Introduction
Aquaculture plays a critical role in global food security by providing a sustainable and efficient source of protein. However, the industry faces ongoing challenges, including high feed costs, environmental concerns, and the limited availability of high-quality protein sources. These issues highlight the need to explore alternative feed ingredients that can reduce production costs while enhancing the efficiency and sustainability of aquaculture practices (Fantatto et al., 2024; Hernandez de-Dios et al., 2022).
Among the most promising alternatives are plant-based feed ingredients rich in bioactive compounds, capable of improving growth performance, health, and reproductive success in fish while reducing reliance on conventional fishmeal. One such ingredient is Moringa oleifera, known for its excellent nutritional profile, which includes high levels of proteins, essential amino acids, vitamins, minerals, and antioxidants (Momın & Memiş, 2023; Su & Chen, 2020).
Several studies have demonstrated the positive effects of Moringa oleifera leaf meal (MLM) supplementation on growth performance, immune responses, and reproductive efficiency in various aquaculture species, including Nile tilapia (Oreochromis niloticus), common carp (Cyprinus carpio), and African catfish (Clarias gariepinus) (Adekilekun et al., 2022; Magouz et al., 2017; Manuel et al., 2019). These findings position MLM as a valuable functional feed additive. However, while the growth-promoting effects of MLM are well documented, its specific role in enhancing reproductive performance, particularly in freshwater species such as Barbodes binotatus (spotted barb), remains underexplored.
B. binotatus is a promising freshwater species recognized for its adaptability and potential in aquaculture. Nevertheless, its commercial production is constrained by challenges such as seasonal spawning and slow gonadal maturation (Mujtahidah et al., 2019). Current aquaculture practices often rely on formulated feeds that may not fully support the reproductive physiology of this species. Addressing these limitations through improved nutritional strategies is crucial for enhancing the sustainability and productivity of B. binotatus culture.
While previous research has largely focused on the general growth-promoting effects of MLM, this study seeks to expand the knowledge by investigating its functional role in reproductive development. Given that M. oleifera leaves are rich in antioxidants such as Vitamin E, which has been linked to improved gonadal quality, accelerated maturation, and reduced oxidative stress (Leone et al., 2015; Moyo et al., 2011), MLM supplementation may offer significant benefits for reproductive performance in fish.
To ensure consistency and validity, the M. oleifera leaves used in this study were sourced from local plantations in Malang, Indonesia, and analyzed for their proximate composition. MLM contained 27.0% crude protein, 5.2% crude fat, 6.3% crude fiber, 9.5% ash, and 8.0% moisture. The amino acid profile per 100g included lysine (1.5 g), methionine (0.8 g), threonine (1.0 g), and arginine (2.2 g), while the vitamin content included Vitamin E (134.41 mg/100g), Vitamin A (13.48 mg/100g), and Vitamin C (245.13 mg/100g) (Leone et al., 2015; Moyo et al., 2011; Mubarak et al., 2017; Sohaimy et al., 2015).
Thus, this study aims to: (1) determine the optimal level of MLM supplementation to enhance the growth rate of B. binotatus, (2) evaluate the effects of MLM on reproductive parameters, including gonadosomatic index (GSI), fecundity, and oocyte development, and (3) assess the potential impacts of anti-nutritional factors at higher MLM inclusion levels. By addressing these objectives, this research contributes to advancing functional feed development and sustainable aquaculture practices, particularly for species facing reproductive challenges.
Materials and Methods
The research was conducted from June to August 2021 at the Fisheries Laboratory, University of Muhammadiyah Malang, Indonesia. The experimental species, spotted barb (B. binotatus), were collected from natural freshwater habitats in Indonesia. Prior to the experiment, the fish underwent a one-week acclimatization period in controlled aquaria to stabilize their physiological conditions.
This study employed a completely randomized design with four treatments and three replicates. A total of 180 prospective female B. binotatus broodfish (average weight: 4–7 g; length: 7–9 cm) were used in this study to assess growth and reproductive performance. The fish were evenly distributed across the experimental treatments, with each replicate consisting of 15 individuals maintained in glass aquaria (40 × 30 × 30 cm; water volume: 30 L). A total of 12 aquaria were used for the experiment.
