Introduction
In many countries, fisheries contribute to food security and poverty reduction among populations living in coastal areas, along riverbanks, and on small islands. Inland fish play a critical role as an essential food and nutritional resource, particularly contributing to the economies of rural areas in developing countries (Welcomme et al., 2010). Thus, to contribute to achieving the 2030 UN Sustainable Development Goals of “Zero Hunger” and “Life Below Water,” it is crucial to ensure the sustainability of fisheries. Indeed, understanding fish population dynamics and conducting stock assessments are fundamental for effective fishery management. However, many inland fisheries have not been assessed due to data limitations.
African countries face challenges related to their inland water resources due to pollution, overfishing, climate change, and eutrophication, which are primarily caused by human activities (Tesfaye et al., 2021). Additionally, there is a lack of stock assessments in numerous freshwater fisheries, even when data collection and analysis capabilities exist (Tesfaye et al., 2021). Therefore, credible scientific evidence is imperative for assessing and managing fishery resources to ensure their effective sustainability.
According to the General Directorate of Fishery Resources in Burkina Faso, the local fishery yield in 2020 is estimated to be 29,752 tons (Compaoré et al., 2023). The national demand for fish is projected to reach 130,000 tons per year. Several challenges affect most reservoirs, including the reduced numbers of rangers, non-compliance with fishing bans during fish spawning periods, and the use of prohibited fishing gear such as purse seines, which negatively affect the dynamics of various fish species. Despite these challenges, few studies have focused on fish stock assessment in Burkina Faso.
Oreochromis niloticus, known as Nile tilapia, is a Cichlidae species. This species is omnivorous and feeds on a variety of foods such as insects, plants, smaller fish, and organic debris. Furthermore, it is characterized by its frequent breeding, occurring three to four times annually (Amponsah et al., 2016). In Burkina Faso, Nile tilapia is one of the most economically important fish, with a high proportion of its specimens caught in many fisheries, such as the Samendéni reservoir (Compaoré et al., 2021; Minoungou et al., 2020), Hippo Pond (Ouedraogo et al., 2021), Balla reservoir, and Bama reservoir (Compaoré et al., 2021). Different fishing gears, such as gillnets with 10 to 40 mm mesh sizes, traps, longlines, and cast nets, are used to exploit this species. Due to its high ability to adapt to various aquatic conditions, O. niloticus is used in local aquaculture systems to bridge the gap in high fish demand in Burkina Faso (Sissao et al., 2019). Thus, most studies on this species have focused on its biological aspects (Compaoré et al., 2021; Minoungou et al., 2020; Ouedraogo et al., 2021) and feed formulation (Sissao et al., 2019). However, few studies have been conducted on growth parameters and stock assessment for sustainable management of this species. The stock assessment of O. niloticus is necessary because it will provide more information about the dynamic of this species and how to manage it properly. Thus, the main aim of this study was to determine the population parameters of O. niloticus and evaluate its exploitation rate in the Samendéni reservoir, which is crucial for effective fishery management. In fact, the Samendéni reservoir, the third largest reservoir in Burkina Faso, has undergone many changes since its opening for exploitation in 2018, such as reduced numbers of rangers, non-compliance with fishing bans during fish spawning periods, and the use of prohibited fishing gear such as purse seine (Ouedraogo et al., 2024). Furthermore, no study has reported the population parameters and stock assessment indicators of O. niloticus in this reservoir.
Materials and Methods
Samendéni reservoir is located in the West Sudanian savannah in the Region of Hauts-Bassins, Burkina Faso and it is built on the Mouhoun River (Fig. 1). The location is between latitudes 11˚23’ and 11˚19’ North and longitudes 4˚34’ and 4˚46’ West. It is the third largest in the country. This reservoir includes a catchment area and an irrigated perimeter of 21,000 hectares with a total capacity of 1.05 billion m3. The Samendéni reservoir area is approximately 68,202 hectares and has a tropical savanna climate with air temperatures ranging from 23.50°C to 31.30°C (Kabré et al., 2023). Meanwhile, the mean annual rainfall was 1,075.86 mm from 2010 to 2020 (Kabré et al., 2023). Two distinct seasons characterize the Samendéni reservoir region: a rainy period from June to September, followed by a dry season from October to May. The vegetation mixes wooded savannah, forest, and a dense semi-deciduous gallery forest. Since October 2020, the Samendéni reservoir has been labelled as a Ramsar site (number 2,439) in Burkina Faso.
Each month, a minimum of 30 fish were collected from October 2021 to September 2022 at three sampling sites: Dioufoulma, Badoville, and Sadina in the Samendéni reservoir. Many fishermen were working in these three sampling sites. Therefore, fish were randomly collected from numerous fishermen to ensure a representative sample of different size classes. All specimens collected were sexed, and were identified using the relevant taxonomic keys by Paugy et al. (2003). After sampling and preservation in ice, all O. niloticus specimens were transported to the fish laboratory at Nazi Boni University for further analysis.
In the laboratory, the total length (TL) of each individual of O. niloticus was measured from the tip of the mouth to the extended tip of the caudal fin using a fish-measuring board. The weight of each fish was also measured to the nearest 0.1 g with a digital balance. A histogram was then used to examine the distribution of O. niloticus individuals based on their TL, with the length data frequency pooled at 1 cm intervals. The sex ratio, which is equal to the total number of males divided by the total number of females, was also estimated. The observed sex ratio was then compared to the expected sex ratio (1:1) to check for any statistical difference using the chi-square test (Sokal & Rohlf, 1987).
