Introduction
Chronic inflammatory diseases, including neoplasms, cerebrovascular diseases, and hypertension, remain the leading causes of mortality in the Philippines (Ornos et al., 2023). Inflammation, the body’s natural defense mechanism, facilitates the removal of harmful pathogens and stimulants but can become detrimental when transitioning from an acute to a chronic state. Chronic inflammation is driven by prolonged exposure to inflammatory stimuli, leading to tissue degeneration and the onset of diseases such as asthma, cancer, diabetes, and osteoarthritis (Chen et al., 2018; Franceschi et al., 2014; Germolec et al., 2018). With this, continuous efforts to generate knowledge on the role of inflammation in chronic diseases and develop effective treatment strategies in combatting inflammation are critical to mitigating this problem (Ornos et al., 2023; PSA, 2023).
Complex pathways mediate inflammatory processes, primarily involving the release of cytokines like interleukins (ILs), chemokines, interferons (IFNs), and tumor necrosis factors (TNFs), which modulate immune responses by either promoting or inhibiting inflammation (Chen et al., 2018; Turner et al., 2014). Pharmacological interventions, including monoclonal antibodies (MABs) and non-steroidal anti-inflammatory drugs (NSAIDs), have been developed to modulate these pathways and control inflammation (Gerriets et al., 2023). However, NSAIDs are associated with gastrointestinal toxicity and cardiovascular risks, while MABs can lead to immunosuppression and hypersensitivity reactions (Pirlamarla & Bond, 2016; Yatoo et al., 2018). These adverse effects underscore the need for safer and more effective alternatives derived from natural products.
One promising natural product is fucosterol (Fig. 1), a bioactive sterol derived from brown seaweed, which has demonstrated significant pharmacological properties, including anti-inflammatory, antioxidant, neuroprotective, and anticancer effects (Jang et al., 2024; Meinita et al., 2021). Moreover, fucosterol has also shown favorable toxicity profiles in cell viability assays, making it a strong candidate for drug development (Meinita et al., 2021). Thus, further evaluation of its pharmacological activities is crucial in establishing the compound’s potential as a pharmaceutical agent.
Recent advancements in drug discovery have facilitated the study of plant-derived compounds using molecular docking and computational modeling, which predict interactions between a compound and target proteins (Kazmi et al., 2019; Meng et al., 2011). While these in silico methods enhance drug discovery efficiency, their findings must be validated through in vitro and in vivo studies to confirm bioactivity. Experimental models, such as the lipopolysaccharide (LPS)-induced inflammation model in Human Embryonic Kidney (HEK293) cells, provide controlled environments to assess fucosterol’s effects on cytokine expression in non-immune cells, while zebrafish models allow for the study of inflammation in physiologically relevant systems (Novoa & Figueras, 2011; Raghubeer et al., 2017; Tan et al., 2021). These approaches provide crucial insights into the pharmacological mechanisms of fucosterol and support its potential development as a safer alternative to synthetic anti-inflammatory drugs.
This study provides relevant insights to establish further fucosterol’s potential in regulating inflammatory cytokines and chronic inflammation. By providing comprehensive insights into its anti-inflammatory activity, this research contributes to developing natural product-derived pharmaceutical products that may effectively manage chronic inflammatory diseases. Furthermore, it aligns with broader efforts to explore natural product-based therapeutics, reinforcing the importance of alternative anti-inflammatory agents in modern medicine.
Materials and Methods
All in silico molecular docking simulations were performed based on previously reported methodologies and using the computers and software in the laboratory of the emerging interdisciplinary research (EIDR) program of the University of the Philippines–Manila, Philippines (Agu et al., 2023; Alos et al., 2020; Aventurado et al., 2020; Bell & Zhang, 2019).
The molecular structures of fucosterol, known inhibitors, and the target pro-inflammatory proteins were downloaded from the PubChem (https://pubchem.ncbi.nlm.nih.gov/) and RCSB (https://www.rcsb.org/) online databases, respectively. Using Spartan ‘14 (1.1.4), the structures of the ligands were optimized through the computation feature’s molecular mechanics and converted to appropriate formats to allow loading in other programs. The structure of the pro-inflammatory proteins was prepared through BIOVIA discovery studio (DS) visualizer (17.2.0.16349, Bio-Techne, Minneapolis, MN, USA) by the removal of the attached heteroatoms or ligands and the addition of polar hydrogen atoms to allow and give space for the docking of new ligands. The following pro-inflammatory proteins were selected for this study: Interleukin-1 Beta (IL-1β, PDB ID: 8C3U), Thromboxane A2 (TXA2, PDB ID: 6IIV), Interleukin-6 (IL-6, PDB ID: 1ALU), Cyclooxygenase-2 (COX-2, PDB ID: 5KIR), Transforming Growth Factor Beta (TGF-β, PDB ID: 1PY5), and Lipoxygenase-3 (LOX-3, PDB ID: 1NO3).
To assess fucosterol’s binding energies with the target pro-inflammatory proteins, docking simulations were performed using PyRx–Python Prescription (0.9.8). The docking protocol was validated using an online tool called DockRMSD, developed by the University of Michigan’s Zhong Laboratories, to compute the root mean square deviation (RMSD) values (Bell & Zhang, 2019). Furthermore, Chimera (1.12) was used to visualize the structures of the original and redocked ligands for further validation. All ligands were docked onto the optimized grid-box dimension for four cycles with the following settings: exhaustiveness = 8 and max number of modes = 9, generating 36 poses per protein-ligand complex. BIOVIA DS was utilized to visualize and enlist the key interacting amino acid residues and evaluate their molecular interactions.
To predict the drug-likeness profile of fucosterol, including its absorption, distribution, metabolism, and excretion (ADME) properties, SwissADME (http://www.swissadme.ch/) was used. Moreover, pharmacokinetic properties like gastrointestinal absorption and blood-brain barrier permeability were also predicted using the Brain or IntestinaL EstimateD permeation (BOILED-Egg) model in SwissADME. To evaluate fucosterol’s predicted toxicity profile, BIOVIA DS’s Toxicity Prediction by Komputer Assisted Technology (TOPKAT) tool and Prediction of Toxicity of Chemicals (ProTox) Version 3 (https://tox.charite.de/protox3/) were used.
