Introduction
The largehead hairtail (Trichiurus japonicus) is an important commercial fish species in East Asia. T. japonicus is known to migrate to specific areas depending on its life history, growth, maturation, and environmental conditions such as water temperature and salinity (Chen et al., 2020; Kwok & Ni, 1999; Shih et al., 2011). It is a subtropical species with a prolonged spawning period and typically begins spawning in its second year of life (Chen et al., 2020; Shih et al., 2011). Nevertheless, T. japonicus resources in the East China Sea and around Jeju Island have declined by more than 50% compared to the year 2000 (MOF & NIFS, 2014).
Monitoring data collected in South Korea between 2012 and 2014 indicated that 65% to 99% of the T. japonicus population consisted of immature individuals (MOF & NIFS, 2014). The observed increase in immature individuals may have induced earlier maturation in young, immature fish as a compensatory response to maintain population stability (Ohlberger et al., 2011; Shih et al., 2011; Sun et al., 2020).
As a result, the timing of maturation in T. japonicus may have been altered. In addition, a large number of floating T. japonicus eggs were observed in coastal waters around Jeju Island between April and June 2020 (Lee et al., 2020). The main spawning period of this species has generally been reported to occur between May and September (Cha & Lee, 2004; Kim, 2020). The occurrence of high egg densities in Jeju waters during this period may suggest shifts in the spawning patterns of both immature and mature T. japonicus.
Fish spawning patterns have been investigated using several methods. A direct method involves investigating fish spawning patterns by measuring the number of oocytes in the gonads, performing histological analyses, analyzing blood hormone concentrations, and measuring blood vitellogenin concentrations. The indirect method involves calculating the maturity index based on the weights of the gonads (gonadosomatic index, GSI) and liver (hepatosomatic index, HSI) (Baeck & Huh, 2004; Baldisserotto et al., 2019; Guzmán et al., 2008; Hara et al., 2016; King & Pankhurst, 2003). However, asynchronous development in fish, similar to that observed in T. japonicus, complicates the estimation of reproductive ecology (Grande et al., 2012; Rajasilta et al., 1993; Reglero et al., 2014; van Beveren et al., 2023). This species exhibits multiple simultaneous stages of oocyte development. Therefore, to identify the reproductive patterns of asynchronous fish, one must conduct simultaneous investigations using indirect methods, such as the GSI and HSI, as well as direct methods, such as histological methods using direct specimens, and fecundity investigations.
In this study, female T. japonicus were obtained from a local fish market in Jeju Island. Although the exact capture locations of these individuals were unknown, most market-distributed T. japonicus are presumed to have been caught in nearby coastal waters. To approximate the environmental conditions likely experienced by these individuals, seawater temperature and salinity were measured in the Hagwi coastal area, located in the northwestern part of Jeju Island, which is known as one of the major fishing grounds for T. japonicus.
This study aimed to investigate potential changes in the main spawning season of female T. japonicus and to identify sexual maturation indicators in both mature and immature individuals.
Materials and Methods
Female T. japonicus were obtained from a local fish market in Jeju Island. Although the exact capture locations were unknown, the fish were presumed to have been caught in nearby coastal waters. Based on previous studies that estimated the main spawning season to occur between May and September (Cha & Lee, 2004; Kim et al., 2020), sampling was carried out during this period in 2022.
The collected fish were divided into two groups based on preanal length. Individuals measuring 40 cm or more were classified as mature, whereas those measuring less than 25 cm were considered immature. Each fish was measured for total length and body weight, following the methods of Kwon & Ni (1999), Cha & Lee (2004), Kim et al. (2011), and Kim et al. (2020). The weights of the liver and ovaries were measured to calculate the HSI and GSI. Ovarian tissues and oocytes were sampled from May to September for analyses of oocyte morphology, absolute fecundity, and histological characteristics.
Surface water temperature and salinity were measured from May to September using a multiparameter water quality meter (YSI, Yellow Springs, OH, USA; Xylem Water Solutions, Washington, DC, USA) in the coastal area of Hagwi, Aewol-eup, Jeju-si.
