Introduction
Pantothenic acid (PA) is also known as vitamin B5, one of the main components in the vitamin B complex. PA is a precursor of coenzyme A (CoA), which is responsible for acetylation process in biochemical reactions (Blanco & Blanco, 2022) and it is an essential component in energy production and lipid metabolism in animal body. CoA can be acetylated to acetyl-CoA by fatty acid β-oxidation (Vasiljevski et al., 2018) and acetyl-CoA plays a key role in energy production through rebuilding citrate from oxaloacetate, which is a byproduct in tricarboxylic acid cycle (Abdel-Salam et al., 2018). Furthermore, acetyl-CoA can be converted into malonyl-CoA as a basic unit for fatty acid synthesis (Clarke & Nakamura, 2004). Different amino acids are associated with the synthesis and reaction of PA and CoA in the body. The biosynthesis of CoA requires cysteine along with PA and the biosynthesis of cysteine requires serine (O’Toole & Cygler, 2003; Rabeh & Cook, 2004). In the mitochondrial tricarboxylic acid cycle, amino acids such as histidine are also converted to α-ketoglutarate and utilized (Lemire et al., 2010). Tryptophan is a precursor of nicotinamide dinucleotide, which is involved with the tricarboxylic acid cycle and electron transport chain (Connell et al., 2021). Thus, PA is vital for maximizing animal growth and facilitating the utilization of nutrients, especially lipids. However, shrimp lack the ability to synthesize PA and must be supplied through feed to meet the requirements. The PA deficiency symptoms reported in aquatic animals include growth retardation, lethargy and mass mortality (Raggi et al., 2016). Shiau & Hsu (1999) reported that grass shrimp (Penaeus monodon) fed low PA supplemented diets showed growth retardation, low survival and high levels of body lipid. In fish, Jian carp (Cyprinus carpio var. Jian) and blunt snout bream (Megalobrama amblycephala) fed PA deficient diets exhibited lower growth, feed utilization and high levels of body lipid (Qian et al., 2015; Wen et al., 2009). Thus, dietary PA requirement should be met for maximum growth and lipid utilization. Dietary requirement for juvenile grass shrimp and fleshy prawn (Penaeus chinensis), has been estimated at approximately 100–140 mg kg–1 and 100 mg kg–1 respectively, to optimize growth and feed efficiency (Shiau & Hsu, 1999; Tiebin et al., 1995). Freshwater species such as Jian carp and blunt snout bream typically exhibit lower requirements in the range of 23–24 mg kg–1 (Qian et al., 2015; Wen et al., 2009). Consistent with the dietary requirements reported for other penaeid shrimp species, juvenile fleshy prawn appear to exhibit a comparatively higher PA requirement than fish species. Shrimps reflect their heightened metabolic activities and unique physiological traits such as exoskeleton formation and immune competence (NRC, 2011).
PA can be found in almost every feed ingredient; however, ingredients do not contain PA with similar concentrations (Casas, 2007). The PA levels in nuts and grains are known as 66–74 mg kg–1, however, in dehulled soybean meals and fish meals are 15–16 mg kg–1. Thus, PA level in the feeds can be different by the formulation and processing of ingredients. For successful aquaculture, the vitamin requirements depending on aquatic animals must be fulfilled in the feed. Vitamins including PA are supplied in feeds in the form of premixes that are pre-formulated to meet the requirements for ease of use. However, dietary PA requirements are variable for different species. Water-soluble vitamins including PA are easily excreted from the body and are known to be non-toxic, so commonly used in feed in higher levels than the requirements to prevent deficiencies (Mai et al., 2022). However, vitamins are expensive ingredients and supplementing more than the requirements in animals can make less competitive in the aquaculture product market, caused by increasing in feed cost. In addition, an excess CoA synthesized from PA in the body can lead negative feedback in the reactions including CoA synthesis from PA (Yun et al., 2000) and fatty acid synthesis from acetyl-CoA (Wang et al., 2022). The growth, feed efficiency, digestive enzyme activity, immunity and anti-oxidant capacity of black carp (Mylopharyngodon piceus) fed diets with excessive levels of PA have been reported to be reduced compared to fish fed diets with adequate levels of PA (Jia et al., 2022). Therefore, for successful aquaculture, the dietary PA requirements of aquatic animals must be accurately determined.