The relationship between length and weight was described by the equation W = a × TLb (Ricker, 1975), where W represented the total weight (g), and TL the total length (cm). The logarithmic form of the equations was used to estimate the intercept and the slope as follows: ln(W) = ln (a) + b × ln (TL), with ln(a) the intercept of the regression line and “b”, the slope. The deviation of “b” from the isometric value 3 was tested with student t-test whether O. niloticus individuals had an isometric growth in the Samendéni reservoir. To calculate the “t” value, this formula was used:
yi weight value of sample i, y̅i, mean weight of all samples, xi length value of sample i and x̅i the mean length of all samples and n total sample size, respectively (Awasthi et al., 2015). The resulting “t” value was then compared to the t-table value for (n-2) degree of freedom at a 5% significance level. If “b” was not significantly different from 3 (p > 0.05), it was concluded that there was an isometric growth. However, if there was a significant difference between “b” and 3 (p < 0.05), it indicated either negative (b < 3) or positive allometric growth (b > 3; Ricker, 1975).
In tropical areas, the length-based method has been widely used to assess the fish stock compared to age data (Osei et al., 2021). The preference for length data is due to the reported difficulty in ageing tropical fish (Osei et al., 2021). Thus, many mathematical models using length frequency data have been developed to estimate growth and mortality parameters (Gayanilo & Sparre, 2005; Osei et al., 2021). Furthermore, in the tropics, seasonal fluctuations of the environment are less marked than in temperate areas. Therefore, this study used the length-based method through the electronic length frequency analysis (ELEFAN) incorporated in the FISAT software program (FISAT II; FAO, Roma, Italia) to estimate the growth parameters.
ELEFAN is a widely used tool for fitting the Von Bertalanffy growth function and estimating growth and mortality parameters in fisheries with limited data, especially in tropical regions. Thus, the total length TL values collected monthly from October 2021 to September 2022 were grouped into a class interval of 1 cm to calculate the population demographic parameters using the ELEFAN routine incorporated into FISAT software (FISAT II; FAO; Pauly & Gayanilo, 1997). Therefore, some parameters, such as the asymptotic length (L∞), the coefficient of growth (K), natural mortality rate (M), fishing mortality rate (F), and exploitation rate (E), were estimated using ELEFAN program incorporated in FISAT. The growth parameters L∞ and K were obtained based on the highest Rn value representing the best fitting of goodness. Thus, the growth of O. niloticus in the Samendéni reservoir was estimated following the von Bertalanffy function (VBGF):
with Lt, the fish size at a specific time t, L∞ asymptotic length of the fish, K growth coefficient representing the rate at which a fish attains its maximum size, t the age of the fish, t0 the age at which the fish length is null.
To determine t0, the theoretical age at length zero, Pauly’s empirical equation (Pauly, 2024) was used:
The life-span of fish (tmax) was also estimated as tmax = (3/K) (Taylor, 1958).
The growth of O. niloticus from the Samendéni reservoir was compared to previous studies by calculating the growth performance index Ф′. Thus, the growth performance index Ф′ was computed using asymptotic length L∞ and growth coefficient (K):
The size at first sexual maturity (Lm50) is the size at which 50% of O. niloticus individuals were mature. This size was determined during the spawning season. Gonad and oocyte maturation stages were identified following the method described by Brown‐Peterson et al. (2011). Thus, individuals at stage 1 were immature, and individuals from stages 2 to 5 were mature. The data were divided into several size groups, and the percentage of mature individuals in each category was computed using logistic regression:
with P, percentage of mature individuals, Lt total length, “α” and “β” are the parameters of the logistic equation. The formula was transformed to ln[P/(1-P)] = α + β × Lt. Thus, by substituting P = 50% in the equation, Lm50 = –α/β.
The length-converted curve is a linear regression incorporated in FISAT and used to estimate the total mortality rate Z, the natural mortality rate M, the fishing mortality rate F, and the exploitation rate E of a fish species. This regression is a plot of ln(N/△t) = ψ+μ×t, where N is the number of fishes in a given length class, △t is the time needed for the fish to grow through that length class, ψ is the intercept, t is the mean relative age of the fishes in that class and μ, is with a sign changed, an estimate of the total mortality rate Z (Pauly, 1990). Growth parameters from the Von Bertalanffy growth function VBGF, such as L∞ and K, are required to estimate:
where Li1 and Li2 are the lower and the upper limits of length class i, respectively. Furthermore, the t value can be obtained as t = (–1/K) × ln(1 – Lt/L∞). In this analysis in FISAT, only the right descending part of the curve was used to determine the total mortality rate Z. Thus, from the highest point of the descending part, a selection then deselection of different points was done until to get the regression line with the highest correlation coefficient r2 corresponding to the best model, with Z = -slope of the regression line. Moreover, the estimation of the natural mortality rate (M) was obtained using the empirical formula of Pauly (1980):
where T = mean surface temperature (29.7°C in this study) (Ouedraogo et al., 2024).
The fishing mortality rate (F) was calculated using the formula:
with Z total mortality rate and M natural mortality rate.