The GSI and HSI were measured for each group using samples collected from April to September. The liver and gonads were removed separately for index calculations.
The following formulas were used to calculate the indices:
Absolute fecundity was estimated by counting oocytes under a stereo microscope (SM2745T, Nikon, Tokyo, Japan). For each individual, three subsamples of approximately 10 mg of oocytes were collected, rinsed with distilled water, and quantified. Relative fecundity (RF) was calculated using the following formula (Bobko & Berkeley, 2004):
where AF is the absolute fecundity (total number of eggs per female), TW is the total body weight, GW is the gonad weight, and RF represents the number of eggs per unit somatic weight.
Ovarian tissues were fixed in 10% neutral buffered formalin (Sigma-Aldrich, St. Louis, MO, USA) at 4 °C for 48 hours. The samples were then transferred to 5% neutral buffered formalin prepared with 0.2 M phosphate buffer (pH 7.4) and fixed for an additional 48 hours. Fixed tissues were sequentially dehydrated in a graded ethanol series (70%, 80%, 90%, and 100%) and embedded in paraffin according to the method described by Namgung et al. (2021). Paraffin blocks were sectioned at 7 μm thickness using a rotary microtome (RM 2125 RTS, Leica, Wetzlar, Germany). The sections were dried on a slide warmer (XH-2004, Premiere, CNA Scientific, Sterling, VA, USA) at 37 °C for 24 hours. Slides were stained with hematoxylin and eosin (H&E), mounted with Canada balsam (Duksan, Seoul, Korea), and observed under a stereo microscope (Z16 APO, Leica, Wetzlar, Germany). The oocyte growth stage is divided into is early oocyte stage (chromatin-nucleolus stage, peri-nucleolus stage), yolk vesicle stage, primary yolk stage, secondary yolk stage, and mature stage.
All sample measurements were repeated in triplicate, and all measurements were expressed as the mean ± SD. Data were analyzed using a one-way analysis of variance (ANOVA), followed by post-hoc analysis with Duncan’s multiple range test. All data analyses were performed using SPSS 24 software (IBM, Armonk, NY, USA). The differences were considered statistically significant at p < 0.05.
Results