Fleshy prawn is an important species which have high values and demands in East Asia shrimp market including Japan, China and Korea. In 2003 to 2023, fleshy prawn capture production increased to 81,000 tons to 219,000 tons, while aquaculture production decreased gradually 56,000 tons to 27,000 tons (FAO, 2025). Before 1990, fleshy prawn was the second leading shrimp aquaculture production and still forms a part of the marine shrimp aquaculture industry. However, due to the prevalence of infectious diseases including white spot syndrome virus, the fleshy prawn aquaculture industry declined rapidly and was replaced with Pacific white shrimp (Penaeus vannamei) (Hennig et al., 2005). With the development of aquaculture technology and information in feed nutrition, the global aquaculture production of shrimp has rapidly increased. However, Pacific white shrimp is a tropical animal, which is hard to produce all year long in regions with low water temperature season including Korea. Fleshy prawn have gained attention as an aquaculture species again due to the high demand in East Asia and tolerance against low water temperature compared to Pacific white shrimp (Hennig et al., 2005). Although fleshy prawn is the species with the highest aquaculture production after Pacific white shrimp and grass shrimp, to the best of our knowledge, the dietary PA requirement of fleshy prawn has not been reported. Therefore, this study was conducted to investigate the dietary PA requirement of fleshy prawn post-larvae and its effects on growth, lipid metabolism and amino acid profile in the body.
Materials and Methods
Prawns (< 0.001 g) were purchased from a domestic hatchery (Taean, Korea) and reared until they reached the appropriate size for the experiment. Prawns were fed a commercial diet (Sajo Donga One, Seoul, Korea) with 39.6% crude protein and 7.9% crude lipid during the acclimation periods. The calcium pantothenate used as the PA source was purchased from Vixxol (Gunpo, Korea). A basal diet (designated as P0) contained 45% crude protein and 11% crude lipid, formulated without PA supplementation. Four other diets were prepared by supplementation of 100, 200, 300 and 400 mg PA kg–1 (designated as P100, P200, P300 and P400, respectively) into P0 diet. The PA concentrations determined by high-performance liquid chromatography (HPLC) were 16, 107, 226, 317 and 424 mg kg–1 diet in P0, P100, P200, P300 and P400, respectively. The dough made by mixing all the ingredients with fish oil and distilled water was pelletized into 1–2 mm pellets (SP-50, Kumkang Engineering, Daegu, Korea). The pellets were dried at 25°C for 8 h using a feed dryer (SI-2400, Shinil General Dryer, Daegu, Korea) and then stored at –25°C until use. The experimental diets were ground to 50–100 μm size. The diet formulation and proximate compositions analyzed by the method of AOAC (2005) are provided in Table 1.
3) Mixture of calcium pantothenate (Vixxol, Gunpo, Korea) and cellulose to obtain graded levels of pantothenic acid.
5) Each kg–1 of mineral premix contains: 12.50 g C4H2FeO4, 12.00 g MnSO4, 20.00 g FeSO4, 6.00 g CuSO4, 0.75 g CoSO4, 25.00 g ZnSO4, 0.75 g Ca(IO3)2, 80.20 g MgSO4 and 0.75 g Al2O3. The mixture was prepared to 1 kg using cellulose.
6) Each kg–1 of vitamin premix contains: 2.4 g retinol, 0.002 g cholecalciferol, 32.0 g tocopherol, 20.0 g menadione, 25.0 g ascorbic acid, 5.0 g thiamine, 10.0 g riboflavin, 32.5 g nicotinic acid, 400.0 g myo-inositol, 20.0 g pyridoxine, 0.5 g biotin, 0.8 g folic acid and 0.5 g cobalamin. The mixture was prepared to 1 kg using cellulose.
