. 9) and WBC content (Fig. 10), respectively.
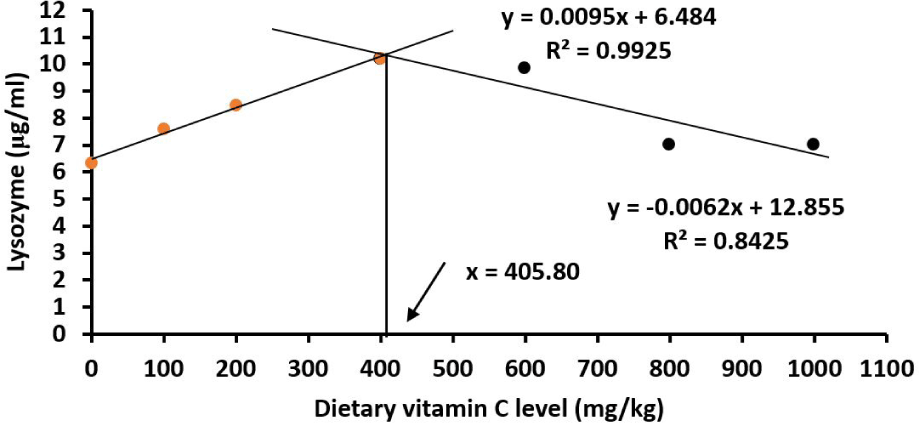
Similarly, the serum lysozyme activity was lower in fish fed a basal diet than those fed vitamin C enriched diets (p < 0.05; Table 6). Juveniles fed 400 mg/kg vitamin C diets had the highest lysozyme activity. The optimal vitamin C requirement for the serum lysozyme activity was 405.80 mg/kg (Fig. 11).
| Parameters | Vitamin C concentrations (mg/kg) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 100 | 200 | 400 | 600 | 800 | 1,000 | |
| Lysozyme (μg/mL) | 6.33 ± 0.47a | 7.58 ± 0.54ab | 8.47 ± 0.43bc | 10.20 ± 0.65d | 9.84 ± 0.75cd | 7.01 ± 0.44ab | 7.01 ± 0.44ab |
Discussion
The survival of the red drum juveniles was 100% in all treatments, suggesting that the level of vitamin C in the basal diet met the requirement for survival, but was not optimal for growth. Indeed, the vitamin C level in the basal diet was 23.2 mg/kg which is higher than the minimal vitamin C requirement of 15 ± 3 mg/kg for red drum juveniles (Aguirre, 1999). The highest WG, SGR, PER and PPV were observed in juveniles fed the diet with a supplement of 400 mg/kg and the estimated optimal vitamin C levels for growth and food utilization parameters were around a narrow range of 343–350 mg/kg which is comparable to the optimal vitamin C levels for other marine farmed fish species such as cobia or parrot fish (Ishibashi et al., 1992; Zhou et al., 2012). It has been shown that vitamin C helps to increase the activity of digestive enzymes such as protease and lipase, which enable an increase in the nutrient absorption efficiency in fish (e.g., discus fish Symphysodon haraldi, Liu et al., 2019). This may explain the higher FCR and protein utilization (PER and PPV), body condition, and most importantly increased growth rate of red drum juveniles in our study.
Despite HSI and VSI being proxies for physiological conditions in fish (Dawood & Koshio, 2018), red drum juveniles did not show differences in HSI and VSI between treatments, suggesting that vitamin C levels in this study did not affect non-fatty energy reserves (e.g., glycogen) of red drum juveniles. These results are similar to our previous study on Psammoperca waigiensis, another farmed species in Vietnam (Le et al., 2021).
Vitamin C supplements in the diet resulted in higher crude protein and lipid contents in red drum juveniles, which may be a result of increased protein and lipid conversion efficiency from the higher protease and lipase activity (Liu et al., 2019). In addition, vitamin C has been known as a cofactor enhancing protein synthesis in fish, which may increase the protein content (Biswas et al., 2013; Sandnes, 1991). The higher protein and lipid contents are essential to building muscle and growth and supply energy to fuel fish activities. Interestingly, the optimal vitamin C levels for the body composition of juveniles were within the same narrow range for the growth and feed utilization parameters.
Vitamin C enhances ferrous iron (Fe2+) speciation and absorption (Bakke et al., 2010) that iron (Fe2+) plays multiple important physiological functions in fish such as a component of hemoglobin in RBCs, myoglobin in muscle, and many enzymes which serve defense functions such as peroxidase, catalase, and cytochromes (Lim et al., 2001). As expected, RBC, WBC, and lysozyme activity were all higher in juveniles fed vitamin C supplements in the diets. The higher levels of WBC and lysozyme activities may improve the resistance of juveniles to pathogens (see e.g., Le et al., 2021). RBC is directly relating to fish respiration. Interestingly, the optimal vitamin C levels for RBC, WBC and lysozyme activity were around 400 mg/kg, which was similar to the finding for other fish species (e.g., Pseudosciaena crocea, Ai et al., 2006). However, the optimal vitamin C levels for these hepatological and immune parameters were slightly higher compared to growth and food conversion parameters, suggesting higher requirements of vitamin C for respiration, and immune defense mechanisms, which is likely due to red drums being a fast-growing species.
Conclusion
In conclusion, the optimal dietary vitamin C requirements of red drum juveniles varied from 342.92 to 405.80 mg/kg diet to improve growth rate, food conversion, and increase innate immunity in red drum juveniles. These results are important to consider when producing artificial feed for rearing red drum juveniles, a species of high commercial and consumption interests in Vietnam. Future studies should also investigate whether these optimal vitamin C levels may be affected by other culture conditions such as extreme temperatures (Le et al., 2021), ocean acidification and diseases (Naylor et al., 2021).