In terms of mortality rates, the exploitation rate (E) representing the percentage of fish captured annually from a population due to fishing activities, was calculated in the ELEFAN program using the formula: E = F/(F + M) = F/Z (Beverton & Holt, 1966); where M was the natural mortality rate and F the rate of fishing mortality. Furthermore, according to Gulland (1971), when the exploitation rate (E) is equal to the optimal exploitation rate (Eopt = 0.5), the exploitation of the stock is optimal (F = M). An exploitation rate E below the optimal exploitation rate (E < 0.5) describes a level of underfishing of the species (F < M). However, when the exploitation rate E is beyond the optimal exploitation rate (E > 0.5), it indicates an overfishing (F > M) of the species.
Pauly’s (1987) method incorporated into FISAT was used to calculate the length probability at the first capture (Lc or L50). The length at first capture Lc is the length at which 50% of O. niloticus individuals in Samendéni reservoir are retained by the fishing gear. Thus, after obtaining the length-converted catch curve, probability data were generated and saved. This probability data file can be employed using either a logistic curve or a moving average to estimate the length at first capture. In this study, the logistic curve was selected because it allows the user to identify at least three data points to be included in the analysis. Therefore, a minimum of three points on the logit plot that displayed the highest correlation coefficient were used to compute L25, L50, and L75.
The virtual population analysis (VPA) method incorporated in FISAT was used to reconstruct the historical dynamics of O. niloticus population based on the catch data through the length converts curve procedure of Jones & van Zalinge (1981). Thus, for this analysis, the length-frequency data, the intercept “a”, the slope “b”, the growth coefficient K, the asymptotic length L∞, the natural mortality rate M, and the fishing mortality rate F values were used as inputs. The results of this analysis were represented by a plot of the reconstructed population (in numbers), the mean stock biomass (kg) by length group, and the fishing mortality for each length group.
To evaluate the influence of various levels of fishing effort on O. niloticus stock, two prediction models were implemented using FISAT II (FAO): the Thompson and Bell model (Thompson, 1934) and the yield per recruit model (Beverton & Holt, 1957). The length-converted Thompson and Bell analysis uses the F-array, corresponding to the fishing mortality rates for each length class as estimated through VPA analysis, and serves as a reference. This analysis evaluates the impact of increasing or decreasing the F-array by a specific factor X. Specifically, it predicts the average long-term catch, assuming that recruitment remains constant. Thus, the Thompson and Bell model was built based on the output of length-based VPA using parameters such as the length group, growth coefficient K, age at zero length t0, asymptotic length L∞, natural mortality rate M, terminal fishing year Ft, intercept “a,” and slope “b” of the length-weight relationship, catch (in numbers) for each length group, and the yield and biomass output of the virtual population analysis. The outputs of this model were predictions of mean biomass, and yield for various combinations of F and M values. In this model, the sum of yields Y= ∑ Yi in weight (kg) was computed from Yi= Ci W̅1 where Ci number cumulative catch for a given mesh size of O. niloticus per length group, the mean body weight W̅1 = and “a” and “b” were the coefficients of the length-weight relationship and Li and Li+1 were the lower and upper limits of the length class, respectively. Furthermore, where the predicted population Ni was given by Ni+1 = Ni × EXP(–(M+Fi) × △ti), and . The biomass was computed from . The F-array table obtained from VPA analysis was also added. F-array corresponds to the fishing mortality rates for each length class. The result from FISAT was used to plot the yields and biomass estimated for a range of F-factors from 0 to 4. In addition, the total mortality was estimated as Zi = M + X × Fi, where X is the multiplier used to raise or reduce the fishing mortality rate sequence, M the natural mortality rate, and Fi the fishing mortality of the length class group i. For this current study, the X-Factor was 1. Furthermore, the terminal fishing mortality Ft obtained from the length converted curve was used as a reference to obtain the new fishing mortality (new F) by multiplying the terminal Ft by the multiplier X. Thus, new F = X × Ft. By this formula, the fishing mortality of Maximum Sustainable Yield FMSY was deducted as FMSY = X × Ft. Furthermore, to determine whether the O. niloticus species was overfished or not, the ratio of Ft/FMSY was calculated. Thus, a ratio of Ft/FMSY over 1 means overfishing. However, a ratio of Ft/FMSY less than 1 indicates an underfishing situation.
A plot illustrating the seasonal pattern of recruitment was obtained by projecting the frequencies backward onto the time axis of a time series of samples following a trajectory determined by the Von Bertalanffy Growth Function (Moreau & Cuende, 1991) using FISAT II. The age at length zero, growth coefficient K, and asymptotic length L∞ were used as inputs.
The relative yield per recruit (Y’/R) of O. niloticus was also assessed using the model of Beverton & Holt (1966) as follows:
where E = F/(F+M) =F/Z exploitation rate, U = 1 – (Lc /L∞) with Lc length at first capture, and m = (1 – E)/(M/K) = (K/Z).
Using the relationship B’/R = (Y’/R)/F, the biomass per recruit (B’/R) was calculated. The first derivation of this function estimated Emax, E10, and E50. Emax is the exploitation rate that maximizes Y/R or Y’/R; E10 and E50 are respectively, the exploitation rate at 10% of the virgin biomass, and the exploitation rate at 50% of the virgin biomass.
Results
A total of 1,268 individuals of O. niloticus were obtained, including 732 males and 536 females as shown in Table 1. The data collected by October, December 2021, April, May, June, and July 2022, both for males, females, and the pooled data, showed a unimodal distribution pattern. However, the data collected in January, March, August, and September 2022 for both males, females, and the pooled data revealed a bimodal distribution. Only, the data collected in November 2021 showed a trimodal distribution pattern (Supplementary Fig. S1). The analysis of both sexes revealed that there were considerably more male specimens than female, resulting in a sex ratio of 1.3656:1. A significant difference was observed between this sex ratio and the expected one (Chi-square test, ᵡ2 = 14.93, df = 1, p < 0.05).