The average total length, preanal length, and body weight of mature female T. japonicus were 121.32 ± 13.87 cm, 45.20 ± 4.41 cm, and 1.46 ± 0.35 kg, respectively. In contrast, immature females exhibited average values of 71.80 ± 4.30 cm for total length, 22.40 ± 1.50 cm for preanal length, and 0.18 ± 0.02 kg for body weight.
The maturation patterns of mature and immature female T. japonicus were examined using GSI, HSI, and absolute fecundity (Fig. 1).
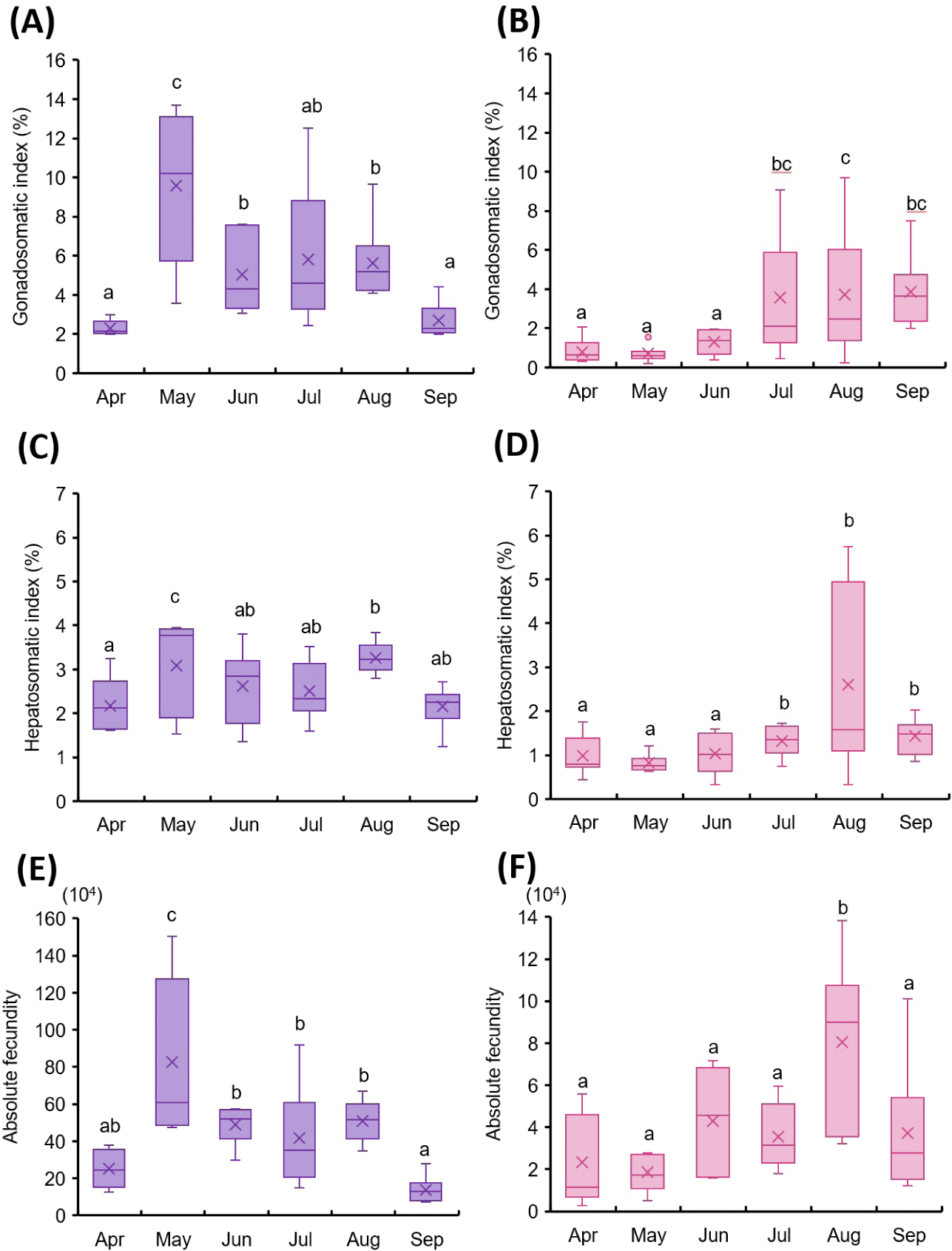
In mature females, GSI showed a significant increase in May (Fig. 1A). Although it declined slightly in June, July, and August, the value remained higher than those recorded in April and September. HSI also peaked in May and August in the mature group (Fig. 1C).
Absolute fecundity was highest in May (Fig. 1E). In immature females, GSI did not differ significantly between April and June, but increased markedly in July and August, followed by a decline in September (Fig. 1B). HSI remained low from April to June, then increased significantly in August (Fig. 1D). Absolute fecundity showed no significant differences from April to June, but a substantial increase was observed in August (Fig. 1F).
In mature females, a large proportion of early developmental stage oocytes was observed in April. Some oocytes had progressed to the primary yolk stage, showing yolk accumulation in the center and peripheral lipid droplets (Fig. 2A). In May, the proportion of oocytes in the secondary yolk stage increased markedly. These oocytes were characterized by a high density of central yolk and peripheral lipid droplets (Fig. 2B). In June, ovaries showed a mixture of primary yolk stage and secondary yolk stage (Fig. 2C). By July, most oocytes had reached the secondary yolk stage, with diameters exceeding 2,000 µm (Fig. 2D), although some individuals maintained the same state as in June (data not shown). In August, vitellogenesis was completed, and the proportion of oocytes entering mature stage increased, with diameters approaching 3,000 µm (Fig. 2E). Abdominal ovulation was also observed in some individuals. In September, the proportion of mature stage oocytes decreased, ovarian lumen spacing widened, and post-ovulatory follicles were observed. Ovaries exhibited a mixed distribution of early oocyte stage and mature stage but unspawned oocytes (Fig. 2F).