A total of 750 prawns (initial mean body weight: 3 mg) were randomly distributed into 15 tanks (10 L), with three replicates per treatment and 50 individuals stocked per tank fed the experimental diets for 40 days. Water quality parameters were measured daily and maintained as follows: salinity, 34.2 ± 0.1 psu; dissolved oxygen, 6.0 ± 0.2 mg L–1; water temperature, 25.9 ± 0.4°C; pH, 7.6 ± 0.2; ammonia level, 0.02 ± 0.01 mg L–1 and 12-hour light/dark photoperiod was maintained using artificial lighting conditions. Prawns were fed their respective diets four times daily (08:00, 12:00, 16:00 and 20:00 h) in the range from 20% biomass at the beginning to 10% biomass at the end of feeding trial based on specific growth rate (SGR). Every 3 days, feces were removed by siphon and 70%–80% of the rearing water in each tank was exchanged with pre-heated clean seawater.
At the end of feeding trial, prawns in each tank were individually weighed and counted. Then, the final body weight (FBW), SGR, feed conversion ratio (FCR), protein efficiency ratio (PER) and survival were calculated. Twelve prawns per tank were captured and anesthetized with ice-cold water. Three prawns were dissected for hepatopancreas collection. Hepatopancreas were immediately frozen into liquid nitrogen and kept in –80°C until use for quantitative reverse transcription polymerase chain reaction analysis. Three other prawns were immediately frozen and used for whole-body PA analysis. Cephalothorax of three other prawns were collected and fixed in Davidson’s solution (consisting of 37% formalin, 100% ethanol, glacial acetic acid and distilled water) used for histopathological analysis. Three other prawns were used for amino acid profile analysis.
Hepatopancreas was homogenized using a tissue homogenizer (Kimble Chase, Vineland, NJ, USA) and RNA was extracted from the tissue suspension. The quantity and quality of total RNA were measured using a Nano drop 2000 (Thermo Fisher Scientific, Waltham, MA, USA). The 260/280 nm ratio for all samples was between 1.85 and 1.96. cDNA synthesis was performed using the PrimeScript™ RT reagent kit (Takara Bio, Kusatsu, Japan). β-actin was used as a reference gene. Gene expression analyses of acyl-CoA binding protein (ACBP), fatty acid binding protein (FABP) and target of rapamycin (TOR) were performed using TB green premix Ex Taq (Takara Bio) on a thermal cycler dice (Real time system III, Takara Bio). The reaction was performed in 20 μL sample containing 10 μL of TB green master mix, 7.2 μL RNase free dH2O, 2 μL cDNA template and 0.4 μL each pair of primer. The PCR was performed for one cycle at 95°C for 30 s, followed by 40 cycles at 95°C for 10 s and 55°C for 30 s. The results were evaluated using the 2–ΔΔCT method. Primer sequences of analyzed genes are provided in Table 2.
PA was extracted from the feed and hepatopancreas by sonication with 20 mM KH2PO4 (P5655, Sigma-Aldrich, St. Louis, MO, USA) solution for 30 min. The samples were centrifuged (1,008×g, 10 min) and the supernatant was filtered through a 0.45 μm membrane filter. An Alliance E2695 HPLC (Waters, Milford, MA, USA) system equipped with a C18 (4 μm, 4.6 × 250 mm) column and UV/Vis detector was used to determine the PA concentration in the samples. The mobile phase (20 mM KH2PO4, pH 2.1) was eluted at a flow rate of 1.0 mL min–1. A wavelength of 200 nm was used to detect PA. The column temperature during the analysis was 25°C.
The cephalothorax was fixed in Davidson’s solution for 24 h and dehydrated with ethanol (70% to 100%). Samples were embedded in paraffin, sliced with a thickness of 5 μm using with microtome (RM2125RTS, Leica, Bensheim, Germany) and stained with hematoxylin and eosin. The number of blasenzellen cell (B cell), fibrillar cell (F cell) and resorptive cell (R cell) in hepatopancreatic tubules were observed with microscope (DM750, Leica). Total of 20 tubules were examined from each section and three sections were obtained from each prawn.