The results obtained from the One-way ANOVA test result indicated that the mean total length of males was significantly higher than that of females (F [1,1266] = 18.8103, p < 0.001). Moreover, the analysis revealed a significant difference in body weight between male and female specimens (F [1,1266] = 18.2334, p < 0.001).
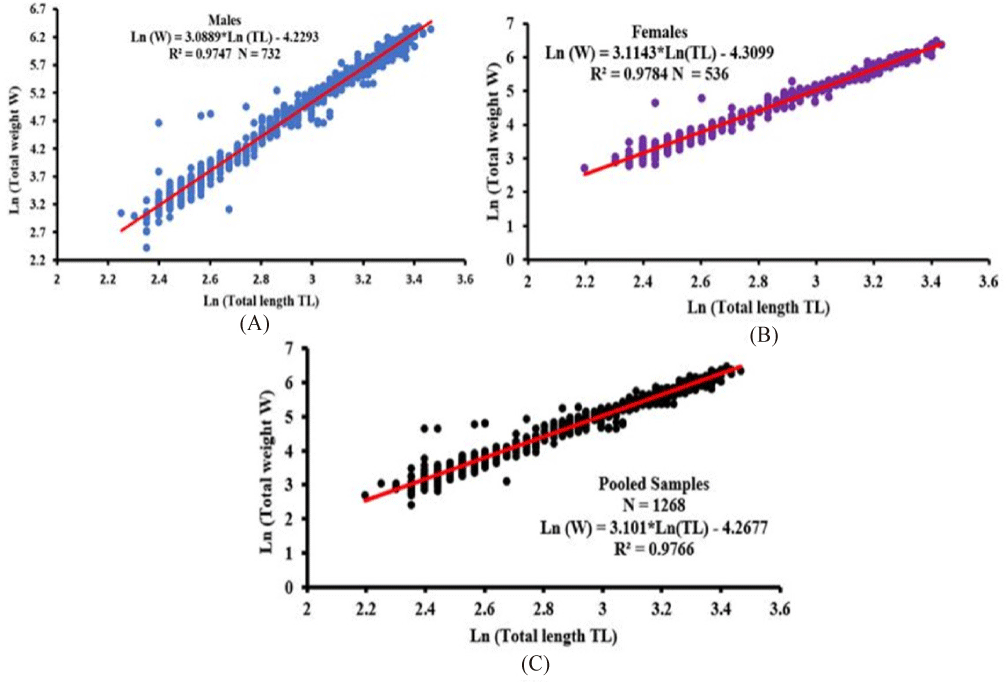
The results of the length-weight relationship of O. niloticus females, males, and pooled samples are shown in Table 2 and Fig. 2. The comparison of the different values of slope “b = 3.0889, 3.1143, and 3.101” to “3” respectively for males (t-test, df = 730, t = 4.816, p < 0.05), females (t-test, df = 534, t = 5.7028, p < 0.05), and pooled samples (t-test, df = 1266, t = 7.4687, p < 0.05), indicated that “b” showed a statistically significant difference from 3 (p < 0.05) for males, females, and pooled samples and b > 3. Therefore, O. niloticus showed a positive allometric growth (Table 2).
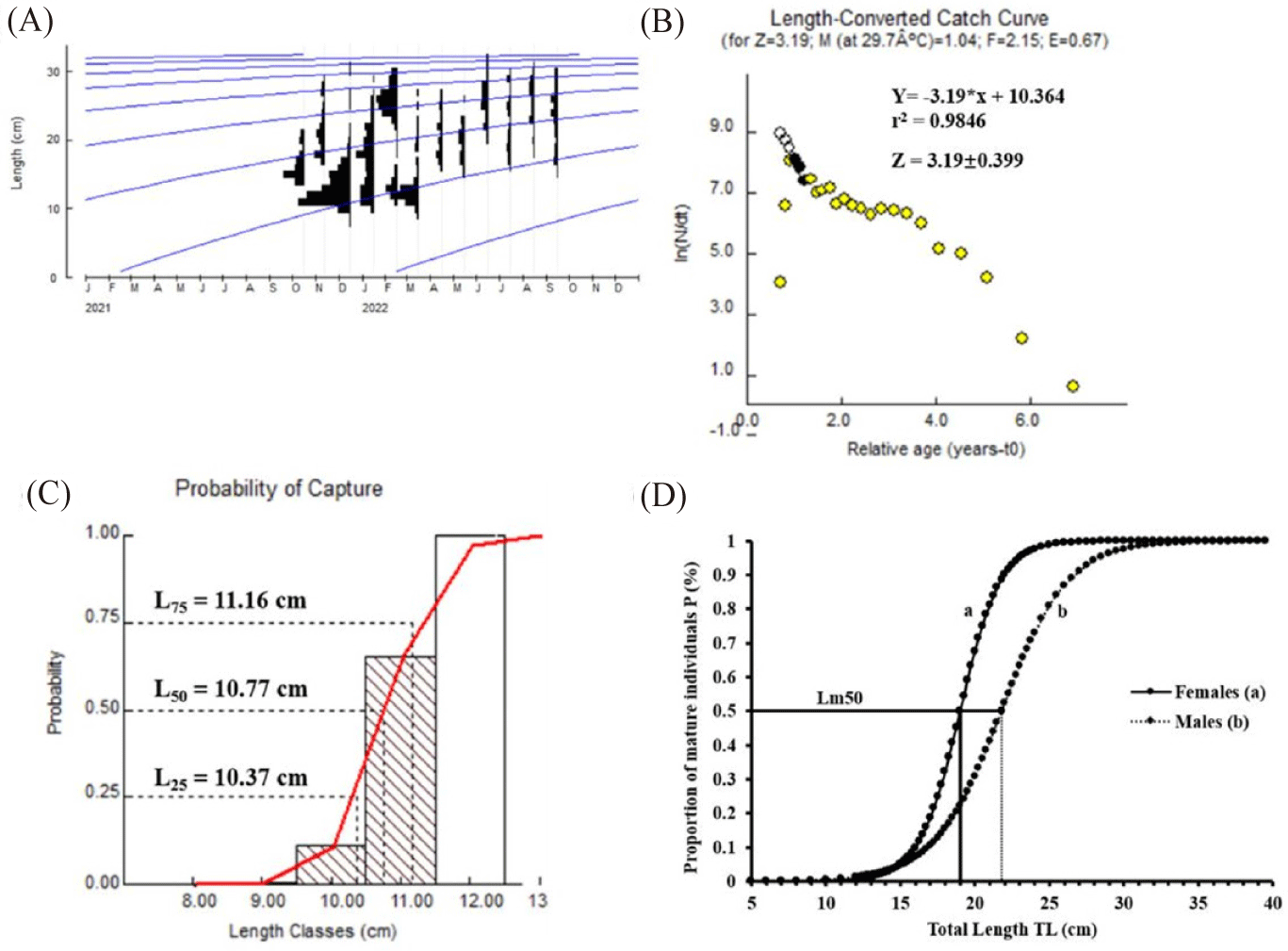
The current results revealed an asymptotic length, growth rate, and life-span tmax of 33.6 cm, 0.44 year-1 with the highest fitness Rn of 0.216, and 6.818 years (Fig. 3A, and Supplementary Fig. S2), respectively. Thus, the Von Bertalanffy equation of O. niloticus obtained from these parameters is written as follows:
The growth performance index Ф’ of O. niloticus in Samendéni reservoir was 2.696.
The catch curve shown in Fig. 3B was used to estimate the total mortality rate (Z) of O. niloticus. The darkened circles in the Fig. 3B correspond to the data points used to calculate the estimated Z value, which was 3.19 ± 0.399, with an r-squared value of 0.9846. Black dots represent the length group fully recruited into the stock and used in the analysis to calculate the mortality rate. Yellow dots indicate small-sized fish and those that were excluded from the estimation. Black outline circles are used as