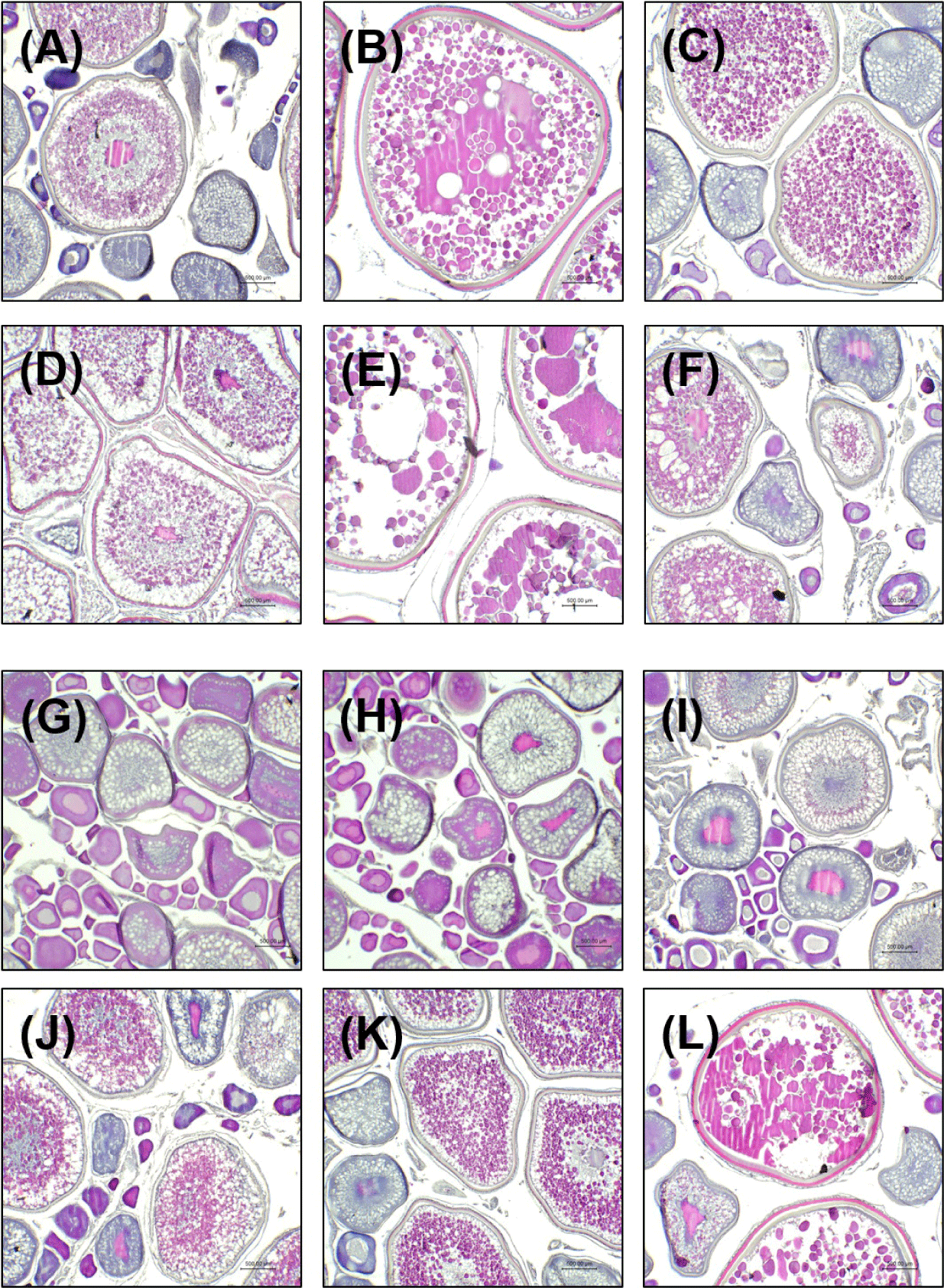
In immature females, most oocytes were in early oocyte stage in April, with the yolk vesicle stage being predominant. Some oocytes were at the beginning of the primary yolk stage, characterized by visible lipid droplets and initial yolk accumulation (Fig. 2G). In May, the proportion of primary yolk stage oocytes increased (Fig. 2H), and this trend continued in June (Fig. 2I). In July, yolk formation progressed further, with yolk volume increasing and moving toward the center of the oocyte (Fig. 2J). In August, most oocytes remained in the primary or secondary yolk stages. In the secondary yolk stage, lipid droplets accumulated along the periphery, while yolk was concentrated in the center (Fig. 2K). In September, a notable increase in the percentage of mature oocytes was observed. Some individuals showed post-ovulatory follicle remnants, while others appeared ready for ovulation (Fig. 2L).