Amino acids profile of the prawn was analyzed by method of Ding et al. (2023). Sample was homogenized with 1 mL of distilled water. The homogenate was transferred into 10 mL glass tube and then, 1 mL of hydrochloric acid was added. The tube was sealed and heated (150°C, 1 h) and completely dried under nitrogen stream. Dried pellet was re-dissolved with 5 mL of distilled water and filtered (0.45 μm). To derivatization, 100 μL of 1M triethylamine (acetonitrile, ACN) and 100 μL of 0.1M phenyl-isothiocyanate (ACN) was added into 200 μL of the sample and incubated (40°C, 1 h). After incubation, 600 μL of distilled water and 600 μL of hexane were added into the mixture and then, centrifuged (21,952×g, 10 min). For amino acids analysis, 10 μL of the infranatant was injected into HPLC (Waters) system equipped with a C18 (4 μm, 4.6 × 250 mm) column and UV/vis detector. A wavelength of 254 nm was used to detect amino acids in the sample. Mobile phase A (50 mM ammonium acetate, pH 6.5) and B (ACN:distilled water = 8:2) were flowed (95:5) at a flow rate of 1.0 mL min–1 following conditions: 0–8 min, 87:13; 8–20 min, 84–16; 20–23 min, 67:33; 23–35 min, 60:40; 35–45 min, 0:100; 45–60 min, 95:5. The column temperature during the analysis was 36°C.
A randomized complete block design was used to assign the experimental tanks. All data were expressed as mean ± SD. Before statistical analysis, the percentage data were transformed to arcsine values. One-way analysis of variance (ANOVA) was plotted to establish the differences among dietary treatments. Before conducting ANOVA, the data normality and homogeneity of variance were tested using the Shapiro-Wilk test and Levene’s test, respectively. When ANOVA identified differences between the means of dietary groups, the significant differences were compared using Tukey’s HSD post-hoc test (p < 0.05). An orthogonal polynomial contrast was performed to determine the effect was linear or quadratic. Statistical analysis was determined using SPSS 24.0 (IBM, Armonk, NY, USA). The optimum dietary PA requirements were estimated with broken-line regression analysis using SGR and PA levels in whole-body. Regression analysis was performed with SigmaPlot version 14.0 (Systat Software, San Jose, CA, USA).
Results
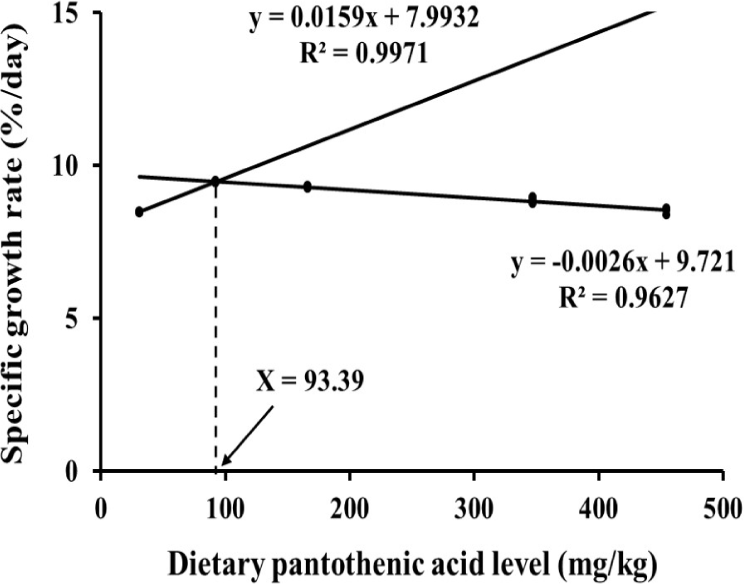
FBW and SGR in P100 and P200 groups were significantly higher (p < 0.05) than those in P0, P300 and P400 groups (Table 3). FCR in P100 group was significantly lower (p < 0.05) than that in P0 group. PER in P100 group was significantly higher (p < 0.05) than in P0 group. FBW, SGR and PER of the prawns were elevated when dietary PA level increased from P0 group to P200 group, however, decreased again to P400 group while FCR showed opposite results. Survival of the prawns were significantly higher in PA supplemented groups compared with P0 group. Based on SGR, optimal dietary PA requirements for fleshy prawn post-larvae are 93.39 mg kg–1 (Fig. 1).