HEK293 cell lines were procured from the Mammalian Tissue Culture Laboratory of the University of Santo Tomas–Research Center for the Natural and Applied Sciences (UST–RCNAS), Manila, Philippines. Cells were grown and cultured following standard procedures based on previous literature (Erukainure et al., 2018; Javadi et al., 2020). The cells were incubated with various concentrations of fucosterol (0.1–2,000 μg/mL), and separate wells were assigned with 20 μg/mL Paclitaxel (positive control). Standard MTT assay procedures were performed based on the study of Yoojam et al. (2021), where the cell viability was evaluated using the absorbances from the readings under the wavelength of 570nm using a microplate reader (Yoojam et al., 2021). HEK293 cells and the positive control paclitaxel were selected for this study based on the works of previously published articles (Raghubeer et al., 2017; Yadav et al., 2020).
Based on the results of the MTT assay, five concentrations of Fucosterol (0.1 μg/mL to 62.50 μg/mL) and the control drug 5 μg/mL dexamethasone (DEX) were added to a 24-well plate containing the pre-seeded HEK293 cell lines (1.5 × 105 cells/mL). Inflammation was induced by administering 1 μg/mL of LPS from Escherichia coli to the wells containing the cells incubated with Fucosterol after 1 hour. After 24 and 48 h, the supernatants from each well were collected and transferred to individual tubes for further assays (Bin et al., 2023; Sun et al., 2015; Yoojam et al., 2021). Using the instructions provided by the manufacturer of uncoated Enzyme-Linked Immunosorbent Assay (ELISA) kits for IL-6 | Cat. No. 430504 and IL-1β | Cat. No. 437004 (Biolegend, San Diego, CA, USA), the expression level of IL-6 and IL-1β in the supernatant fluid was measured. GraphPad Prism (10.4.1) was used to calculate the half-maximal inhibitory concentration (IC50) by plotting the dose-response curve using non-linear regression analysis (Aykul & Martinez-Hackert, 2016; Nadri et al., 2014).
Adult zebrafish were purchased from a local market located in Cartimar, Pasay City, Philippines. The zebrafish were allowed to acclimatize in the Pharmacology Laboratory of the UST-RCNAS following the conditions stated in the Organization for Economic Cooperation and Development (OECD) guidelines (OECD, 2013; Wang et al., 2021). Following the OECD’s acute toxicity testing guidelines (Test 236), the fish embryo acute toxicity (FET) test for fucosterol was done for 96 h. Two trials were performed, and observations were made every 24 h for the following apical parameters: coagulated embryos, lack of somite formation, non-detachment of the tail, and lack of heartbeat (OECD, 2013). Using the data from the observations, the medial lethal concentration (LC50) was computed using non-linear regression analysis in GraphPad Prism.
Similarly, five concentrations of fucosterol (6.25 μg/mL to 100 μg/mL) and the control drug 5 μg/mL DEX were administered to each well containing the embryos. After an hour, they were treated with 10 μg/mL LPS to induce inflammation. After 24 and 48 h, the embryos were transferred to individual tubes, homogenized using cold saline, and centrifuged at 5,000 repetitions per minute (rpm) for 10 min at 4°C. The supernatants were collected from each tube and were kept for further analysis (Wang et al., 2021, 2022; Yang et al., 2014). Expression of IL-6 and IL-1β in the supernatants was determined by following the manufacturer’s instructions using pre-coated ELISA kits for IL-6 | ELK9663 and IL-1β | ELK8505 (ELK Biotechnology, Wuhan, China) where the percentage inhibition at 450nm was used to determine the IC50 through non-linear regression analysis in GraphPad Prism.
All tests were conducted in triplicates and were tested for significant differences using one-way ANOVA and Post Hoc Tukey’s test (p ≤ 0.05) using Microsoft Excel (Redmond , WA, USA), the Statistical Package for the Social Sciences (SPSS, Chicago, IL, USA), and RStudios (Posit, Boston, MA, USA).
Results and Discussion
Docking simulations were performed to determine the accuracy of the redocking process of the originally bound ligands to their respective target proteins where RMSD values ranged from 0.465 to 1.968 Å, which are within the acceptable range of 0 to 2.0 Å (Fig. 2; Agu et al., 2023). The proteins’ active sites were defined using the optimized grid-box dimensions, which guided the docking of fucosterol and known inhibitors (Table 1).
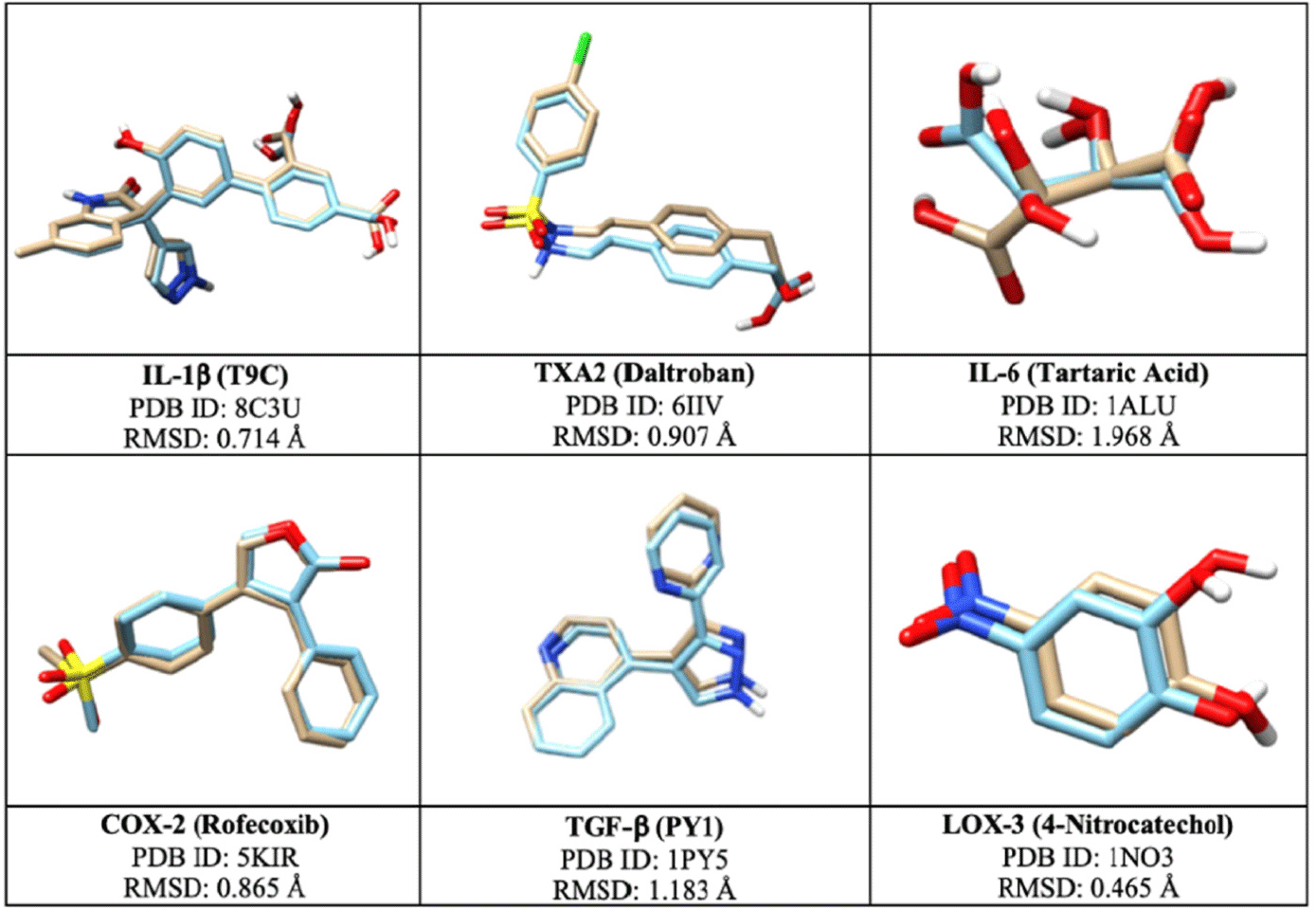
The binding mode with the most negative binding energy among the several cycles was selected as the most favorable docking pose and was included in the analysis. Fucosterol exhibited negative binding energies with all proteins, indicating its ability to form stable complexes with the targets. Notably, fucosterol’s lowest binding energies were reported with IL-1β, TXA2, and IL-6, at –7.4 kcal/mol, –6.7 kcal/mol, and -6.3 kcal/mol, respectively (Table 2). However, fucosterol showed more negative binding energy with IL-6 and IL-1β when compared with the known inhibitors’ results. These suggest that fucosterol can effectively bind and inhibit the progression of these proteins, potentially delaying the progression of chronic inflammatory diseases mediated by these targets. The anti-inflammatory activity of fucosterol was evaluated through both in vitro and in vivo assays by quantifying the expression levels of IL-6 and IL-1β, which were selected based on the findings from in silico studies.