M. oleifera leaves used in this study were sourced from local vegetables plantations in the Malang area. The leaves were air-dried, ground into a fine powder, and sieved. This powder was ixed with commercial fish feed to create experimental diets, which were formulated as follows:
The mixtures were blended with water to form a dough, shaped into pellets, and sun-dried for subsequent use. Determination of MLM dosage was based on findings from previous studies (Farid et al., 2023; Kurniawan et al., 2019).
The proximate composition of experimental diets and fish tests is presented in Table 1.
Fish were reared in aquaria under controlled conditions. Water quality was monitored daily, maintaining a temperature of 26 ± 1°C, pH of 7.5 ± 0.3, and dissolved oxygen above 5 mg/L. Fish were fed twice daily at 07:00 and 17:00, with feeding rates set at 4% of their body weight. The rearing period lasted for 40 days, during which sampling was conducted every 10 days to monitor growth, assess gonadal development, and adjust feeding doses as needed.
Observations were conducted every 10 days to monitor the growth and gonadal development of B. binotatus (spotted barb). The test parameters included:
The length gain (L) was calculated using the formula:
where:
L = Length gain (cm)
Lt = Total length at the end of the study (cm)
Lo = Initial length at the start of the study (cm)
The absolute weight gain (W) was calculated using the formula:
where:
W = Weight gain (grams)
Wt = Total weight at the end of the study (grams)
Wo = Initial weight at the start of the study (grams)
The specific growth rate (SGR) was calculated using the formula:
where:
SGR = Specific growth rate (%)
Wo = Initial weight of the test fish (grams)
Wt = Final weight of the test fish (grams)
t = Duration of the study (days)
The feed conversion ratio (FCR) was calculated weekly by the following formula:
The percent survival represents the percentage of organisms that survive at the end of the maintenance period relative to the initial number of organisms stocked in the container.
The GSI was calculated using the formula:
where:
GSI = Gonado Somatic Index (%)
Wg = Weight of the gonads (grams)
Wt = Body WEIGHT of the fish (grams)(Pham & Nguyen, 2019).
Fecundity refers to the number of mature eggs in the ovaries that are ready to be released during spawning in female broodstock.
Histological analysis of gonadal tissue was employed to determine the developmental stages of oocytes, following the procedures outlined by Siegfried & Steinfeld (2021). The gonads were carefully dissected and fixed in 10% formalin to preserve tissue structure. Thin sections were prepared using a microtome and stained with acetocarmine solution, which binds specifically to chromatin and highlights nuclei and yolk granules in red. After staining, sections were dehydrated through a graded ethanol series, cleared with xylene, and mounted on glass slides for microscopic examination.
To enhance the objectivity of gonadal assessment, a quantitative scoring system was adopted. Oocyte developmental stages were categorized based on defined histomorphological criteria, and each sample was evaluated using a standardized scoring scale ranging from Stage I (primary growth) to Stage IV (mature oocytes).
The data were statistically analysed using analysis of variance (ANOVA) to identify significant differences among treatments. Post-hoc comparisons were performed using Duncan’s multiple range test at a significant level of 0.05. Statistical analyses were conducted using IBM SPPS Statistics software (version 25.0, IBM, Armonk, NY, USA).
Results
The results indicated that the incorporation of MLM as a raw material in commercial feed mixtures had a significant effect on the growth of spotted barb (B. binotatus). The highest growth was observed at a 5% inclusion level of Moringa leaf meal in the commercial feed. The growth performance of B. binotatus is illustrated in Figs. 1 and 2.