The correlation between oocyte maturation stage ratios and GSI was analyzed (Fig. 3). In mature females, the proportion of oocytes in the early oocyte stage was highest in April, while the proportions of secondary yolk and mature stage remained low. In May, the ratio of the early oocyte stage decreased, whereas the primary yolk stage, secondary yolk stage, and mature stage increased. This trend continued into June, with a further increase in yolk vesicle stage, primary yolk stage, and a decrease in mature stage oocytes to 17.83%. In July, early oocyte stage oocytes increased again, while primary yolk stage, secondary yolk stage, and mature stage oocytes decreased. In August, the early oocyte stage oocytes decreased, and oocytes in the primary yolk stage, secondary yolk stage, and mature stage increased. In September, the early oocyte stage and secondary yolk stage oocytes further increased. Although the patterns were not entirely consistent, the overall trend in oocyte maturation stage ratios generally mirrored changes in GSI (Fig. 3A).
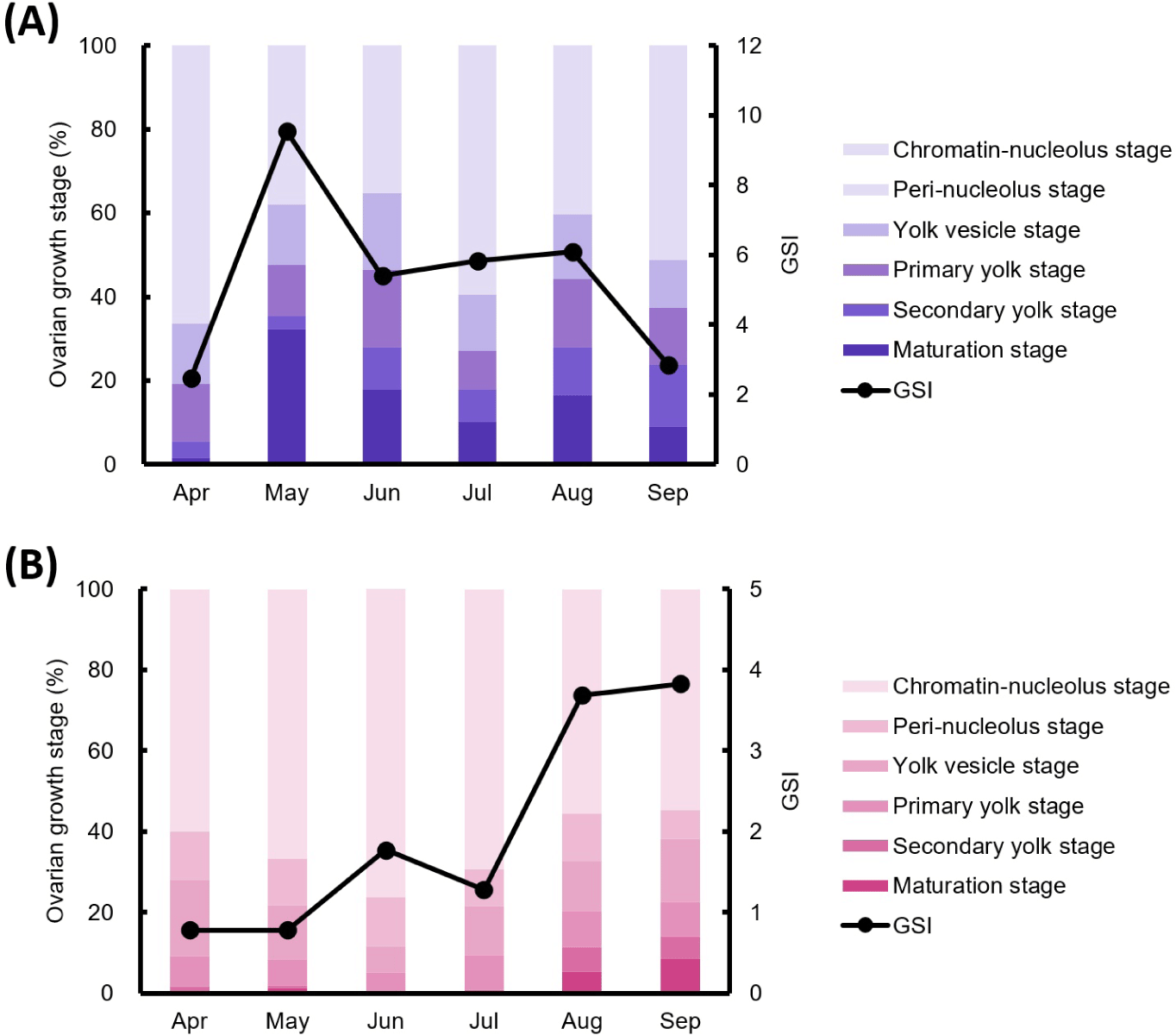
In immature females, the proportion of early oocyte stage oocytes was highest in April and increased in May, while other stages remained similar to April. However, mature oocytes were observed in a few individuals. In June, the early oocyte stage oocytes remained dominant. In July, the proportions of the primary yolk stage and mature stage oocytes increased. In August and September, the overall oocyte maturation ratios remained similar to July, though the proportion of mature oocytes gradually increased. In immature individuals, the oocyte maturation stage ratios did not align with GSI trends in May, June, and July. However, in April, August, and September, oocyte development stages showed patterns similar to the GSI (Fig. 3B).
Discussion
