Introduction
In recent years, functional foods have gained increasing attention, particularly the use of seaweeds as a promising source for promoting gut health. Seaweeds are rich in bioactive compounds such as polysaccharides fucoidan, alginate, carrageenan, and ulvan, polyphenols, and essential minerals, which play a crucial role in modulating gut microbiota (Lee et al., 2023). These compounds act as prebiotics, selectively promoting the growth of beneficial gut bacteria while inhibiting harmful microbes. Recent research has highlighted the potential of seaweed-derived prebiotics in enhancing digestive health, improving immune function, and mitigating metabolic disorders. Unlike conventional prebiotics, seaweed polysaccharides possess unique structural properties that resist digestion in the upper gastrointestinal tract, allowing them to reach the colon, where they undergo fermentation by gut microbiota (Sedgwick et al., 2025). This fermentation process generates short-chain fatty acids (SCFAs), which play a key role in maintaining gut barrier integrity, modulating immune responses, and reducing inflammation. Beyond microbial modulation, seaweeds also contain antioxidant and anti-inflammatory compounds that contribute to overall gut health (Kim et al., 2021). However, despite their growing recognition as functional foods, a deeper understanding of their specific mechanisms of action and optimal application is still required. The versatility and multifaceted properties of seaweeds have been utilized in various sectors, ranging from food and medicine to agriculture and environmental management, highlighting their abundance, nutritional value, and applications as valuable marine resources with immense potential for further exploration (Premadasa et al., 2024).
Gut Microbiota in Human Health
Human gut microbiota is a complex and dynamic community of microorganisms, with bacteria, viruses, fungi, and archaea mainly existing in the gastrointestinal tract (Jayapala & Lim, 2023). These diverse microbial organisms play a vital role in maintaining overall health by contributing to digestion, immune regulation, metabolic processes, and neurological functions (Hou et al., 2022). The composition and balance of gut microbiota are essential for health, as disruptions in this microbial community have been linked to inflammatory diseases, metabolic disorders, and neurological dysfunctions (Durack & Lynch, 2019). One of the primary functions of the gut microbiota is aiding digestion and nutrient absorption, as the human body lacks enzymes to break down certain dietary fibers and complex carbohydrates, gut bacteria ferment these indigestible components, converting them into SCFAs, which are essential for gut barrier integrity and immune modulation (den Besten et al., 2013). Additionally, gut microbiota plays a significant role in metabolic health, influencing energy balance, fat storage, and insulin sensitivity, making its maintenance crucial through a fiber rich diet, probiotics, and lifestyle modifications (Sender et al., 2016).
Seaweeds as Functional Food
The term “seaweed” refers to large marine algae, classified into brown (Phaeophyceae), red (Rhodophyceae), and green (Chlorophyceae) algae, each exhibiting distinct characteristics based on anatomy, pigmentation, morphology, and chemical composition. Seaweeds hold immense potential for use in functional foods and with their bioactive compounds, mainly polysaccharides, polyphenols, and antioxidants, making them promising candidates for gut health enhancement. These compounds seem to have antioxidant, anti-inflammatory, anticancer, antimicrobial, and antidiabetic properties (El-Beltagi et al., 2022). Due to their diverse bioactive properties, seaweeds have been widely utilized across food, medicine, agriculture, and environmental sectors.
Historically, seaweeds have been used in medicine for treating iodine deficiency, intestinal disorders and as hypocholesterolemic and hypoglycemic agents (El Gamal, 2010). They serve as thickening and gelling agents (phycocolloids) in the food and pharmaceutical industries and are commonly consumed in East Asian countries due to their high nutritional value (Mwalugha et al., 2015). Seaweeds are a rich source of macronutrients protein, fiber, carbohydrates, fatty acids, lipids and micronutrients minerals and vitamins, along with significant bioactive compounds (Edwards et al., 2012). Each seaweed group contains distinct bioactive compounds that contribute to gut health, with brown algae being rich in polysaccharides (fucoidan, alginate, laminarin), polyphenols (phlorotannins), and other unique bio actives that exhibit prebiotic and anti-inflammatory properties, red algae containing sulfated polysaccharides (carrageenan, agar), proteins, and pigments that help maintain gut microbiota balance, and green algae containing ulvans (prebiotic properties), sulfated polysaccharides (gut microbiota balance), carotenoids (antioxidants), and proteins (anti-inflammatory effects) (Jang et al., 2024). Seaweeds serve as excellent sources of functional food, making them essential for both human and animal nutrition, while ulvans, a key component in green algae, enhance beneficial gut bacteria, sulfated polysaccharides contribute to gut microbiota balance, carotenoids act as antioxidants protecting gut epithelial cells, and proteins from green algae help reduce gut inflammation.