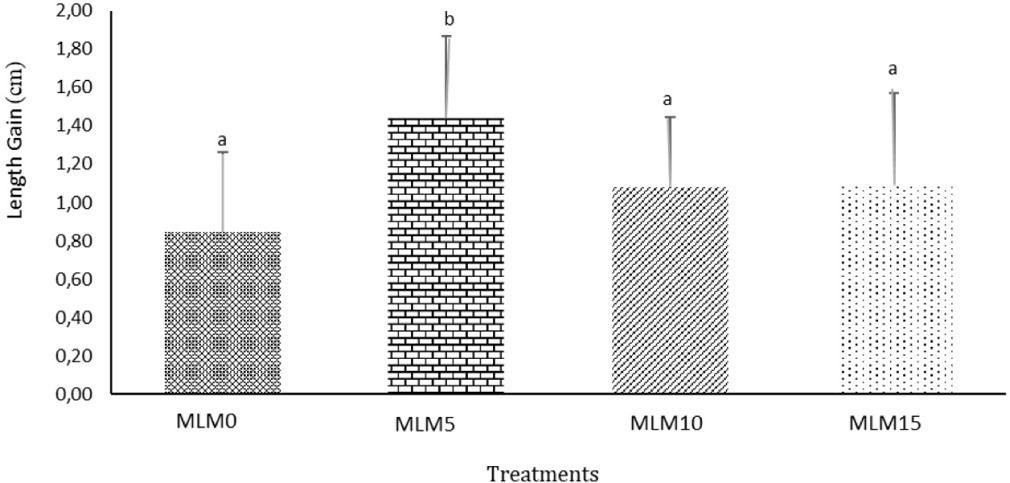
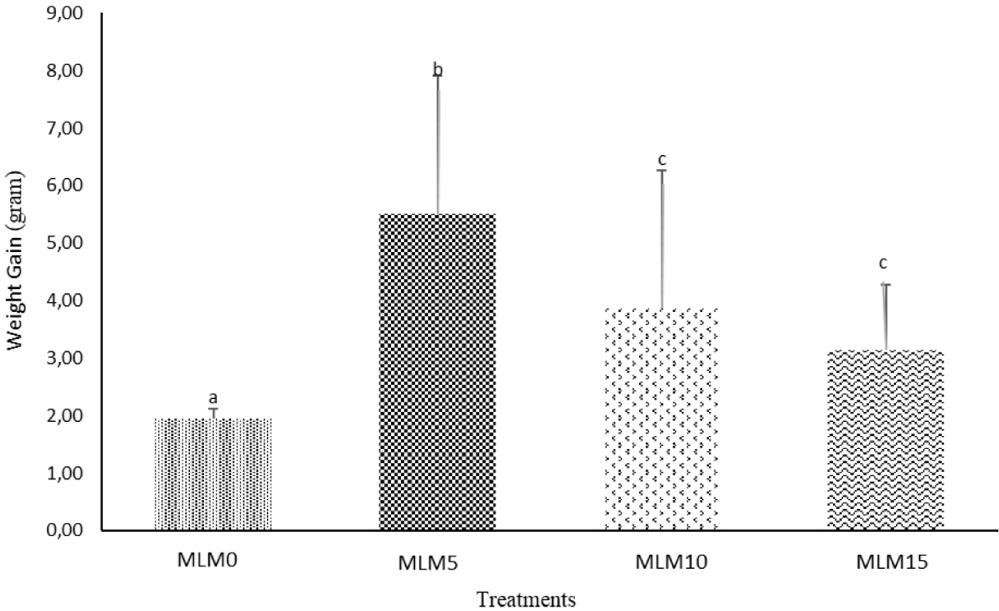
The highest increase in length gain of spotted barb was observed in the MLM5 treatment (5%), with a value of 1.44 cm, followed by MLM15 (15%) at 1.09 cm. The value for MLM10 (10%) was 1.08 cm, which was not significantly different from MLM15, while the lowest value was observed in the MLM0 treatment, with a value of 0.85 cm. The ANOVA test indicated that the treatments resulted in significant differences (p < 0.05). The highest increase in weight gain was found in the MLM5 treatment (5%), with a weight of 5.50 g, followed by MLM10 (10%) at 3.86 g, MLM15 (15%) at 3.14 g, and the lowest in the MLM0 treatment, with a value of 1.94 g. The ANOVA test for body weight also showed significant differences between treatments (p < 0.05). Based on the growth measurements, the MLM5 treatment (5%) resulted in the highest growth in both length gain and weight gain, while the MLM0 treatment resulted in the lowest growth.