For IL-6, residues including GLN175, SER176, LEU178, and ARG179 were found to be commonly interacting with the various ligands (Fig. 3). Moreover, the overlay of fucosterol with methotrexate and oxaprozin helps visualize how fucosterol fits within the binding pocket in comparison to known inhibitors. Since fucosterol aligns well with these inhibitors, it suggests that it could exhibit comparable inhibitory effects on IL-6.

The interaction with SER176 and ARG179 involves hydrogen binding, enhancing binding affinity and stability, while LEU178 contributes to hydrophobic interactions, further strengthening ligand engagement. These interactions strengthen ligand affinity and prevent dissociation, which is essential for prolonged inhibition of the cytokine; thus, these residues play vital roles in stabilizing ligand binding, contributing to the inhibition of the IL-6 signaling pathway (Tran et al., 2022). Fucosterol’s binding with IL-6 involved residue interactions with PHE74, GLN175, SER176, and ARG179. PHE74 facilitates pi-stacking interactions, which occur when aromatic rings interact with other rings or electron-rich systems, stabilizing the ligand within the binding site. In addition, GLN175 influences IL-6’s conformational dynamics, potentially interfering with its signaling pathway by inducing structural shifts in IL-6. Moreover, SER176 and ARG179 provide electrostatic stability, aiding in the prolonged inhibition of IL-6 expression (Tran et al., 2022). These interactions suggest that fucosterol can effectively regulate inflammation by modulating IL-6 signaling pathways, crucial in controlling chronic inflammatory diseases like rheumatoid arthritis (Gabay, 2006; Rahman et al., 2021).
For IL-1β, fucosterol’s residues are primarily reported through hydrophobic interactions with VAL3, PHE46, VAL47, ALA59, and VAL100 (Fig. 4). These nonpolar residues contributed to hydrophobic forces, which lead to ligand stabilization, thus, reducing IL-1β activity through the alteration of the binding of receptors or signaling proteins.

Like in IL-6, fucosterol occupies a similar binding region as anakinra or ustekinumab, suggesting potential inhibitory effects on IL-1β. Since anakinra primarily inhibits IL-1β by blocking the receptor interaction and ustekinumab downregulating interleukin’s signaling pathway, this suggests that fucosterol affects inflammatory pathways through direct hydrophobic engagement. Thus, by creating a hydrophobic environment that stabilizes the ligand-binding site, IL-1β activity is inhibited. Moreover, hydrophobic interactions like these are critical in reducing the production of similar inflammatory cytokines like IL-6 and TNF-α, thus managing inflammatory response (Abdul et al., 2016; Mo et al., 2018).
Overall, the docking simulations underscore the therapeutic potential of fucosterol in the modulation of key inflammatory pathways through cytokine inhibition. The interactions of fucosterol with critical residues in IL-6 and IL-1β provide insight into its mechanism of action, emphasizing its role in managing chronic inflammatory diseases, which needs to be further validated by performing succeeding assays on cellular or animal models. The selected proteins for molecular docking were chosen based on their vital roles in the development and progression of major chronic inflammatory diseases like diabetes and cancer. Their roles in sustaining prolonged inflammatory signaling make them relevant targets for assessing the potential anti-inflammatory activity of natural compounds. This selection is well-supported by existing literature emphasizing their importance as biomarkers and therapeutic targets in chronic inflammation-related research (Chen et al., 2018; Turner et al., 2014).
Fucosterol’s physicochemical and pharmacokinetic properties were also predicted and evaluated, focusing on its gastrointestinal absorption potential and ability to cross the blood-brain barrier (BBB). Results show that fucosterol exhibits poor pharmacokinetic properties, including low GI absorption due to its poor water solubility profile. Moreover, fucosterol also exhibits poor BBB permeability due to its lipophilicity profile, which is favorable, especially for drugs that are not meant to enter the brain and exhibit neurological effects (Table 3). Nevertheless, fucosterol satisfied Lipinski’s Rule of Five with only one violation (MLogP > 4.15), indicating its potential utility as a pharmaceutical agent through its favorable drug-likeness profile.