Bioactive Compounds from Seaweeds and Their Mechanism on Gut Health
As seaweeds are a rich source of diverse bioactive compounds, including polysaccharides, polyphenols, carotenoids, and proteins, each playing a crucial role in gut health. Polysaccharides, such as fucoidan, alginate, and laminarin from brown algae, carrageenan and agar from red algae, and ulvans from green algae, act as prebiotics by selectively promoting the growth of beneficial gut bacteria like Bifidobacteria and Lactobacillus (Cherry et al., 2019). These compounds enhance gut microbiota diversity, stimulate SCFAs production, and protect gut barrier integrity, thereby reducing inflammation and supporting overall digestive health (Fusco et al., 2023). Polyphenols, particularly phlorotannins from brown algae and other phenolic compounds found in red and green algae, exhibit strong antioxidant properties that help modulate gut inflammation by inhibiting pathways like nuclear factor-kappa B (NF-κB), which plays a key role in oxidative stress and inflammatory responses. Carotenoids, such as fucoxanthin from brown algae and beta-carotene from green algae, further contribute to gut health by protecting gut epithelial cells from oxidative damage, maintaining gut barrier integrity, and reducing the risk of inflammatory disorders (Simón et al., 2023). Additionally, proteins and bioactive peptides derived from red and green algae possess immunomodulatory properties, regulating immune responses in the gut and reducing the production of pro-inflammatory cytokines like tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) (Oliyaei et al., 2025). These bioactive compounds collectively contribute to gut homeostasis, making seaweed a promising functional food ingredient for gut health (Behera et al., 2024). Given their prebiotic, antioxidant, and anti-inflammatory properties seaweeds derived bioactives hold significant potential for application in functional foods and therapeutics aimed at improving gut microbiota balance and overall digestive health.
Polysaccharides on Gut Microbiota
Polysaccharides derived from seaweeds, including fucoidans, alginates, laminarins, carrageenans, and ulvans, play a significant role in gut microbiota modulation, immune regulation, and gut barrier function (Zang et al., 2023). These complex carbohydrates are resistant to digestion in the upper gastrointestinal tract and reach the colon intact, where they serve as prebiotics by selectively stimulating the growth of beneficial gut bacteria such as Bifidobacteria and Lactobacillus while inhibiting harmful microbes (Lee et al., 2023). Seaweed-derived polysaccharides contribute to gut health through multiple mechanisms. Their prebiotic effects serve as fermentable substrates for beneficial bacteria, increasing microbial diversity and promoting SCFAs production, which helps regulate inflammation and maintain gut homeostasis (den Besten et al., 2013). Additionally, they enhance gut barrier integrity by upregulating tight junction proteins such as claudin, occludin, and zonulin, reducing intestinal permeability and preventing conditions like leaky gut syndrome (Oomizu et al., 2006). These polysaccharides also play a key role in immunomodulation, interacting with gut immune cells to promote anti-inflammatory responses while suppressing excessive immune activation, which is particularly beneficial for managing inflammatory bowel diseases (Pereira & Valado, 2024). Moreover, their antioxidant and anti-inflammatory properties help neutralize free radicals, protecting gut epithelial cells from oxidative stress and reducing the risk of inflammation-induced gut disorders (Michalak et al., 2022). Overall, seaweed polysaccharides hold immense potential for functional food and therapeutic applications aimed at improving gut health.