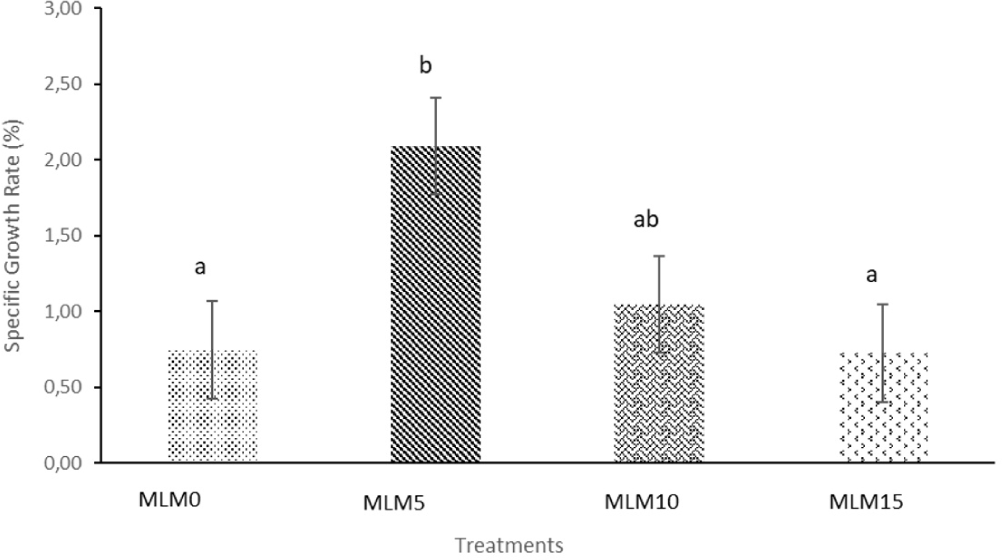
The study results showed that the highest SGR was observed in treatment MLM5, with a value of 2.09 ± 0.55 (5% addition of Moringa leaf meal to the feed), followed by treatment MLM10, with a value of 1.05 ± 0.45 (10% addition of Moringa leaf meal to the feed). The SGR values for all treatments are presented in Fig. 3.
The study results showed that the FCR values in the MLM5 and MLM10 treatments were not significantly different (1.04 and 1.03 respectively). The highest FCR value was obtained in the control treatment (1.8) and followed by the MLM15 treatment (1.4). The FCR values for all treatments are presented in Fig. 4.

The results of the study revealed that the HSI reached its highest value in treatment MLM15 (15%) and its lowest value in treatment MLM0 (0%). Although the HSI values were relatively consistent across all treatments, statistical analysis using ANOVA showed no significant differences (p > 0.05). Detailed HSI values for each treatment are presented in Table 2.
The study indicated that the visceralsomatic index (VSI) was highest in treatment MLM15 (15%) and lowest in treatment MLM5 (5%). Similar to the HSI results, VSI values showed little variation between treatments, and ANOVA analysis confirmed no significant differences (p > 0.05). A comprehensive overview of VSI values for each treatment is available in Table 2.
No significant differences were observed in HSI and VSI among treatments (p > 0.05). This suggests that MLM did not induce hepatic or visceral stress at the tested inclusion levels. Moreover, the lack of difference between treatment groups suggests that the MLM supplementation did not have a substantial effect on the liver and visceral organ sizes in B. binotatus under the conditions of this study. This could indicate that, while MLM improved certain growth and reproductive parameters, it may not directly influence liver and visceral organ growth, or the impact may only become evident under different conditions, such as longer feeding periods or higher doses of MLM.
Percent survival (%) remained at 100% across all treatments, indicating that MLM supplementation was safe and well-tolerated by B. binotatus. Statistical analysis using ANOVA confirmed that the results were not significantly different (p > 0.05). Details of the percent survival for each treatment can be found in Table 2.
The results of the study showed that the highest GSI values were observed in the MLM5 (5%) and MLM10 (10%) treatments, both with a value of 0.16%. The lowest GSI value was recorded in the MLM15 (15%) treatment, at 0.14%. The GSI values across treatments were relatively similar, but the ANOVA test indicated non-significant differences among the treatments (p > 0.05). The gonadosomatic index values for each treatment are presented in Table 2.
The results of the study, as presented in Fig. 5, show that the highest fecundity was observed in the MLM5 (5%) treatment, with a value of 2,605 eggs. This was followed by MLM0 (control) with 2,310 eggs, MLM15 (15%) with 2,294 eggs, and the lowest value was in MLM10 (10%) with 2,104 eggs. The ANOVA test results indicated that the MLM5 (5%) treatment had a highly significant effect (p < 0.005).