Toxicity profiling provided further insights into fucosterol’s safety profile (Table 4). Fucosterol was predicted to be a single-carcinogen in female rats but a non-carcinogen in males. This sex-specific carcinogenic effect warrants further investigation into underlying hormonal or metabolic pathway differences between sexes to establish fucosterol’s carcinogenic potential further. Moreover, fucosterol was determined to be a non-mutagenic, moderately skin irritant, weak sensitizer, and non-irritant for ocular applications, making it suitable for use in pharmaceutical formulations like cosmetics. Fucosterol also showed moderate toxicity through a predicted oral LD50 of 1.92 g/kg body weight and high inhalational LC50 (6,057.88 mg/m3/h), which suggests safety for oral and inhalational delivery methods.
Oral toxicity predictions also revealed that fucosterol is classified under Class 4, which generally corresponds to compounds with low to moderate toxicity. In addition, fucosterol was also evaluated for organ toxicity, where it was found to have no hepatotoxic, carcinogenic, mutagenic, or cytotoxic effects; however, it may have immunotoxic effects, meaning it could impact immune function by immunosuppression or immunostimulation (Table 5).
Fucosterol’s ADMET profile predictions highlight the compound’s potential in therapeutic contexts and should not limit the clinical applicability of the compound. Thus, further studies are needed to elucidate and reinforce its pharmacological use.
Fucosterol’s effect on the cell viability of HEK293 cells was evaluated at concentrations from 0.1 to 2,000 μg/mL. Paclitaxel was used as a positive control due to its well-established cytotoxic activity, allowing the benchmarking of cell viability responses (Yadav et al., 2020). Results revealed that concentrations above 62.50 μg/mL were toxic to the cells, where lower concentrations exhibited ≥ 80% viability, indicating low toxicity and promotion of cell viability. Fig. 5 shows the IC50 value, which was determined to be 279.90 μg/mL, where a concentration-dependent decrease in cell viability was found to be statistically significant (p ≤ 0.01).

Similar studies demonstrated that fucosterol promotes cell viability at concentrations ≤ 100 μg/mL and was also reported to have minimal toxicity on normal human cells (Fernando et al., 2019; Mao et al., 2019). Compared with previous reports, fucosterol showed similar results by exhibiting non-toxic effects on A549 human lung epithelial, dermal fibroblast, and keratinocyte cultures at concentrations lower than 100 μg/mL (Fernando et al., 2019). Moreover, fucosterol was also shown to inhibit cancer cell lines by inducing cell apoptosis and cell cycle arrest, suggesting that fucosterol is a compound capable of promoting cell viability and a concentration-dependent effect on cytotoxicity (Mao et al., 2019).
The inhibitory effect of fucosterol on IL-6 for 24 and 48 h was evaluated through ELISA across five concentrations ranging from 0.1 μg/mL to 62.50 μg/mL. It was compared with the control drug 5 μg/mL DEX, as shown in Fig. 6. DEX was selected as a positive control due to its established anti-inflammatory properties. As a synthetic glucocorticoid, dexamethasone is known to inhibit the production of pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6, making it an appropriate reference for assessing anti-inflammatory responses (Patil et al., 2018). This well-established pharmacological profile of dexamethasone allowed for the validation of the assay’s sensitivity and provided a reliable benchmark for evaluating the anti-inflammatory activity of the test compounds.
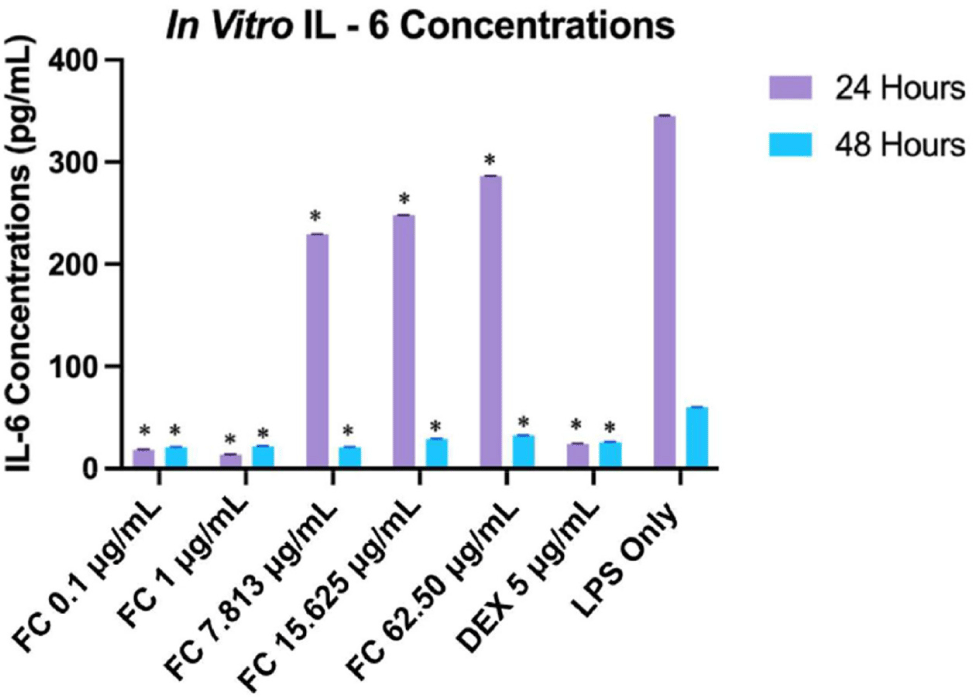
Results indicate that fucosterol exhibited a significantly more substantial inhibitory effect at lower concentrations, with 1 μg/mL showing the least expression after 24 h (p ≤ 0.01). This suggests a biphasic concentration-response relationship, where lower concentrations of fucosterol exhibit beneficial effects, but higher concentrations may lead to reduced efficacy or toxicity (Trela-Makowej et al., 2024). This response has been documented with other phytochemicals like resveratrol and sulforaphane, which exhibit similar biphasic responses (Jodynis-Liebert & Kujawska, 2020). After 48 h, the IL-6 level was significantly reduced, and its expression was substantially higher during the first 24 h, as seen in the LPS-treated cells (p = 0.03). Moreover, LPS-treated cells showed a significant difference between 24 and 48 h, indicating that IL-6 expression peaked and was reduced after 48 h (p ≤ 0.01). As seen in Fig. 6, at 24 h, fucosterol concentrations at 0.1 μg/mL and 1 μg/mL showed similar significant inhibitory effects with the positive control DEX when compared through the expression level of IL-6 (p = 1.0 and p = 0.99).