extrapolated points to estimate the probability of capture. The fishing mortality (F) and natural mortality (M) rates were also calculated to be 2.15 year-1 and 1.04 year-1, respectively. The exploitation rate (E) of 0.67 appeared to be higher than the optimal exploitation rate (Eopt = 0.5) (Fig. 3B).
A size of 10.77 cm was estimated as the length at first capture (Lc or L50) (Fig. 3C). In addition, the probabilities of capture values were estimated at L25 = 10.37 cm and L75 = 11.16 cm.
Females of O. niloticus exhibit a size at first sexual maturity (Lm50) of 19 cm. In comparison, males reach this maturity at 21.78 cm (Fig. 3D). Furthermore, 61% of captured females and 64.207% of captured males have a TL less than 19 cm and 21.78 cm, respectively.
Recruitment occurred throughout the year for O. niloticus in Samendéni reservoir. Furthermore, the major peak was observed by July (26.52%), which corresponds to the rainy season (Fig. 4A).
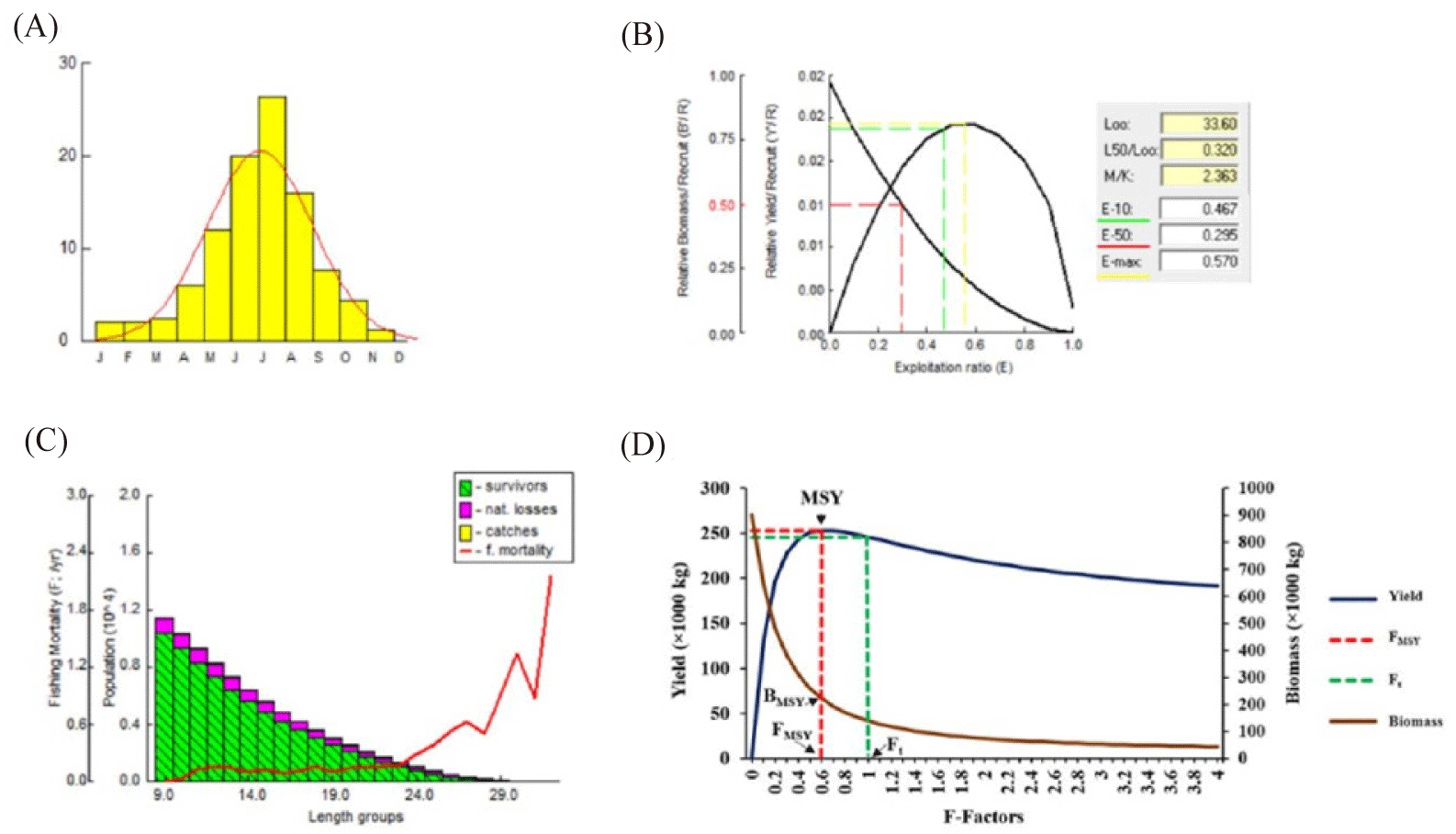
The evaluation of the yield per recruit (Y’/R) for O. niloticus was based on the ratio of Lc/L∞ = 0.320 and M/K = 2.363 (Fig. 4B). The biological target reference points, including E10, E50, and Emax, were 0.467, 0.295, and 0.570, respectively. These results indicated that the current exploitation rate (E) of 0.67 was higher than that of both biological target reference points (Fig. 4B).
Natural causes are considered the main reason for the mortality of O. niloticus for the length range of 9 cm (Fig. 4C and Supplementary Table S1). However, from 10 cm, the fish is vulnerable to fishing gear and peaked at 32 cm with fishing mortality F values ranging from 0.1344 to 2.15.