Understanding the reproductive dynamics of migratory fish is essential for predicting fishery productivity and establishing sustainable conservation strategies.
In this study, we investigated the maturation characteristics and spawning patterns of female T. japonicus populations migrating around Jeju Island, South Korea, aiming to support the management of this declining resource. Migratory fish species exhibit plasticity in reproductive cycles depending on environmental conditions (Servili et al., 2020). Even within a single species, populations may display variation in maturation timing, growth rates, and spawning periods. Although maturity indices alone may not fully capture this complexity, they remain widely used indicators for estimating reproductive timing, as demonstrated in studies of tuna (Stéquert et al., 2001), mackerel (Yukami et al., 2009), and herring (McPherson et al., 2011).
In mature females, GSI, HSI, and absolute fecundity peaked in May, with moderately elevated values observed again in August (Fig. 1A, 1C, and 1E). Histological data were consistent with these findings, showing abundant mature oocytes in May, a decline through June and July, and partial recovery in August (Fig. 2B–2E). These results suggest that May represents the primary spawning peak, and some individuals retain reproductive potential into August.
In immature females, low GSI and HSI values and histologically undeveloped oocytes were observed from April to June (Fig. 1B, 1D and 2G–2I). Oocyte maturation progressed during July and August, with fully mature oocytes appearing in September (Fig. 2J–2L). These results suggest that the main spawning period for immature females occurs from July to September. Since some individuals appeared to still be developing reproductive capability in September, spawning may extend beyond this period. Long-term monitoring beyond September is therefore necessary to clearly define the full reproductive window in this group.