This reduction of IL-6 may be related to suppressing the NF-κB pathway, a vital pathway involved in IL-6 transcription, as reported in previous studies (Jayawardena et al., 2020). The biphasic response could be linked to the activation of receptor pathways at lower concentrations and cellular stress responses that may be overwhelmed at higher doses, leading to diminished efficacy or even toxicity (Kageyama & Nemoto, 2022). Fig. 7 shows the IC50 values, which were determined to be 5.86 μg/mL (at 24 h) and 73.36 μg/mL (at 48 h), which were significantly different from each other (p ≤ 0.05) and lower than previously reported IC50 values, indicating more substantial inhibitory effect under the experimental conditions of this study (Rocha et al., 2022; Wong et al., 2018).

Similarly, fucosterol’s inhibitory effect on the expression of IL-1β within 24 and 48 h was evaluated using ELISA (Fig. 8). At 24 h; fucosterol was shown to inhibit IL-1β expression; however, the concentration-response curve showed relatively stable concentrations across various concentrations, suggesting that these concentrations may not significantly alter IL-1β at this period. While the degree of inhibition did not vary significantly among the increasing concentrations, results still highlight a meaningful reduction in IL-1β expression (p ≤ 0.01). On the other hand, the difference was not significantly different in comparing the IL-1β expression between 24 and 48 h (p = 0.9861). Moreover, LPS-treated cells for 24 and 48 h showed significant differences compared to all fucosterol concentrations (p ≤ 0.01). At the same time, fucosterol did not exhibit a significant difference in its IL-1β expression inhibitory potential than the positive control DEX (p ≥ 0.05).
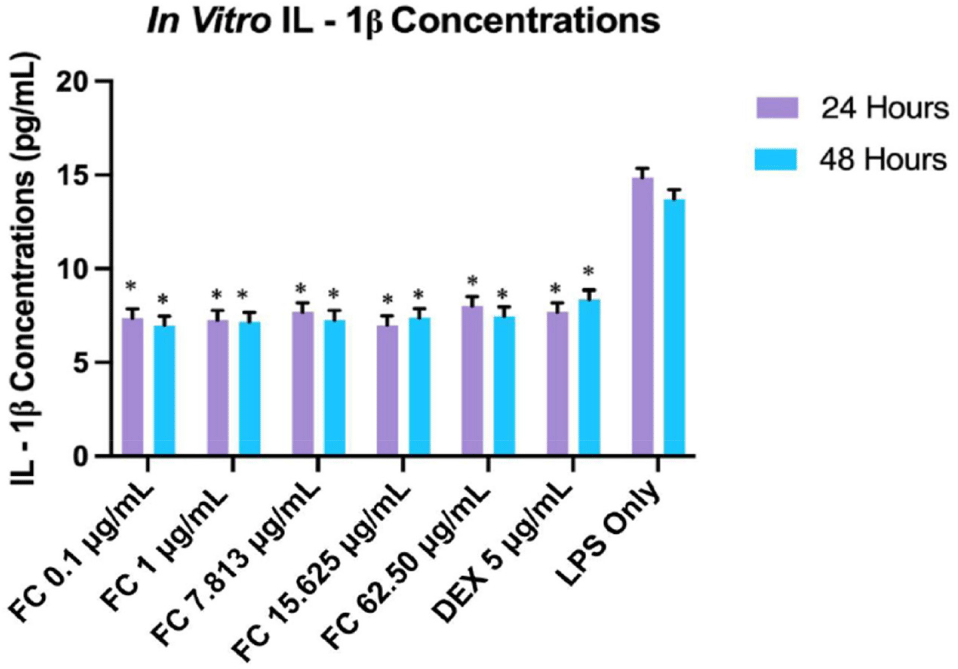
Similar to the results of the IL-6 evaluation, the results suggest early saturation of the signaling pathways since lower concentrations are more effective in inhibiting IL-1β. Although no studies have published that fucosterol exhibits this response in IL-1β, prior studies showed that fucosterol can inhibit inflammatory cytokines by modulating the NF-κB and MAPK pathways, thus reducing IL-1β expression (Ghallab et al., 2024; Mo et al., 2018). This suggests that fucosterol’s anti-inflammatory effect could be attributed to the modulation of these pathways, although the concentration-response relationship with IL-1β is not as evident as in IL-6. IC50 values were computed to be 2.59 μg/mL (at 24 h) and 0.02 μg/mL (at 48 h), as shown in Fig. 9, which were significantly different from each other (p ≤ 0.05).