Fucoidans, complex carbohydrates found in brown algae such as kelp, have garnered increasing attention due to their potential health benefits, particularly in gut health and microbiota modulation. Although traditionally considered indigestible by bacterial and human enzymes due to their unique chemical structures (Nagamine et al., 2014), recent studies indicate that certain gut microbiota species can digest fucoidans, impacting gut microbiota composition and intestinal homeostasis (Xie et al., 2017).
Fucoidans act as prebiotics by promoting beneficial bacterial growth (Table 1), enhancing gut microbial diversity, and regulating the balance of microbial populations (Shang, 2020). Studies have shown that dietary fucoidan influences gut microbiota by increasing beneficial bacteria such as Lactobacillus and Bifidobacterium while reducing harmful bacteria like Peptococcus and Clostridiales (Shang et al., 2016; Yoshinaga et al., 2018). Notably, different fucoidan types (Fig. 1) exhibit distinct effects, type I fucoidan from Laminaria japonica increases Lactobacillus, whereas type II fucoidan from Ascophyllum nodosum enhances Ruminococcaceae while reducing Clostridiales and Alistipes (Smith et al., 2013). Fucoidans contribute to gut health beyond microbiota regulation by enhancing short-chain fatty acid (SCFA) production, which influences immune function and intestinal integrity. Increased SCFAs production supports Th1 and Treg cell regulation, promoting intestinal homeostasis and reducing inflammation (Smith et al., 2013; Sun et al., 2018). Additionally, fucoidans have been shown to alleviate gut dysbiosis, improving Akkermansia muciniphila abundance and reducing metabolic endotoxemia (Shang et al., 2017).
| Fucoidan sources | Fucoidan type | Effect on gut | Reference |
|---|---|---|---|
| Isostichopus badionotus | Type I | - Regulates obesity, inflammation, and hyperlipidemia due to high-fat diets - Maintains gut dysbiosis by improving Oscillibacter, Acetatifactor, and Desulfovibrionales population |
Li et al. (2019) |
| Pearsonothuria graeffei | Type I | - Reduces the impacts of high-fat diets (e.g., Obesity, Hyperlipidemia) - Increases the populations of Bacteroides and Parabacteroides (i.e., Prevotellaceae and Olsenella) - Decreases Desulfovibrio and Dorea populations |
Li et al. (2019) |
| Laminaria japonica | Type I | - Enhance the population of Lactobacillus - Decrease the Peptococcus population - Alleviated a metabolic syndrome in high-fat diet-fed mice - Enhance the population of probiotic bacteria (Akkermansia) - Decrease the population of pathogens (e.g., Rikenellaceae) |
Shang et al. (2016)
Shang et al. (2017) |
| Acaudina molpadioides | Type I | - Triggers healing of a mucosal injury - Enhanced the Butyricicoccus, Rikenella, and Coprococcus populations - Reduce the abundance of Bacillus and Streptococcus - Increases the resistance of insulin - Regulates gut dysbiosis via minimizing the population of Mucispirillum schaedleri like pathogenic bacteria and enhancing the probiotic bacteria Bifidobacterium abundance |
Jang et al. (2024) |
| Sargassum fusiforme | Type II | - Influences the population of Roseburia, Alloprevotella, and Alistipes, - Reduces the abundance of pathogenic bacteria and Parabacteroides. - Regulates the hyperglycemia caused by streptozotocin |
Cheng et al. (2019) |
| Ascophyllum nodosum | Type II | - Increases the population of probiotic bacteria (e.g. Blautia, ClostridialesvadinBB60, Akkermansia) - Decreases the population of Alistipes and Rikenellaceae |
Shang et al. (2017) |
| Undaria pinnatifida | Type II | - Lowers the low-density lipoprotein cholesterol, serum total cholesterol, and lipopolysaccharide - Increase the Oscillospira, Prevotella, Bacillus, and Ruminococcus populations and reduce that of Staphylococcus and Clostridium - Enhances liver steatosis - Regulates gut dysbiosis by reducing Staphylococcus population and increasing Alloprevotella abundance |
Cheng et al. (2019) |
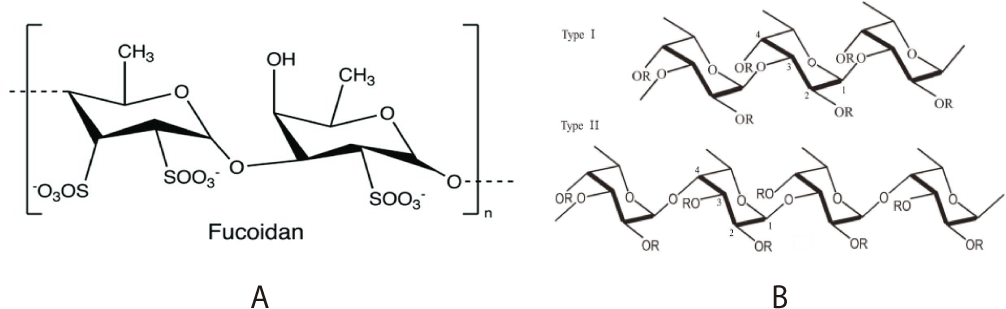