Histological observations revealed distinct differences in ovarian development among treatment groups. As illustrated in Fig. 6, oocyte development in B. binotatus after 40 days of feeding varied significantly with the level of MLM supplementation. In the control group, oocytes predominantly remained in early stages of development, with minimal vitellogenesis observed. In contrast, the MLM5 group exhibited more advanced stages of oocyte development, including late vitellogenic oocytes with prominent yolk granules and clearly defined follicular structures. These findings suggest that moderate supplementation (5%) effectively enhanced gonadal maturation.

Higher supplementation levels (MLM10 and MLM15) showed signs of oocyte degeneration, with several atretic oocytes observed. The presence of these atretic oocytes, along with reduced maturity in oocyte structure, indicates potential negative effects of excessive MLM inclusion, possibly due to anti-nutritional factors such as saponins, tannins, or phytates that are known to interfere with nutrient absorption and hormonal regulation.
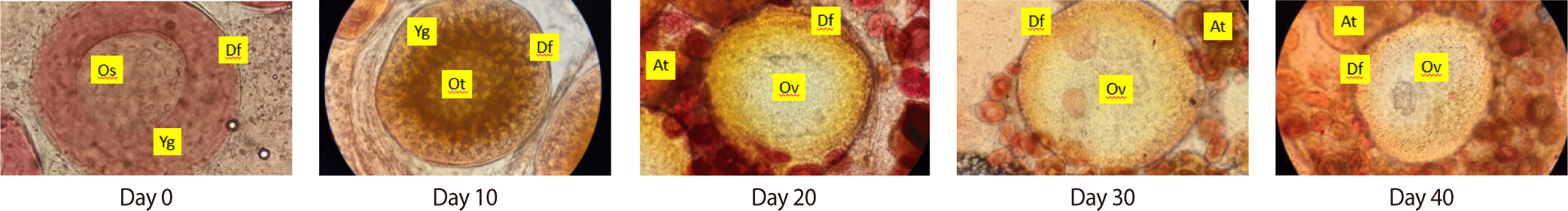
To further understand the temporal progression of ovarian development, Fig. 7 presents a time-series histological analysis of the MLM5 group from Day 0 to Day 40. Initially, at Day 0, ovaries displayed predominantly previtellogenic oocytes. By Day 10 and 20, early vitellogenic stages became evident, marked by the appearance of yolk vesicles. Continued supplementation led to progressive maturation, with mature oocytes and well-developed follicular layers observed by Day 30 and Day 40. No atresia was detected throughout the MLM5 timeline, highlighting the suitability of this supplementation level for supporting sustained and healthy gonadal development.
Discussion
The findings of this study provide valuable insights into the role of MLM as a functional feed ingredient in aquaculture. The significant improvements in growth performance and reproductive traits observed in fish fed with MLM5 highlight the effectiveness of M. oleifera in enhancing physiological processes in B. binotatus. This section discusses the underlying mechanisms contributing to these improvements and compares them with previous studies in related fish species.
Many factors affect the growth of fish, including environmental conditions, genetic factors, and nutrients in their feed. The nutritional needs of fish are different and depend on the species. Key nutrients, such as protein, fat, and carbohydrates, are important in building the body’s structure and producing energy. In addition, water-soluble vitamins and minerals are important components of coenzymes (Anti et al., 2018). Moringa leaves, with a protein content of 27% and a balanced amino acid profile, have the potential to positively impact the growth of spotted barb.
The different composition of moringa leaves added to the diet of spotted barb fish has an impact on decreasing their daily growth at a higher level of giving. This is in line with the findings of (Anti et al., 2018), who reported that an increase in the composition of moringa leaf flour in feed can reduce the daily growth rate in gourami fish. This can happen because of the fish’s response to the feed and the composition of the ingredients in the feed formulation which can affect the ability of fish to utilize the feed optimally for their growth. In addition, the low response of fish to the feed given has an impact on the low rate of feed consumption which causes the weight of fish not to increase significantly, even though water quality and environmental conditions are well maintained.
In this study, the optimal increase in the growth of spotted barb fish was the treatment of adding MLM5 in feed. The improved growth performance in MLM5 can be attributed to the high protein and essential amino acid content in MLM, which supports muscle development and energy metabolism. The presence of bioactive compounds, such as flavonoids and phenolic acids, likely contributed to improved nutrient assimilation and overall fish health. Previous studies have reported that dietary inclusion of plant-based proteins, particularly those with antioxidant properties, can enhance growth rates in fish species such as O. niloticus and Clarias gariepinus (El-Sayed & Izquierdo, 2022; Momın & Memiş, 2023).