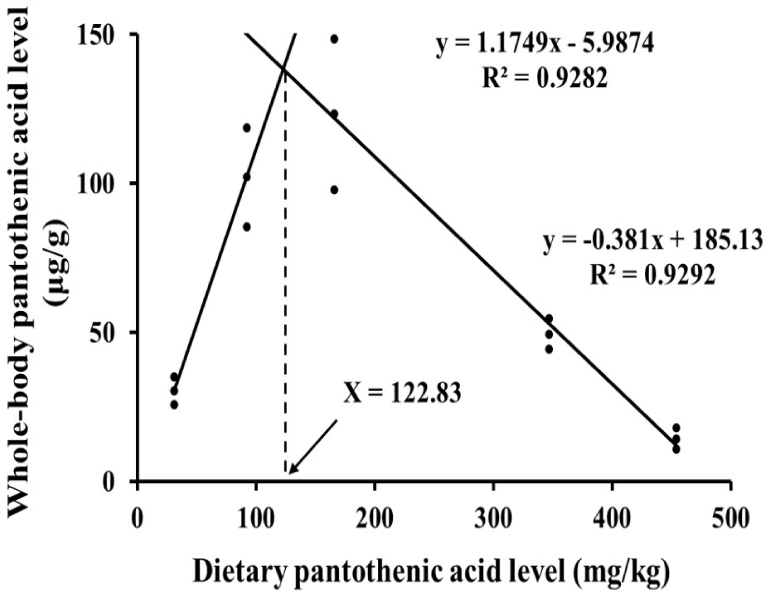
PA level in prawn whole-body was increased from P0 group to P200 group and then decreased again to P400 group (Table 4). Whole-body PA level in P200 group was significantly higher (p < 0.05) than in P0 group. Moreover, P100 and P200 groups showed significantly higher (p < 0.05) whole-body PA level compared to P400 groups. Based on whole-body PA levels, optimal dietary PA requirements for fleshy prawn post-larvae are 122.83 mg kg–1 (Fig. 2).
In prawn hepatopancreas, B cell counts per tubule in P100 group were significantly higher (p < 0.05) than that in P0 group. P100 and P200 groups showed significantly higher (p < 0.05) LD counts per tubule compared to P0 group. R cell counts per tubule in P100 and P200 groups were significantly higher (p < 0.05) than those in P0 group.