In T. japonicus, asynchronous ovarian development results in the simultaneous presence of oocytes at multiple developmental stages (Kwok & Ni, 1999). This characteristic makes it difficult to precisely predict spawning periods using histology or maturity indices alone. Although the proportion of mature oocytes increases as the peak spawning period approaches (Chen et al., 2020), the asynchronous development requires detailed age-based analysis and high-resolution monitoring of mature (May to August) and immature (August to September and beyond) females to improve predictive accuracy.
Environmental variables such as water temperature and salinity play important roles in regulating fish reproduction. These factors can influence not only gonadal development but also growth, mortality, food availability, and the timing and location of spawning (Pankhurst & Porter, 2003; Tobin & Wright, 2011; Watson et al., 2022). The temperature (19.26°C–21.23°C) and salinity (32.95–33.54 psu) recorded in this study fall within the range reported by Lee et al. (2020) for coastal waters around Jeju during the presumed spawning season. However, due to differences in study location, sampling period, and methodology, direct comparisons should be interpreted with caution. While environmental similarity may suggest suitable spawning conditions for T. japonicus, further studies are required to determine whether this correspondence reflects similar reproductive dynamics.
A notable finding of this study is that ovulation was observed in immature females under two years of age (preanal length < 25 cm). While previous studies have reported that T. japonicus typically reaches sexual maturity in its second year (Shih et al., 2011), our results suggest that some individuals may mature, and spawn earlier than previously understood. This early maturation may reflect a compensatory mechanism triggered by population stress.
In marine species, stock depletion often leads to reduced age and size at maturity as a reproductive strategy (Ramírez-Amaro et al., 2020). Therefore, the emergence of early maturing females in this study may indirectly indicate a decline in stock abundance. The simultaneous presence of mature and immature spawning females in the Jeju area may also imply changes in migratory behavior or timing. Previous studies described T. japonicus migration from Korea’s western coast to the southern regions, including Jeju, in winter (KMA, 2020; Seol et al., 2020). Our results suggest a possible shift or expansion in spatial spawning patterns. However, because this study was limited to Jeju coastal waters, future studies should conduct broader geographic monitoring and assess migration routes in relation to environmental conditions.
Previous studies have identified the southern coast of Korea and the waters around Jeju as major spawning grounds of T. japonicus (KMA, 2020; Shih et al., 2011). Based on the presence of favorable temperature and salinity ranges observed in this study, along with historical data from nearby areas such as the East China Sea, it is possible that the spawning grounds may have expanded geographically to include a broader area than previously recognized.
At the same time, increased densities of T. japonicus eggs in Jeju coastal waters, observed during certain periods, suggest a potential shift in the center of spawning activity toward southern regions. This may be a temporary response to environmental fluctuations. In this study, expansion refers to a broadening of the spatial range of spawning activity, and shift indicates a change in the central spawning location.
To further understand the reproductive ecology of T. japonicus, future research should incorporate molecular and hormonal analyses, including the expression of sex-related genes and endocrine profiles. Additionally, long-term and seasonal monitoring across size classes and life stages is recommended to clarify the impact of environmental stressors on reproductive success. Findings from this study provide important baseline data for developing effective resource management plans, such as fishing restrictions or size-based regulations.
Conclusion
The spawning season of T. japonicus around Jeju Island is estimated to extend from April to September, with peak activity from May to August in mature females and from July to September in immature females. Spawning activity in younger individuals and early maturation suggest that population decline may be accelerating reproductive schedules.
To understand and manage this change, future studies should integrate molecular, physiological, and ecological approaches and implement long-term environmental monitoring. These efforts are essential not only for effective stock assessment and resource management, but also for future development of aquaculture strategies for T. japonicus.