The results also showed that lower fish growth rates occurred at higher levels of inclusion (MLM10 and MLM15). This decline in effectiveness can likely be attributed to the presence of anti-nutritional factors in Moringa leaves, which may interfere with nutrient absorption, metabolism, and overall health of the fish (Abdel-Latif et al., 2022). The diminishing returns observed at higher MLM inclusion levels (MLM10 and MLM15) are likely a result of an accumulation of these anti-nutritional factors in the fish’s diet. The experiment investigates the impact of MLM supplementation on the growth and reproductive performance of B. binotatus (spotted barb), highlighting MLM’s potential as a sustainable feed ingredient in aquaculture. By determining the optimal inclusion level of 5%, the study demonstrates that MLM can enhance both growth and reproductive efficiency, offering practical insights for improving fish productivity through cost-effective and sustainable aquaculture practices. When compared to previous studies, the findings align with research on species like Nile tilapia and African catfish, which also benefit from MLM supplementation, supporting its role as a functional feed additive. However, this study also highlights differences, particularly the diminishing returns seen at higher MLM inclusion levels (MLM10 and MLM15), a phenomenon not always observed in other species. This reinforces the importance of optimizing MLM levels to minimize the adverse effects of anti-nutritional factors, which have been noted in studies involving gourami fish (Anti et al., 2018).
In addition to growth performance, feed efficiency is a crucial parameter in aquaculture, as it determines how well fish utilize dietary nutrients for biomass production (Glencross, 2009). The FCR results in this study further support the findings on growth efficiency, highlighting the optimal supplementation levels of MLM. The study results indicate that the supplementation of MLM in the diet of B. binotatus significantly influenced feed utilization efficiency, as measured by FCR. The best FCR values were observed in the MLM5 (5% MLM) and MLM10 (10% MLM) treatments, with values of 1.04 and 1.03, respectively, indicating that fish were able to convert feed into biomass more efficiently at these supplementation levels. A low FCR value indicates a high level of feed efficiency. This improved feed efficiency is likely due to the high protein and essential amino acid content in M. oleifera, which enhances nutrient utilization. M. oleifera has emerged as a promising plant-based feed ingredient in aquaculture, offering both nutritional and medicinal benefits, as its rich composition of protein, essential amino acids, vitamins, and minerals enhances growth performance and nutrient utilization in various fish species (Hridoy et al., 2025; Olaniyi et al., 2013). Studies on Clarias gariepinus, C. carpio, and O. niloticus have shown improved feed conversion efficiency and weight gain when fed diets containing MLM or seed protein extracts (Olaniyi et al., 2013; Stadtlander et al., 2013).
Conversely, the highest FCR values were recorded in the control treatment (MLM0) at 1.8 and in the MLM15 treatment (15% MLM) at 1.4, suggesting that at higher supplementation levels, feed efficiency declined. This reduction in efficiency may be attributed to the presence of anti-nutritional factors in M. oleifera, such as saponins, tannins, and alkaloids, which could interfere with nutrient absorption and increase the metabolic burden on fish (Egwui et al., 2013). Excessive levels of MLM supplementation can induce anti-nutritional effects that negatively impact feed efficiency and fish growth performance. The bioactive compounds in MLM, including saponins, tannins, and alkaloids, have been shown to disrupt digestion and nutrient absorption in fish. Saponins form complexes with cholesterol and damage intestinal cell membranes, reducing the absorption of essential nutrients. Additionally, they inhibit digestive enzymes, lowering feed conversion efficiency. Tannins can bind to proteins, causing their precipitation in the gastrointestinal tract, which reduces the availability of absorbable proteins, potentially suppressing growth and reproduction. Alkaloids in MLM can disrupt metabolic processes and decrease appetite, leading to reduced feed consumption and lower feed utilization efficiency. As the levels of these anti-nutritional compounds rise with higher MLM supplementation, they may cause a decline in fish growth and reproduction, as well as an increase in the FCR. Therefore, MLM supplementation should be limited to optimal levels to maximize nutritional benefits and minimize the negative effects of these anti-nutritional factors (Abdel-Latif et al., 2022; Egwui et al., 2013).