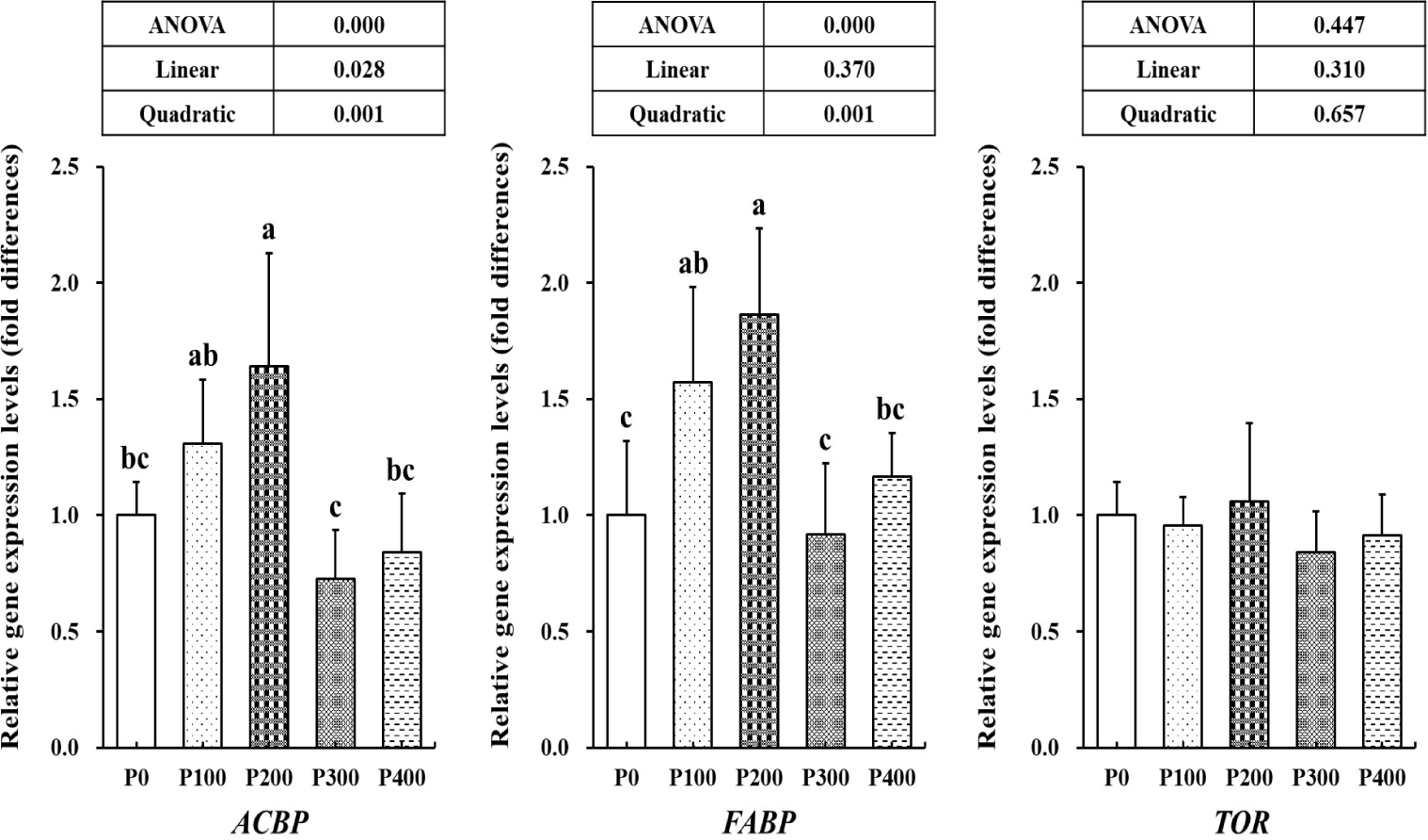
P200 group showed significantly higher (p < 0.05) ACBP gene expression level compared to P0 group (Fig. 3). FABP gene expression level in P100 and P200 groups were significantly upregulated (p < 0.05) than in P0 group. TOR gene expression level was not significantly different (p > 0.05) among all the groups.
In amino acid profiles in prawn whole-body, cysteine level in all the PA supplemented groups were significantly lower (p < 0.05) than that in P0 group (Table 5). Histidine, serine and tryptophan levels in P200, P300 and P400 groups were significantly lower (p < 0.05) than that in P0 group. P300 group showed significantly higher (p < 0.05) isoleucine, methionine and threonine levels compared to P0 group. Valine level in P400 group was significantly lower (p < 0.05) than that in P0 group.