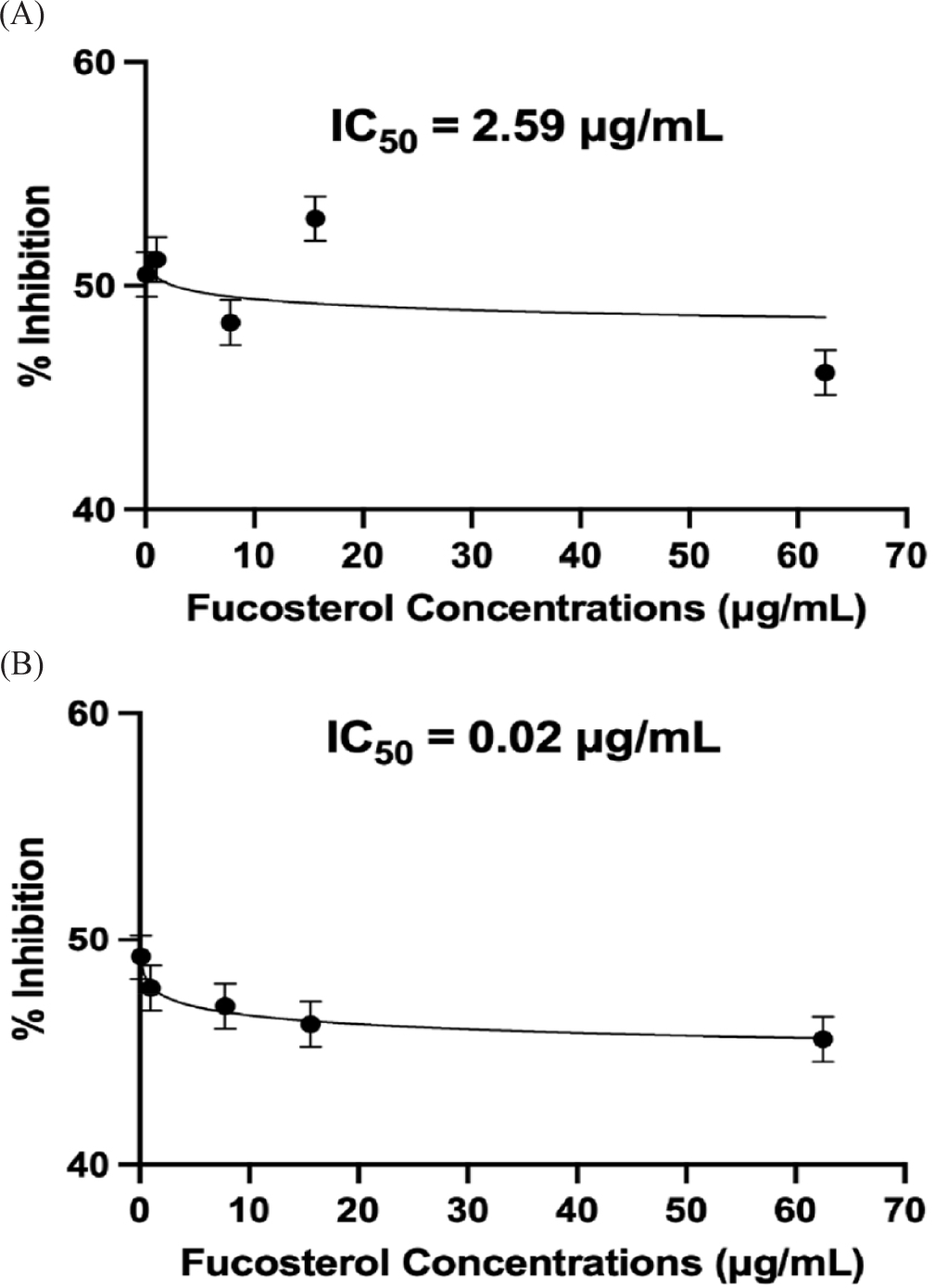
The delayed time-dependent effect of fucosterol, as seen by a lower IC50 value at 48 h, suggests that its potency and anti-inflammatory activity with IL-1β may involve complex pharmacokinetics and cellular interactions. These findings differ from the results presented in previous studies wherein fucosterol exhibited an immediate concentration-dependent effect on RAW264.7 macrophage cell lines and rat model (Li et al., 2015; Yoo et al., 2012). This discrepancy may be caused by variations in experimental conditions, the cell type, and the inflammatory stimuli used in various studies. One possible explanation for this delayed effect is that fucosterol may require metabolic activation or accumulation in the cells before exerting its inhibitory effect on the cytokine. Furthermore, the biphasic response indicates that fucosterol may act on several pathways or work indirectly by modifying the transcription factors generating these cytokines in HEK293 cells (Hayakawa & Qiu, 2010).
Furthermore, these findings highlight the potential of fucosterol as a promising anti-inflammatory agent, even at lower concentrations, which could have significant implications for therapeutic applications. Effective lower concentrations may reduce the risk of cytotoxicity and side effects, making fucosterol a favorable candidate for drug development (Meinita et al., 2021). However, further investigations are necessary to elucidate the molecular mechanisms underlying its time-dependent activity and biphasic response. Future research should identify specific signaling pathways, receptor interactions, and possible metabolic modifications contributing to the observed effects to optimize dosing strategies for clinical applications.
A significant lethality rate was observed at higher concentrations of fucosterol in the acute toxicity assay using zebrafish embryos (p ≤ 0.01). Fig. 10 shows the comparison between the development of zebrafish embryos treated with ≤ 100 μg/mL (healthy) and > 100 μg/mL (dead) of fucosterol.
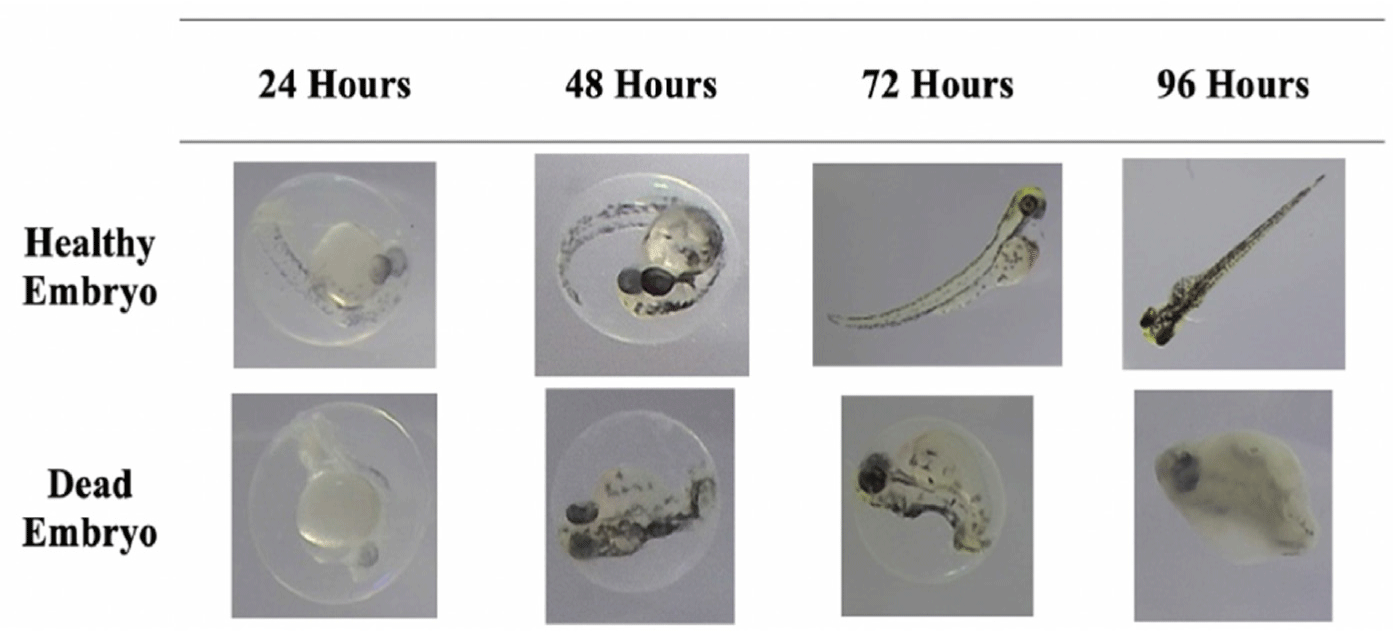