The decline in feed conversion efficiency at higher MLM inclusion levels indicates that while M. oleifera provides significant nutritional benefits, its inclusion in fish feed must be optimized to avoid negative effects from anti-nutritional factors. In the context of sustainable aquaculture, these findings suggest that moderate MLM supplementation (5%–10%) can maximize feed efficiency without compromising fish growth. Improved feed efficiency not only enhances fish productivity but also reduces dependence on fishmeal, which is costly and has substantial environmental impacts. These findings align with previous studies demonstrating that herbal supplementation in fish feed can enhance growth efficiency when used at appropriate levels. In Nile tilapia, MLM inclusion up to 30% did not negatively impact growth or feed efficiency (Manuel et al., 2019). Similarly, juvenile Puntius altus fed diets containing MLM exhibited increased body weight and muscle protein profiles (Sirimongkolvorakul et al., 2015). For Asian sea bass, a carnivorous species, MLM inclusion up to 10% in fish meal-based diets yielded acceptable growth performance without adverse effects (Ganzon-Naret, 2014). Further research is recommended to explore processing strategies for MLM, such as fermentation or detoxification methods, to mitigate the impact of anti-nutritional factors and enable higher inclusion rates without compromising feed efficiency.
The HSI and VSI are important indicators for assessing the health of fish and their nutritional status, often reflecting the metabolic response to changes in feeding patterns. HSI and VSI serve as indicators of liver function and energy storage, respectively. In this study, the MLM15 treatment recorded the highest HSI value at 15%, while the MLM0 treatment recorded the lowest value at 0%. Although there was a difference in HSI values between treatments, statistical analysis showed that the difference was not significant (p > 0.05). The absence of significant differences among treatments indicates that MLM supplementation did not induce metabolic stress or excessive fat accumulation in the liver. This aligns with findings from Abdel-Latif et al. (2022), who reported that moderate levels of plant-based protein sources do not adversely affect hepatic function in farmed fish. Future studies should consider analyzing liver histology and enzyme activity to further assess metabolic responses to MLM supplementation.
The VSI value shows a similar trend to HSI, where the highest value is found in the MLM15 treatment (15%) and the lowest value is recorded in MLM5 (5%). Statistical analysis showed that the difference between treatments was not significant (p > 0.05), indicating that the addition of moringa oleifera leaf meal (MLM) in the feed, even at a high level of inclusion, did not substantially affect visceral fat deposition. The increase in VSI in treatments with higher levels of MLM is likely due to the body’s response to storing excess nutrients or adaptation to the presence of anti-nutrient compounds by increasing the activity of visceral organs. These findings are consistent with previous reports that anti-nutrient compounds in plant-based feed ingredients can trigger an increase in VSI because of additional metabolic load on the digestive organs (Kurniawan et al., 2019).
Survival refers to the percentage of organisms that survive until the end of the maintenance period compared to the initial number of organisms stocked. In this study, the survival rate of spotted barb fish in all treatments reached 100%. This high survival rate can be caused by a variety of factors, including internal factors such as sex, heredity, age, reproductive capacity, and resistance to disease, as well as external factors such as water quality, stocking density, and amino acid content in feed (Ajo et al., 2020).
Perfect survival across all treatments showed that moringa oleifera leaf meal (MLM) supplementation at the tested inclusion level was safe for B. binotatus. These results are in line with previous studies that reported that moringa supplementation does not have a negative effect on the health of various other fish species (Kurniawan et al., 2019). This finding further strengthens the potential of moringa as a safe, sustainable, and non-toxic feed ingredient in modern aquaculture practices.
The GSI, calculated as the ratio of gonad weight to body weight, is a widely used indicator to assess gonadal maturity and spawning readiness in fish (Ibrahim et al., 2020). In this study, the inclusion of MLM at 5% (MLM5) yielded the highest reproductive performance, as indicated by significantly increased GSI, fecundity, and advanced oocyte development. These improvements reflect the fish’s enhanced reproductive capacity, as GSI and fecundity correlate with the quantity and maturity of ova produced per spawning cycle.
Histological observations in the MLM5 group confirmed more developed oocyte stages, supporting the notion that moderate MLM supplementation can accelerate gonadal maturation. This positive effect is likely attributed to the high vitamin E content in moringa leaves, which functions as a potent antioxidant. Vitamin E is known to enhance egg quality, promote oocyte maturation, and protect reproductive cells by reducing oxidative stress—a key factor that can disrupt steroidogenesis and impair reproductive efficiency (El-Sayed & Izquierdo, 2022; Ghosh et al., 2024; Putra et al., 2018; Tao et al., 2023).