The recruitments of O. niloticus in the Samendéni reservoir were estimated at 11,393. Following this, a decrease in population size was noted as the length of classes increased. In particular, length categories ranging from 19 to 24 cm of O. niloticus were subject to significantly higher levels of exploitation in the fishery, highlighting the need for management strategies. The vulnerability of the length group 32 cm to fishing was found to be greater (2.15 year-1), followed by length groups 30 cm (1.337 year-1) and 29 cm (0.9157 year-1). The observed maximum steady-state biomass of O. niloticus was 0.06 tons, corresponding to the length group of 19 – 24 cm.
The biomass (B) and yield (Y) of O. niloticus at various fishing levels are presented in Fig. 4D and Supplementary Table S2. The maximum sustainable yield (MSY) was attained at an X-factor of 0.6, which corresponds to a total of 253,336 kg. The corresponding biomass at Maximum sustainable Yield BMSY was estimated at 221,854 kg with a virgin biomass B0 of 902,530 kg. As the fishing mortality at Maximum Sustainable Yield FMSY = XMSY × Ft, a value of 1.29 year-1 was obtained. Furthermore, as the fishing terminal of this study Ft = 2.15 year-1, the ratio of Ft/FMSY = 1.666. In addition, the biomass at a fishing terminal year Bt was 139,088 kg; therefore, the ratio of Bt/BMSY = 0.626 with Bt as the biomass at the fishing terminal year Ft.
Discussion
The monthly pooled, female and male data of O. niloticus exhibited different modes, which could be explained by the sample size and the seasonal pattern. In fact, from April 2022 to September 2022, the monthly catch was lower. Furthermore, from March to May 2022, the O. niloticus population faced the dry season characterized by lower levels of water and a scarcity of food resources. In addition, from June to September 2022, corresponding to the heavy rainfall period and a higher level of water in Samendéni reservoir, most of the mature individuals of this species were laying and breeding. Moreover, fish were widely dispersed in the newly inundated areas during this period, which would reduce fishing efficiency (Akongyuure et al., 2016).