Most available research on fucoidans and gut microbiota is based on animal models, primarily mice, raising concerns about their direct applicability to human gut microbiota (Nguyen et al., 2015). However, human trials have demonstrated promising effects. A study by Kan et al. (2020) found that a diet containing 0.3 g of fucoidan and 1.0 g of wheat peptides daily reduced gastric mucosal damage and stomach pain while increasing beneficial bacteria such as Bacteroides intestinalis, Bifidobacterium pseudocatenulatum, and Eubacterium siraeum, alongside a decrease in Prevotella copri abundance. Beyond microbiota modulation, fucoidan exhibits significant therapeutic properties. It reduces gastric mucosal damage, promote epithelial cell regeneration, mitigates liver steatosis and obesity, and reduces tissue damage and inflammation (Huang et al., 2021). Furthermore, fucoidans have demonstrated anti-cancer properties, reducing colon cancer cell proliferation and decreasing TNF-α and IL-6 levels, contributing to reduced gut inflammation (Lee et al., 2018). Additionally, fucoidans enhance paracellular permeability, regulate gut immunity, and stimulate intestinal epithelial cell regeneration, offering potential therapeutic applications for inflammatory bowel diseases such as ulcerative colitis and Crohn’s disease (Fernando et al., 2017). Fucoidan’s immunomodulatory effects further enhance gut health by promoting anti-inflammatory cytokine IL-10 production while reducing pro-inflammatory markers like TNF-α and IL-6 (Shimizu et al., 2005). Fucoidans also reinforce gut barrier integrity by upregulating tight junction proteins, such as zonulin and occludin, reducing intestinal permeability and preventing leaky gut syndrome (Oomizu et al., 2006). Additionally, fucoidans have demonstrated potential benefits in alleviating psoriasis and IgE-associated allergic diseases, further emphasizing their immunoregulatory capabilities. Beyond gut health, fucoidans may play a role in metabolic disorders, particularly type II diabetes mellitus. Studies on Sargassum fusiforme derived fucoidans indicate their ability to lower fasting blood glucose levels, enhance glucose tolerance, and reduce oxidative stress and epididymal fat deposition in diabetic models (Wu et al., 2021). Furthermore, fucoidans mitigate inflammatory responses, preventing cryptosporidiosis by inhibiting Cryptosporidium parvum oocyst adhesion to intestinal epithelial cells (Maruyama et al., 2007).
Alginate, another bioactive compound found in seaweed, can absorb water and body fluids up to 20 times its weight, forming a hydrophilic gel and helping maintain a moist environment, which is beneficial for wound healing (Hillier & Rakkar, 2008). Recent research suggest that the ability of human gut Bacteroides to degrade alginate originated from an ancient gene acquisition. This system, encoded within polysaccharide utilization loci, includes PL6 and PL17 alginate lyase enzymes, as well as hypothetical proteins involved in alginate recognition, internalization, and breakdown. Additionally, bacterial ABC transporter proteins assist in alginate uptake across the bacterial membrane. Alginates, composed of mannuronic are linear block copolymers composed of β-d-mannuronic acid (M) and α-l-guluronic acid (G) residues. and guluronic acid units, are another major polysaccharide found in brown algae, such as Laminaria and Ascophyllum (Hillier & Rakkar, 2008). They act as prebiotics by increasing the populations of beneficial gut bacteria while decreasing the prevalence of harmful bacteria such as Clostridium perfringens (Cherry et al., 2019). Alginates have been shown to form a gel-like structure in the gut, which helps slow digestion, enhance satiety, and improve metabolic processes. Fucoidans contribute to gut detoxification by binding to heavy metals and toxins, facilitating their removal from the body. Additionally, they enhance mucin production, strengthening the gut lining’s protective mucus layer (Hillier & Rakkar, 2008).