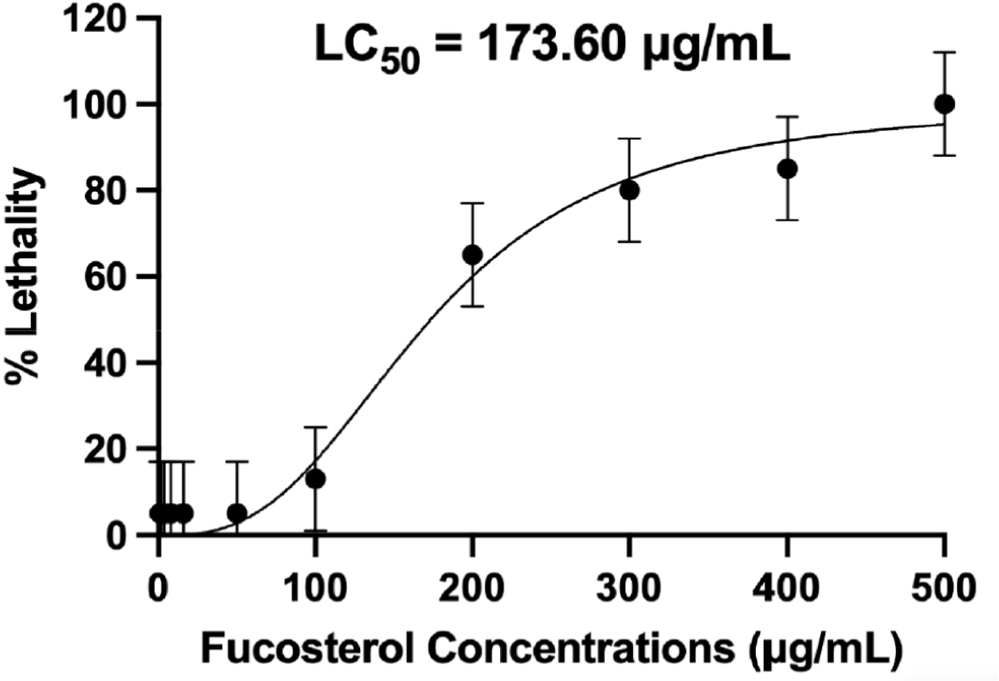
As seen in the figure, the healthy embryo could fully develop the somite or the backbone of the fish at 48 h and exhibit a heartbeat, marked by the reddish part near the head of the fish, at 72 h. These features were lacking in the embryo treated with higher concentrations of fucosterol, which can be considered dead already due to lack of somite formation at 48 h and the coagulation of the whole body after 96 h. At 96 h, a healthy embryo could fully develop its tail, show a heartbeat, and exhibit locomotion, which signifies survival and continuous development. After 48 h, concentrations above 100 μg/mL already showed 50% lethality in the zebrafish embryos, indicating toxicity. At 96 h, 13% mortality was observed at 100 μg/mL, which falls under the acceptable limit to be considered non-toxic. The significant difference (p ≤ 0.01) in lethality rates between various concentrations highlights fucosterol’s concentration-dependent response to fucosterol in the model. Moreover, 3,4-dichloroaniline, an environmental pollutant known to cause oxidative stress, was used as the positive control based on OECD guidelines which resulted in a 90% lethality rate after the 96-hour testing period (OECD, 2013). LC50 was determined to be 173.60 μg/mL, which supports the result of the study that higher concentrations have a more substantial lethal effect, as shown in Fig. 11. Since concentrations ≤ 100 μg/mL were considered to be non-toxic, concentrations lower than this were used for subsequent assays to ensure the safety of the embryos during the testing process.
Various concentrations of fucosterol (6.25 μg/mL to 100 μg/mL) and the control drug DEX were used to evaluate IL-6 and IL-1β expression on zebrafish embryos through ELISA. For both cytokines, a concentration-dependent reduction was observed with increasing inhibition at higher concentrations of fucosterol. For IL-6, both 24 and 48-hour observations consistently showed a significant reduction in IL-6, suggesting its effective role in modulating the inflammatory cytokine (p ≤ 0.01; Fig. 12).