The observed sex ratio (1.365:1) found for O. niloticus was significantly different from the 1:1 ratio, similar to the results of Njiru et al. (2006), who observed a predominance of male individuals in the Kenyan section of Lake Victoria and a significant deviation from the expected sex ratio. Offem et al. (2007) also reported a predominance of male individuals with a sex ratio of 2:1 in Cross River, Nigeria. However, this result was not observed at Wadi Hanifah, Arabia Saudia, by Mortuza & Al-Misned (2013), who reported a sex ratio of 1:0.85 for the same species. This variation in the sex ratio could be due to the behavioral characteristics of male fish, which tend to exhibit higher levels of aggression and territorial behavior than females (Otieno et al., 2014). Moreover, the prevalence of males in the sample was likely attributed to their potential migratory behavior from spawning areas to shallow feeding grounds after egg fertilization. At the same time, the females moved toward rocky and submerged vegetation to incubate the eggs. This allowed the females to protect their offspring in these hidden areas where they are difficult to catch (Otieno et al., 2014).
Pooled samples, female and male specimens, both in this study exhibited a positive allometric growth pattern, indicating that O. niloticus tends to grow a little more in weight than in length. Generally, the values of “b” do not fall outside the range of 2.5 to 3.5 respectively, depending on the species and suggesting the validation of the result of this present study (Novaes & Carvalho, 2012). However, before the opening of the Samendéni reservoir for exploitation, Minoungou et al. (2020) reported a negative allometric growth (b = 2.86) for this species. Furthermore, Ouedraogo et al. (2021) reported an isometric growth (b = 3.088) for the same species at the Mare aux hippopotames in Burkina Faso. All these reports contradict our results. Novaes & Carvalho (2012) suggested that various factors might affect the regression coefficient, including natural intra- and interspecific variation, seasonal fluctuations, food availability, and reproductive cycles. However, the result obtained at Samendéni reservoir could be due to the size of this reservoir, the third largest in Burkina Faso providing more secure space for fish, and various food opportunities. Furthermore, in Samendéni reservoir, Kabré et al. (2024) recorded a total of 96 microalgae species. Moreover, 34 and 41 phytoplankton species have been respectively observed during the dry and rainy seasons (Kabré et al., 2024). About periphyton, 41 species were recorded during dry and rainy seasons. As O. niloticus species is known to feed on phytoplankton, periphyton, aquatic plants, small invertebrates, and benthic fauna, detritus, and has a high tolerance to environmental conditions, Samendéni reservoir can be considered as a favorable environment for O. niloticus.
O. niloticus grew at a growth coefficient K of 0.44 year-1 with an asymptotic length L∞ of 33.6 cm. However, before the opening of the Samendéni reservoir for exploitation, Minoungou et al. (2021) reported respectively for L∞ and K, 25.07 cm and 0.5 year-1. Therefore, the asymptotic length L∞ of this study is slightly higher. However, the growth coefficient K was almost similar. The asymptotic length L∞ attained in the Samendéni reservoir was lower than the report from the Tapoa reservoir (L∞ = 36 cm) (Baijot et al., 1996), but higher than all other Burkina Faso reservoirs as reported respectively by Baijot et al. (1996) at Boromo reservoir (L∞ = 21.3 cm), at Boulmiougou reservoir (L∞ = 23.7 cm), at Kokolo reservoir (L∞ = 23.3 cm), at Ramitenga reservoir (L∞ = 18.2 cm), at Sourou reservoir (L∞ = 22 cm), and Tanguiga reservoir (L∞ = 17.6 cm). The value of the asymptotic length L∞ obtained in this study was lower than the results reported by some authors such as Tesfaye et al. (2021) at Lake Chamo, Ethiopia (L∞ = 59.4 cm), Tesfaye & Wolff (2015) at Lake Koka, Ethiopia (L∞ = 44.5 cm) in Ethiopia, Montcho et al. (2015) at Lake Toho, Benin (L∞ = 42.3 cm). These differences could be attributed to geographical locations (Amponsah et al., 2016), metabolism, reproduction, the genetic constitution of the individual, fish sizes, fishing pressure, and the method used for data sampling (Panda et al., 2018).
The estimated growth rate (K) for O. niloticus in the Samendéni reservoir (K = 0.44 year-1) was within the range of 0.34 and 0.67 year-1. This suggests that the species might be characterized as an intermediate-growing fish species (Amponsah et al., 2020). The growth coefficient (K) of O. niloticus was lower compared to that of some reservoirs in Burkina Faso, such as Sourou reservoir (K = 0.7 year-1), Boromo reservoir (K = 0.522 year-1), Ramitenga reservoir (K = 0.58 year-1) (Baijot et al., 1996), and Lake Toho, Benin (K = 0.56 year-1) (Montcho et al., 2015). However, comparatively to the Samendéni reservoir, some low growth rates of K were reported by Baijot et al. (1996) in others reservoirs in Burkina Faso, such as Tapoa reservoir (K = 0.39 year-1), Boulmiougou reservoir (K = 0.286 year-1), Kokolo reservoir (K = 0.196 year-1).
According to Baijot et al. (1996), a low growth performance index Ф′ was recorded for most African freshwater fishes with a range of 2.65 to 3.65. The Ф′ value of 2.696 obtained in this study was not out of this range, suggesting a low growth performance index for O. niloticus in Samendéni reservoir. However, a growth performance index out of the range of 2.65 to 3.65 was reported in some reservoirs in Burkina Faso, including Boromo reservoir (Ф′ = 2.374), Boulmigou reservoir (Ф′ = 2.206), Kokolo reservoir (Ф′ = 2.027), Ramitenga reservoir (Ф′ = 2.284), Sourou reservoir (Ф′ = 2.53), and Tanguiga reservoir (Ф′ = 2.154) (Baijot et al., 1996), and in Sakumo II lagoon, Ghana (Ф′ = 2.31) (Amponsah et al., 2020). The difference in the growth performance index between Samendéni reservoir and other Burkina Faso reservoirs could be explained by the favorable environment and availability of various microalgae species with an abundance of phytoplankton and periphyton for O. niloticus in this reservoir (Kabré et al., 2024). Thus, some changes in environmental conditions, food availability, composition, genetic composition, fishing pressure, and digestibility could impact the growth pattern of O. niloticus in natural aquatic environments (Njiru et al., 2006; Sossoukpe et al., 2016).