Laminarins, primarily found in Laminaria species, consist of β-glucans that serve as fermentable substrates for gut microbiota, particularly Bacteroidetes and Firmicutes, leading to an increase in SCFAs production (Cherry et al., 2019). These SCFAs, such as butyrate, acetate, and propionate, play essential roles in reducing inflammation, maintaining gut pH, and enhancing energy metabolism. Laminarins also exhibit antimicrobial properties, limiting the growth of pathogenic bacteria such as Salmonella and Escherichia coli (Shannon & Abu-Ghannam, 2016). Furthermore, they help modulate immune function by influencing dendritic cells and macrophages, thereby promoting a balanced immune response. The enzymes in human gut Bacteroides responsible for breaking down laminarin are likely different from those that degrade mixed-linkage β-1,3-1,4-glucans, such as those found in cereals. For example, the degradation of cereal-derived glucans is facilitated by enzymes like BoGH16MLG. This distinction suggests that gut bacteria have evolved specific enzymatic pathways to target different polysaccharides based on their structural variation (Tamura et al., 2017).
Carrageenans, sulfated galactans found in red algae (Chondrus crispus, Kappaphycus alvarezii), have been widely studied for their effects on gut health. These polysaccharides are composed of sulfated 1,4-β-D-galactose, 1,3-α-D-galactose, and 3,6-anhydro-D-galactose, constituting 30%−75% of the dry weight of red seaweeds (Weiner, 2014). Carrageenans serve as a structural component of the extracellular matrix. Among the 15 different forms, iota (ι), kappa (κ), and lambda (λ) carrageenans are the most widely used as phycocolloids in the food industry (Shannon et al., 2021). All carrageenan forms are soluble in water above their gel-melting temperatures, which range between 40°C–70°C. In cold water, only λ-carrageenan and the sodium salts of κ- and ι-carrageenan are soluble (Olatunji, 2020). Additionally, carrageenans regulate immune responses by interacting with toll-like receptors and modulating cytokine production, which helps reduce gut inflammation when consumed in controlled amounts. However, excessive intake of carrageenans has been associated with potential inflammatory responses in some studies, highlighting the need for careful dietary consideration (Bhattacharyya et al., 2010).
Ulvans, found in green algae such as Ulva lactuca, are sulfated rhamnose-containing polysaccharides with strong prebiotic and immunomodulatory properties Green seaweed is primarily composed of ulvans, which are sulfated polysaccharides consisting of 1,3-α-l-rhamnose, 1,4-β-d-glucuronic acid, and 1,4-β-d-xyloglucan. These compounds constitute approximately 29%–38% of the seaweed’s dry weight mass (Lahaye & Robic, 2007). They enhance gut microbiota balance by increasing populations of beneficial bacteria while reducing pathogens. Ulvans are particularly effective in modulating gut immunity, as they stimulate macrophages, T cells, and natural killer cells, thereby enhancing the body’s defense against infections. Their antioxidant, antiviral and antibacterial properties also contributes to gut epithelial protection by reducing oxidative stress and enhancing tight junction integrity, ultimately preventing gut barrier dysfunction (Cindana Mo’o et al., 2019). A recent in vitro fecal fermentation study demonstrated that ulvan stimulated the growth of Bifidobacterium and Lactobacillus while promoting lactate and acetate production, whereas a murine study revealed that Enteromorpha and Enteromorpha polysaccharides alleviated Loperamide-induced constipation by increasing alpha diversity, Firmicutes, and Actinobacteria in the fecal microbiota of seaweed-supplemented mice compared to the constipated control (Cherry et al., 2019).