Discussion
PA is an essential trace element for the growth and survival of aquatic animals. To maximize the yield in shrimp farming, dietary PA levels should be customized according to life stage and culture environment of shrimps. However, the lack of recently published data is a major concern to establish accurate dietary PA level for diet formulations (Liu et al., 2024). Therefore, the current study demonstrates that dietary PA requirement of fleshy prawn post-larvae range between 93–123 mg kg–1. The practical significance of determining an optimal dietary PA level for fleshy prawn post-larvae is substantial for cost-effective feed formulation and sustainable aquaculture production. Tailoring PA inclusion to this optimal range can maximize growth performance and feed efficiency, reducing feed costs by avoiding unnecessary supplementation above the optimal level that leads to diminished returns (Liu et al., 2024). This precision in formulation helps decrease wasted feed and nutrient excretion, promoting environmentally sustainable production by minimizing nutrient pollution in culture systems (Koshio, 2010). Moreover, optimizing PA levels supports improved survival and health of post-larvae, thereby enhancing overall farm productivity and profitability in shrimp farming operations. Similar to this study, Shiau & Hsu (1999) discovered that dietary PA requirement for grass shrimp was 100–139 mg kg–1, based on the growth performance. In this study, the growth and feed efficiency of the prawns were significantly increased by dietary PA supplementation at 100–200 mg kg–1. While in prawns fed PA 300–400 mg kg–1 supplemented diet, the growth performance was comparable to that in prawns fed a diet without PA supplementation. Mechanistically, the reduced growth performance observed at high dietary PA levels (> 300 mg kg–1) may be attributed to enzyme kinetics and adenosine triphosphate (ATP) competition affecting CoA biosynthesis. Excessive PA supplementation likely leads to surplus CoA accumulation, which competitively inhibits pantothenate kinase, the rate-limiting enzyme in CoA synthesis by binding ATP, thereby reducing enzyme activity and CoA production (Leonardi & Jackowski, 2007). This ATP-dependent feedback inhibition disrupts critical metabolic pathways reliant on CoA, including branched-chain amino acid catabolism. This reduction in CoA availability could impair energy metabolism and growth. These hypotheses about ATP competition and enzymatic inhibition in PA uptake and metabolism should be experimentally tested in future studies, for example, through enzyme activity assays and metabolic flux analysis in prawn hepatopancreas under varying PA levels and also competitive inhibition of CoA synthesis and other ATP-dependent metabolic pathways by the surplus CoA generated by the excessive PA supplementation. PA is converted to CoA by combining with cysteine and ATP via enzymatic pathways including pantothenate kinase and phosphopantothenoylcysteine synthetase (Genschel, 2004). The activity of pantothenate kinase is known to decrease as CoA competitively binds to ATP during CoA biosynthesis (Yun et al., 2000). Thus, high cysteine levels in the amino acid profiles of the prawns fed P0 diet in this study may indicate low CoA synthesis in the body. In addition, the growth of the prawns in P400 group was comparable to P0 group in this study, which may indicate a potential disruption of PA metabolism especially in CoA production. Since CoA is a cofactor for branched-chain amino acids (BCAA) hydrogenase, PA contributes to BCAA catabolism in the body (Neinast et al., 2019). An increase of BCAA levels in the body is one of the well-known symptoms of CoA deficiency (Blair et al., 2021). Additionally, the altered amino acid profiles in PA supplemented prawns, such as decreased cysteine and changes in histidine, serine and tryptophan, suggest important biological consequences for protein synthesis and growth. Lower cysteine in supplemented groups may reflect higher utilization for CoA synthesis. Changes in essential amino acids could influence overall protein turnover and muscle development, potentially impacting prawn growth rates and flesh quality, which are critical for market value in aquaculture (Qian et al., 2015). A more thorough discussion of these amino acid alterations could inform how dietary PA modulates nutrient availability for protein accretion and the biochemical quality of aquaculture products, highlighting the need for integrated nutritional and biochemical assessments in feed development. Along with this, PA deficiency could lead to the light body coloration and formation of light and thinner exoskeleton of shrimps, specially making them more vulnerable to predators and other environmental stressors (Liu et al., 2024). The whole-body PA levels of prawns in P100 and P200 groups were significantly higher than in P0 group, while in P300 and P400 groups were comparable to P0 group. Growth retardation has also been reported in fish when feeding higher than optimal levels of dietary PA. The growth of black carp improved with increasing levels of PA in the feed from 0, 5 and 10 mg kg–1, and then decreased again with increasing levels of 20, 40 and 80 mg kg–1 (Jia et al., 2022). However, growth retardation due to high dietary PA was not observed in grass shrimp and Malabar grouper (Epinephelus malabaricus), suggesting that this may depend on factors such as species and growth stage (Lin et al., 2012; Shiau & Hsu, 1999). This result suggests that supplementing excessive PA above 300 mg kg–1 into fleshy prawn feeds may lead to loss of growth and feed efficiency in post-larval stage.