According to Tesfaye et al. (2021), natural mortality in fish is often caused by competition, predation, cannibalism, spawning stress, disease, starvation, and pollution stress. In this study, the natural mortality rate (M = 1.04 year-1) of O. niloticus was respectively higher than the report of Montcho et al. (2015) at Toho lake in Benin (M = 0.74 year-1) and the report of Tesfaye et al. (2021) at lake Chamo in Ethiopia (M = 0.558 year-1). However, the natural mortality rate M was lower than the value of M = 1.35 year-1 reported at Bontanga reservoir in Ghana by Kwarfo-Apegyah et al. (2009). Fast-growing fish often have high mortality rates because of the correlation between the growth coefficient K, and fish lifespan, which is directly related to mortality. Generally, when the Z/K ratio is less than 1, the population is growth-dominated; when it is greater than 1, it is mortality-dominated. If the Z/K ratio equals 1, the population is considered to be in equilibrium, indicating a balance between mortality and growth (Sossoukpe et al., 2017). In this current study, the Z/K ratio is 7.25 indicating a mortality-dominated situation in the Samendéni reservoir. Furthermore, many predator species in the Samendéni reservoir could also contribute to an increase in the natural mortality rate. It was indicated that the high natural mortality of O. niloticus in lakes Chamo and Victoria in Ethiopia might be due to some predators, such as Lates niloticus and Clarias gariepinus, which consume O. niloticus as prey (Tesfaye et al., 2021). Minoungou et al. (2021) confirmed the presence of these species in the Samendéni reservoir. The current exploitation rate (E) of O. niloticus obtained in this study was 0.67. This value showed that 67% of the population of O. niloticus stock in the Samendéni reservoir was annually exploited. This value was higher than the optimal exploitation rate of 0.5 (50%) per year and both of the biological target references points E10 and Emax, and according to Gulland (1971), an overfishing of O. niloticus is occurring in this reservoir. However, comparing this study with the properties of 4 quadrants of relative isopleth diagrams, as Pauly & Soriano (1986) mentioned, a Lc/L∞ of 0.320 and an exploitation rate of 0.67 fall in quadrant D, indicate an overfishing situation. This result means that smaller fish are caught at a high effort level. In addition, the ratio of Ft/FMSY is higher than 1, and Bt/BMSY was also lower than 1, indicating that O. niloticus was facing overfishing in Samendéni reservoir. This could impact sustainability in fisheries, as catching mainly smaller fish affects the overall population and reproduction. Therefore, it is crucial to decrease the exploitation rate to ensure sustainable exploitation and conservation. Furthermore, because 61% of captured females and 64.207% of captured males have a size less than Lm50, these results mean that the fishing system used in the Samendéni reservoir restricts a significant portion of fish from reproducing at least once before capture. Under such conditions, increasing the mesh size of fishing gear should accompany fishing efforts.
The period from June to August corresponded to the peak recruitment of O. niloticus in Samendéni reservoir. It also occurred during the major spawning season for this species in the reservoir. In Burkina Faso, the period from May to August experiences higher rainfall during the rainy season, which also corresponds to the main flooding period in the country. This period, characterized by more nutritional resources, favoured the recruitment of juveniles into the population. Montcho et al. (2015) also reported a peak of recruitment for the same species from May to July at Lake Toho, Benin.
According to VPA, most O. niloticus catches in this study occurred in the mid-length range of 19–24 cm. However, in our data, this length range represented only 22.87% of the total sample. The first sexual maturity is essential to the life history of the animal and should be considered for effective management. A difference was observed between the length at first sexual maturity (Lm50 = 19 cm for females and 21.78 cm for males) and the length at first capture (Lc = 10.77 cm), indicating that overfishing occurs in the fishery, potentially due to the absence of a sufficient period for young fish to mature and join the stock before being caught by fishing gear (Amponsah et al., 2016). Furthermore, this result also means that O. niloticus catches did not meet the criteria for good management (Lc < Lm50). Consequently, increasing the mesh size of fishing gear is necessary to capture only adult fish for management purposes. This would allow females to actively participate in mating activities and ensure resource availability and sustainability.
Conclusion
This study investigated the population dynamics of O. niloticus from Samendéni reservoir, Burkina Faso. The population of this species could be considered as suffering from overfishing. However, the catch consisted of more juveniles than adults. Therefore, without decisive action, the fish stock could collapse if the ban on illegal gear is not reinforced, an increase in mesh size to limit the catch of immature individuals, and vocational training for the fishers is needed to protect the O. niloticus stock. This action would be an excellent chance for O. niloticus individuals to spawn at least once, as the Lc and Lm50 differ. Otherwise, a breakdown might occur due to the lack of spawners. Furthermore, to ensure the recovery and sustainability of the harvest, the formation of a diverse stakeholder group, including community leaders, fisheries scientists, fishermen, and government representatives, will contribute to the successful recovery of the fishery.