Agar is a polysaccharide primarily derived from red algae, commonly used as a gelling agent in microbiological and food applications. It consists mainly of agarose and agaropectin, which are generally considered indigestible by human enzymes. However, certain gut microbiota, particularly in individuals with diets rich in seaweed, have evolved to degrade agar and utilize it as a prebiotic. Specific bacterial species, such as Bacteroides plebeius, have been identified as possessing agarolytic enzymes that break down agar into bioavailable oligosaccharides, which in turn support the growth of beneficial gut bacteria (Bhattarai & Kashyap, 2016). Agar-derived oligosaccharides can positively influence gut microbiota by promoting the growth of beneficial bacteria, such as Bifidobacterium and Lactobacillus, while reducing pathogenic bacteria. These oligosaccharides also enhance the production of SCFAs, such as butyrate, acetate, and propionate, which contribute to gut health by modulating inflammation, improving intestinal barrier integrity, and supporting immune function. Additionally, agar-derived prebiotics have been linked to anti-inflammatory effects, reduced gut dysbiosis, and potential benefits in metabolic disorders such as obesity and diabetes (Bhattarai & Kashyap, 2016). While research on agar’s impact on gut microbiota is still emerging, studies suggest that its polysaccharides may serve as a functional dietary component supporting gut health.
Short-Chain Fatty Acid Production through Polysaccharides and Gut Microbiota
Polysaccharides, especially those derived from dietary fiber, play a vital role in the production of SCFAs in the large intestine (Cherry et al., 2019). When polysaccharides reach the colon, they serve as substrates for microbial fermentation. This process is basically carried out by the gut microbiota, which breaks down these complex carbohydrates into SCFAs, including acetate, propionate, and butyrate (Cummings et al., 1978; den Besten et al., 2013). The amount and type of SCFAs created depend on the particular polysaccharide and the microbial strain involved (Yang et al., 2021). For illustration, resistant starch and non-starch polysaccharides are particularly effective in promoting butyrate generation, which could be a preferred energy source for colonocytes and has been connected to anti-inflammatory and anti-carcinogenic properties. The fermentation process moreover brings down the pH of the colonic environment, which can repress the development of pathogenic microbes and enhance the absorption of nutrients (Cummings et al., 1978).
SCFAs also control the function of innate immune cells, such as macrophages and dendritic cells, by tweaking the expression of pro-inflammatory cytokines and improving the barrier function of the intestinal epithelium (Liu et al., 2023; Song et al., 2023). This helps in avoiding the translocation of harmful microscopic organisms and toxins from the gut lumen into the circulation system, in this manner lessening systemic inflammation (Song et al., 2023). Polysaccharides improve the integrity of the gut barrier by advancing the generation of SCFAs, which in turn stimulate the expression of tight junction proteins. The anti-inflammatory properties of SCFAs are intervened through their capacity to inhibit the activation of NF-κB, a key controller of inflammation. By decreasing NF-κB action, SCFAs diminish the generation of pro-inflammatory cytokines such as IL-6 and TNF-α (Liu et al., 2023; Silva et al., 2020). Moreover, polysaccharides impact the composition and diversity of the gut microbiota, favoring the development of beneficial microbes such as Bifidobacteria and Lactobacilli. These microscopic organisms are known to produce SCFAs and other metabolites that support intestinal well-being and immune function (Tang et al., 2022; Yang et al., 2021). By promoting an adjusted microbial community, polysaccharides help prevent the abundance of pathogenic microbes, which can lead to dysbiosis and related well-being issues (Song et al., 2023).
Polyphenols on Gut Microbiota
Polyphenols, which constitute up to 20% of the dry mass in brown seaweed and 1%–5% in green and red seaweeds, protect the seaweed thallus against biotic and abiotic stresses such as herbivore predation, microbial infection, oxidation, and UV damage (Mannino & Micheli, 2020). Among the factors influencing the human gut microbiota, diet has emerged as a significant determinant, with recent research specifically investigating the consumption of seaweeds. In this context, polyphenols found in seaweeds have garnered attention for their potential impact on gut microbiota and subsequent benefits for bowel health shown in Table 2.