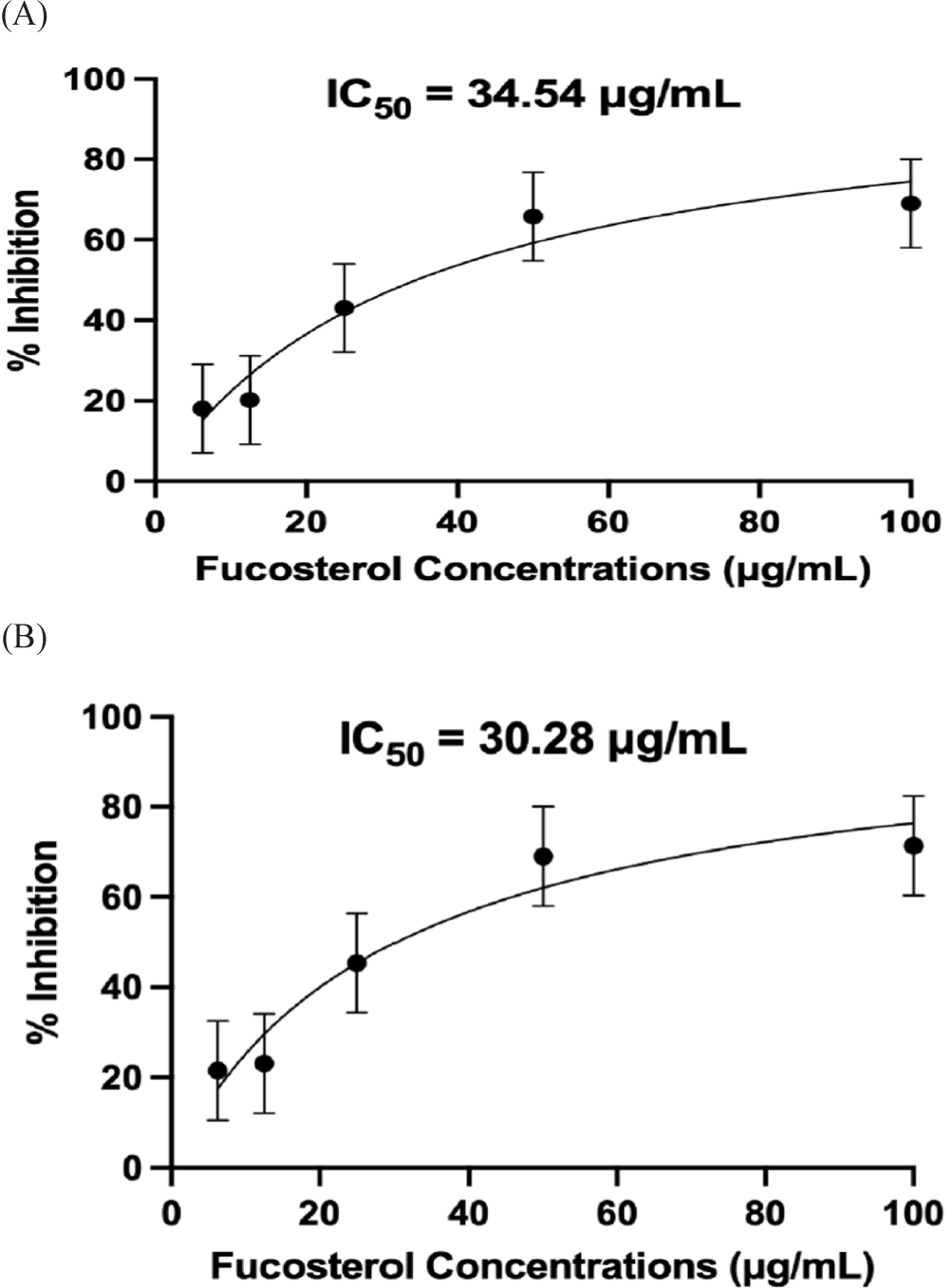
At 50 μg/mL and 100 μg/mL, fucosterol exhibited better inhibition than DEX, indicating that fucosterol may be more active than the standard anti-inflammatory treatment at these concentrations (p ≤ 0.01). Moreover, at 12.50 μg/mL, fucosterol exhibited a similar inhibitory effect to the control drug DEX (p = 0.826). The results support a concentration-dependent relationship, indicating that fucosterol’s efficacy increases as the concentration increases. However, this effect reached a plateau at higher concentrations, likely due to receptor saturation, which needs further evaluation. The findings for IL-6 inhibition in this study align with previous experiments examining the anti-inflammatory properties of fucosterol in other animal models where IL-6 was reduced in mice with liver injury and skeletal muscle atrophy by modulating the Akt/mTOR/FoxO3α pathway (Hwang et al., 2024; Mo et al., 2018). Moreover, previous studies also highlighted fucosterol’s potential to suppress NF-κB and MAPK signaling pathways, which play key roles in cytokine production, and further validate the results of this study (Meinita et al., 2021). IC50 values for IL-6 were computed to be 34.54 μg/mL (at 24 h) and 30.28 μg/mL (at 48 h), with no significant difference from the two incubation periods (p = 0.33; Fig. 13).
Fucosterol’s effect on the expression of IL-1β was also revealed to be concentration-dependent where higher concentrations, like 50 μg/mL and 100 μg/mL, significantly suppressed IL-1β levels, comparable to or even surpassing the effect of DEX (p ≤ 0.01; Fig. 14).

In addition, fucosterol concentration of 50 μg/mL showed no significant difference compared to the effects of the control drug DEX (p = 0.998). Moreover, IL-1β levels in LPS-treated embryos for 24 and 48 h showed significant differences compared to all fucosterol and DEX treatments (p ≤ 0.01). Interestingly, IL-1β expression was slightly lower after 48 h, suggesting either an adaptive immune response or prolonged efficacy of fucosterol over time. Similarly, IC50 values for IL-1β for 24 and 48 h were found to have no significant difference and were determined to be 29.39 μg/mL and 29.61 μg/mL, respectively (p = 0.38; Fig. 15).

A comparison between the IC50 values from the in vitro assay revealed a notable discrepancy, as fucosterol appeared to be more effective at lower concentrations in cell line assays and was found to have a significant difference between the IC50 values from 24 and 48 h incubation. This difference likely results from fucosterol directly interacting with isolated cells in cell line assays, thus achieving maximal efficacy at lower concentrations. In contrast, in an in vivo setting, fucosterol must be absorbed first and metabolized, requiring higher concentrations to elicit beneficial effects and achieve bioavailability. The findings of the in vivo studies support previously reported data that fucosterol can reduce IL-1β levels in a liver injury model through modulation of the P38 MAPK and NF-κB pathways (Hwang et al., 2024; Mo et al., 2018). Moreover, fucosterol has also been found to possess antioxidant properties, which may contribute to the reduction of oxidative stress, which is vital in the synthesis of IL-6 (Meinita et al., 2021). Further studies are needed to explore the broader applications of fucosterol, especially in clinical settings, and evaluate its efficacy and safety in human patients. The consistency of results across various studies shows that fucosterol can elicit an anti-inflammatory effect due to its ability to modulate the production of IL-6 and IL-1β by inhibiting the NF-κB pathway. The potential for fucosterol to be developed as a pharmaceutical product is underscored by its concentration-dependent effects and ability to modulate critical inflammatory pathways. Thus, further studies, including pre-formulation studies and comprehensive mechanistic evaluations, will help establish the compound’s potential as a pharmacological product.
Conclusions
The study demonstrated fucosterol’s potential as an anti-inflammatory agent based on insights from reverse molecular docking, in vitro, and in vivo evaluations. Molecular docking revealed strong binding affinities with pro-inflammatory proteins IL-6 and IL-1β, with potential inhibitory effects validated through ELISA using LPS-induced HEK293 cells and zebrafish embryo models. Fucosterol also showed favorable drug-likeness profiles. However concerns regarding its water solubility limit its clinical application, emphasizing the need for advanced delivery systems to enhance its bioavailability and reduce toxicity. Recommendations for future research include exploring the molecular mechanism of IL-6 and IL-1β inhibition and performing long-term toxicity studies by validating in higher animal models.