In addition to vitamin E, MLM provides essential amino acids such as arginine and lysine, which support protein synthesis and cellular proliferation in gonadal tissues (Andersen et al., 2016). These nutrients likely contribute to the observed increase in reproductive parameters at the 5% inclusion level. However, further molecular studies are required to verify the specific pathways involved.
At higher inclusion levels (10% and 15%), reproductive performance declined, as evidenced by reduced GSI, fecundity, and the presence of atresia in oocyte histology. This reduction is likely due to the accumulation of anti-nutritional factors—such as tannins, saponins, and phytates—which are known to interfere with nutrient absorption and hormonal regulation (Kurniawan et al., 2019). These findings highlight the importance of optimizing inclusion levels to maximize the nutritional benefits of MLM while minimizing potential adverse effects.
Previous research has shown that moringa leaf supplementation can promote growth and health in various species fish species. In Colossoma macropomum, a 30% inclusion of moringa leaf powder in feed resulted in significant increases in body weight, width, and length, while maintaining fish health (Safrida et al., 2020). Similarly, for Clarias gariepinus, a 30% replacement of fish meal with moringa leaf meal showed no adverse effects on growth performance (Adekilekun et al., 2022). Another study found that 10g of moringa leaf powder per kg of feed yielded optimal growth results and improved immunity in Clarias gariepinus (Ekelemu et al., 2023). The optimal level of inclusion of moringa leaves in feed varies, which is between 10%–30% for omnivorous and herbivorous fish, and 10%–20% for carnivorous fish withoght negative impact (Momın & Memiş, 2023). In addition to playing a role in supporting growth, moringa leaves have antioxidant properties that can protect fish oil while maintaining the stability of the quality of polyunsaturated fatty acids (Nascimento et al., 2015). As a sustainable alternative source of protein, moringa leaves not only reduce dependence on conventional protein raw materials such as fish meal but also improve the immune function of fish. Despite its promising potential, more research is needed to determine the optimal level of inclusion for various fish species so that their nutritional and physiological benefits can be maximized (Abdel-Latif et al., 2022; Egwui et al., 2013; Gabriel et al., 2024). These findings suggest that moringa leaf supplementation is a promising strategy for enhancing fish growth and health in aquaculture.
As a sustainable alternative protein source, M. oleifera not only reduces dependence on fishmeal but also offers functional benefits for fish health and reproduction. Nonetheless, precise species-specific formulation and further mechanistic studies are necessary to ensure its effective and safe use in aquaculture feeds (Abdel-Latif et al., 2022; Gabriel et al., 2024).
Fishmeal remains a significant cost factor in aquafeeds, with prices fluctuating due to overfishing and supply chain instability. The use of M. oleifera as a partial replacement for fishmeal has been suggested in previous studies as an economically viable alternative (Abdel-Latif et al., 2022; Momın & Memiş, 2023). Although this study did not conduct a direct economic analysis, prior research suggests that reducing fishmeal dependency could lower feed costs by up to 20% (Aaqillah‐Amr et al., 2024; Arru et al., 2019). The local availability and fast-growing nature of Moringa further enhance its cost-effectiveness, providing an affordable and sustainable option for aquaculture farmers. By partially replacing fishmeal with Moringa, farmers can not only reduce costs but also mitigate the financial instability caused by fluctuating fishmeal prices, making aquaculture more resilient to market changes.
In addition to its economic benefits, shifting towards plant-based feed ingredients such as M. oleifera can reduce the environmental impact of aquaculture.
However, it is important to note the limitations of this study, including the lack of molecular-level analyses or long-term effects of Moringa supplementation on fish health and performance. These gaps highlight the need for further research to better understand the full impact of Moringa on fish at the molecular level and over extended periods. Additionally, the presence of anti-nutritional factors in Moringa leaves, such as saponins, tannins, and alkaloids, may reduce feed efficiency at higher supplementation levels. Future studies should focus on methods to mitigate these anti-nutritional compounds, such as pre-treatment strategies like fermentation or the use of enzymes, to enhance the bioavailability of nutrients and minimize the negative effects of these compounds on fish health and growth.