| Seaweed sources | Polyphenol type | Effect on gut | Reference |
|---|---|---|---|
| Sargassum fusiforme | Polyphenol A | Modulates gut microbiota, reduces inflammation | Cheng et al. (2019) |
| Laminaria japonica | Polyphenol B | Enhances Lactobacillus, decreases Peptococcus | Shang et al. (2016) |
| Ascophyllum nodosum | Polyphenol C | Increases Blautia, Clostridiales, Akkermansia | Shang et al. (2017) |
| Undaria pinnatifida | Polyphenol D | Lowers cholesterol, enhances Oscillospira, Prevotella | Cheng et al. (2019) |
| Fucus vesiculosus | Polyphenol E | Regulates obesity, inflammation, and hyperlipidemia | Li et al. (2019) |
| Chondrus crispus | Polyphenol F | Reduces impacts of high-fat diets | Li et al. (2019) |
Polyphenols, abundant in various seaweed species, possess diverse chemical structures (Fig. 2) and bioactivities. While their direct digestion by bacterial and human enzymes may vary, recent studies (Nagamine et al., 2014) suggest that polyphenols interact with gut microbiota, influencing microbial composition and contributing to overall intestinal well-being. The impact of polyphenols on specific bacterial taxa may depend on the source of seaweed and the type of polyphenol present. For example, polyphenols from certain seaweeds may enhance the abundance of beneficial bacteria such as Lactobacillus while reducing the prevalence of pathogenic species (Cheng et al., 2019). The chemical variability of polyphenols in seaweeds can lead to distinct effects on gut microbiota. Different types of polyphenols may uniquely influence microbial abundance and diversity. For instance, studies have reported that polyphenols extracted from specific seaweed varieties can modulate the abundance of particular bacterial families, potentially impacting the overall balance of the gut microbiota (Cheng et al., 2019). These variations highlight the need for a nuanced understanding of the chemical structures of polyphenols and their specific effects on gut microbial communities. Aside from their influence on gut microbiota, polyphenols from seaweeds have demonstrated direct effects on gut health, which include structural improvements, regulation of inflammatory responses, and potential protective roles against conditions such as colorectal cancer (Maruyama et al., 2007). The multifaceted nature of polyphenols makes them promising candidates for therapeutic interventions against intestinal disorders.
N-3 Polyunsaturated Fatty Acids on Gut Microbiota
Seaweed lipids, comprising 1%–5% of dry weight, include n-3 polyunsaturated fatty acids (PUFAs) such as eicosapentaenoic acid and docosahexaenoic acid. These fatty acids exhibit anti-inflammatory properties, reducing cardiovascular disease risk and supporting brain function through the microbiota-gut-brain axis. While research primarily focuses on fish-derived n-3 PUFAs, seaweed-derived n-3 PUFAs offer significant prebiotic potential. N-3 PUFAs influence gut microbial diversity, enhance the intestinal barrier, and promote beneficial bacteria like Bifidobacteria and Lactobacillus, while reducing proinflammatory Enterobacteria (Jandhyala et al., 2015; Menni et al., 2017). Deficiency in n-3 PUFAs is associated with gut microbial dysbiosis, obesity, and chronic diseases such as cardiovascular diseases, diabetes, depression, and neurodegenerative disorders (Jayapala & Lim, 2023). As essential bioactive compounds, n-3 PUFAs from seaweeds contribute to gut homeostasis, making them valuable for dietary interventions targeting microbiota regulation and overall gut health.