The whole-body PA levels of prawns in this study were notably higher in P100 and P200 groups compared to P0 group. Furthermore, the counts of B cells, F cells and R cells in hepatopancreatic tubules increased in P100 and P200 groups, as well as in the whole-body PA levels. The hepatopancreas is a central organ of nutrient digestion, storage and metabolism in prawn (McGaw & Curtis, 2024). F cells are responsible for the synthesis of digestive enzymes, B cells for the secretion of digestive enzymes and R cells for the storage of digested nutrients (Iswarya et al., 2022). These results suggest that supplementing 100–200 mg kg–1 of PA to the feeds facilitate the digestion and storage of dietary nutrients in fleshy prawn post-larvae, which could benefit the growth. Wen et al. (2009) reported that the activities of digestive enzymes trypsin, lipase and amylase in the intestine of Jian carp were highest when 20–60 mg kg–1 of PA was supplemented into the feeds. The activities of the digestive enzyme protease, lipase and amylase were reported to be highest in Indian major carp (Catla catla) when PA was supplemented at 30 mg kg–1 into the feeds (Khan & Khan, 2022). However, the whole-body PA levels and the counts of B cells, F cells and R cells in the hepatopancreas were comparable at P300 and P400 groups as at P0 group. This may be due to the ATP group of CoA binding competitively with ATP-dependent enzymes involved in the absorption of PA and nutrients in the intestine of fleshy prawn. Therefore, further studies are needed to investigate the effects of supplementing more than 300 mg kg–1 of PA to the fleshy prawn diet on the CoA synthesis, digestive enzyme activities associated in nutrient absorption in the intestinal epithelium and nutrient accumulation in the hepatopancreas.
As a precursor of CoA, PA is essential in lipid metabolism in the body (Rucker et al., 2008). CoA combined with fatty acid and forms to acyl-CoA (Schulz, 2013). ACBP is a protein that binds acyl-CoA esters and acyl-CoA is susceptible to β-oxidation that form acetyl-CoA (Knudsen et al., 2000). FABP is responsible for cellular transportation (Furuhashi & Hotamisligil, 2008). Thus, the upregulated ACBP and FABP gene expression levels of prawns in this study suggested facilitated lipid metabolism. The effects of dietary PA supplementation on lipid metabolism in aquatic animals have already been well documented in previous studies. The hepatopancreatic lipid levels in grass shrimp were reported to decrease by dietary PA supplementation (Shiau & Hsu, 1999). Qian et al. (2015) reported that body and liver lipid levels in blunt snout bream were decreased by dietary PA supplementation. In β-oxidation of fatty acids, cofactors such as NADH and FADH2 were also produced along with acetyl-CoA (Bartlett & Eaton, 2004). These are essential compounds in energy production through the tricarboxylic acid cycle and electron transport chain in the mitochondria that allows rapid growth (Kumari, 2018). Thus, the growth of fleshy prawns in P100 and P200 groups may be promoted by upregulation of lipid utilization.
Conclusion
These findings suggest that the optimal dietary PA concentration for fleshy prawn post-larvae would be 93–123 mg kg–1 based on the growth performance and whole-body PA level. High levels of PA supplementation in feeds above 300 mg kg–1 may not be beneficial in growth, feed efficiency, lipid metabolism and hepatopancreatic development of fleshy prawn in post-larval stage.