Proteins and Peptides on Gut Microbiota
Seaweeds are a good source of protein, and the amount of protein can change depending on the type of seaweed and the season when it is collected. In general, the protein content of seaweeds is described as low in brown seaweeds (3%–15% DW), moderate in green algae (9%–33% DW) and high for red seaweeds, reaching levels of up to 47% DW (Fleurence et al., 2018). Seaweeds contain various proteins, such as phycobiliproteins, lectins, and glycoproteins, which serve as substrates for gut bacteria, selectively promoting the growth of beneficial microbial species. These proteins exhibit prebiotic effects by acting as a nitrogen source for gut microbiota, enhancing microbial diversity and metabolic activity (Shannon et al., 2021). Additionally, dietary seaweed proteins influence SCFA production, which is essential for maintaining gut barrier integrity and reducing inflammation (Zang et al., 2023). Seaweed proteins and peptides have been found in various seaweed species, exhibiting antibacterial, antiviral, anticancer, anti-inflammatory, and antioxidant properties, with some peptides inhibiting harmful bacteria and strengthening mucosal defenses to prevent leaky gut syndrome (Zang et al., 2023). Moreover, these bioactive peptides regulate immune responses by modulating cytokine production, which helps maintain gut homeostasis and prevents inflammatory disorders such as irritable bowel syndrome and colitis. Specific seaweed sources such as red seaweeds (Porphyra, Palmaria palmata) are rich in phycobiliproteins with antioxidant and prebiotic properties, while brown seaweeds (Undaria pinnatifida, Laminaria) contain lectins and glycoproteins known for their antimicrobial and immunomodulatory effects. Green seaweeds (Ulva, Enteromorpha) also provide unique peptides that positively influence gut microbial diversity (Thiviya et al., 2022). Most seaweed species provide all essential amino acids, such as histidine, methionine, leucine, and lysine, which humans need to get from their diet since they cannot produce them naturally. The ability of seaweed-derived proteins and peptides to promote beneficial bacteria, support gut barrier function, and regulate immune responses makes them valuable components in functional foods.
Seaweed-Derived Vitamins, Minerals, and Their Influence on Gut Microbiota
Seaweeds are rich in essential vitamins and minerals that play a crucial role in maintaining gut microbiota balance and overall digestive health. These marine plants contain significant amounts of vitamins A, C, E, K, and B-complex (including B12), along with essential minerals such as iodine, calcium, magnesium, iron, zinc, and selenium, which contribute to microbial diversity and gut homeostasis (Bekah et al., 2023; Hagan & Anyangwe, 2023).
Vitamins derived from seaweeds, particularly vitamins C and E, exhibit strong antioxidant properties that help reduce oxidative stress in the gut, promoting the survival of beneficial bacteria while inhibiting the growth of pathogenic species (Nemoto et al., 2017). Vitamin A plays a key role in immune system regulation and gut barrier integrity, supporting the growth of Bifidobacterium and Lactobacillus while reducing inflammation-related dysbiosis. Additionally, B vitamins, including B12 (which is naturally rare in plant-based sources but present in some seaweeds), contribute to energy metabolism in gut bacteria, supporting their growth and metabolic functions (Shang et al., 2016). Minerals such as iodine, magnesium, and zinc have also been shown to influence gut microbiota composition. Iodine, an essential element for thyroid hormone synthesis, indirectly affects gut microbial balance through its role in metabolism and immune function. Magnesium supports microbial diversity by enhancing the activity of beneficial bacteria and reducing inflammation in the gut lining. Zinc is known for its role in gut integrity and immune modulation, preventing gut permeability issues that can lead to dysbiosis (Lee et al., 2018).
Conclusion
The intricate relationship between gut microbiota and various bodily functions underscores the importance of studying microbiota functionality today. In line with this, current research has primarily delved into the prebiotic effects of marine compounds, particularly complex polysaccharides. Exploring dietary interventions that modulate gut microbial composition and function for enhanced host health is a promising avenue. Seaweeds, which are rich in prebiotic compounds, present an intriguing option for fostering selective metabolism among gut commensals. These efforts can involve in vitro and in vivo experiments aimed at elucidating how host organisms utilize seaweed components and their secondary bioactive metabolites. Incorporating seaweed into the diet as a functional food can improve gut microbiota diversity, promote digestive health, and provide essential micronutrients necessary for overall well-being. However, further human studies are needed to determine the optimal intake levels and bioavailability of these nutrients from seaweed sources. While preclinical studies strongly support the benefits of seaweed-derived bioactive compounds, more human clinical trials are necessary to establish their efficacy and determine optimal consumption levels. Their potential as functional food highlights the importance of further research to optimize their use in human diets. Future studies should focus on standardizing seaweed-derived functional food formulations, assessing long-term safety, and expanding clinical trials to validate their therapeutic applications.








