Introduction
Eels are ray-finned fish belonging to the order Anguilliformes. They inhabit the western Pacific Ocean and are mainly found in Korea, Japan, and the East China Sea. The eels farmed in Korea are mainly from East Asia (Anguilla japonica), Europe (Anguilla anguilla), and the United States (Anguilla rostrata) (Daverat et al., 2006).
The major bacterial diseases causing economic damage in the eel farming industry include Edwardsiellosis and Aeromonas infection (Lee & Wendy, 2017). Edwardsiellosis is a disease that occurs at all life stages in eels when aquaculture environmental conditions are poor, such as when tanks are dirty, there is insufficient water exchange, or the feed supply becomes contaminated (Meyer & Bullock, 1973). Edwardsiellosis is a bacterial disease whose causative agent was originally thought to be Edwardsiella tarda (Loch et al., 2017). However, after additional study of Edwardsiella tarda, two new species were identified—Edwardsiella piscicida (Abayneh et al., 2013) and Edwardsiella anguillarum (Shao et al., 2015)—and the more common E. piscicida was identified as the true causative agent (Buján et al., 2018; Griffin et al., 2014; Shafiei et al., 2016).
Edwardsiellosis in eels is characterized by redness of the fins and abdomen, enlargement and protrusion of the anus, and/or red swelling at the edges and ulceration (Abayneh et al., 2013; Kim et al., 2011, 2012; Park et al., 1993). Internal symptoms include inflammation of the liver, kidneys, and spleen, and purulent inflammation is observed as pus flows (Xu & Zhang, 2014). Edwardsiellosis, which occurs year-round, is prevalent from eels to adult fish and requires long-term drug treatment.
Antibiotic administration is recommended for the treatment of Edwardsiellosis. Excessive administration of antibiotics, however, can cause environmental pollution and the emergence of multidrug-resistant bacteria, thereby weakening consumer confidence in farmed fish species. Therefore, developing a vaccine using the fish’s immune system.
The first fish vaccine used in Korea, an immersion vaccine that prevents the infection of E. tarda of Paralichthys olivaceus, was approved in 2003. Since its approval by the Korean government, 29 vaccines against 10 fish pathogens have been commercialized (Hwang et al., 2020). Injection vaccines, which are the majority of Korean fish vaccines, are a method of immunization in which the vaccine is administered intramuscularly or intraperitoneally. Since they can be used with adjuvants the immune response to these vaccines is strong, meaning that they are quite effective, but it is very difficult to treat a large number of fish in the field. An inactivated injection vaccine for preventing Edwardsiellosis in P. olivaceus has been recently developed and marketed, but its effectiveness in eels, a freshwater fish, has not been proven. In addition, although intraperitoneal vaccination is recommended for the current Edwardsiellosis vaccine for P. olivaceus, it is difficult to administer to eels due to the characteristics of the species—in particular, the round body and the secretion of mucus during anesthesia. To date, no vaccine has been developed for Edwardsiellosis of eels, so damage from diseases is reduced by relying on culture management.
It has been reported in previous studies that mucosal immunity provides more effective protection than injection immunity against pathogenic microorganisms infecting via mucosa (Neirynck et al., 1999; Rao et al., 2010; Shim et al., 2011). The most significant advantage of inducing mucosal immunity compared to the currently available injection vaccines is that the latter will cause only a systemic immune response, with lillle or no mucosal responses. Mucosal vaccines, however, provoke a mucosal immune response that produces antigen-specific secretory Ig on the mucosal surface and systemic immunity that produces antigen-specific serum IgG (Holmgren & Czerkinsky, 2005; Neutra & Kozlowski, 2006).
Oral vaccines induce a local immune response in the intestinal mucosa, an important route of infection for many pathogens (Azizi et al., 2010). Although studies on the presence of organized lymphoid tissue in the fish gut are lacking, evidence is emerging that, as in the mammalian gut, it serves as an immune organ for adaptive immunity generation (Yu et al., 2020). However, gastric acid and gallstone salts often destroy oral vaccine antigens in the intestines, meaning they do not reach the necessary location to provoke an immune response (Jung et al., 2015).
In response to this problem, liposome delivery and coated liposome technology are attracting attention as promising oral vaccine delivery systems. Liposomes are a system that can deliver drugs to various organs or cells, by helping orally administered antigens overcome chemical, biological, and physical degradation. They are absorbed through Payer’s patch in the small intestine in the stomach to produce antibodies in the body (Ling et al., 2006). Liposomes comprise a phospholipid bilayer membrane and can be manufactured in various sizes (Jousma et al., 1987; Shaker et al., 2017).
This study aimed to develop an effective oral vaccine for Edwardsiellosis that can reduce the mortality rate of farmed eels in Korea. Liposomes were used as the antigen carrier, and the mortality rate of eels following different doses of the oral liposomal vaccine and subsequent artificial infection of E. piscicida were compared and evaluated.
Materials and Methods
E. piscicida, the endemic strain of Edwardsiella, was isolated (in 2022) from eels that died naturally due to the disease from a fish farm in Gochang, Jeollabuk-do, Korea. Isolated bacteria were inoculated on tryptic soy agar and subcultured at 30°C for 24–48 h. Buffered formalin (0.5%) was added to the strain suspension and incubated overnight at 4°C to inactivate the strain. The inactivated strains were harvested, washed with sterile phosphate-buffered saline (PBS), and centrifuged at 5,000 ×g at 4°C for 5 min. The washing step was performed three times to ensure that all formalin was removed. The inactivated cells were resuspended in sterile PBS, and about 1 mL of the suspension was inoculated on blood agar to confirm sterility. The mixture was finally incubated at 30°C for 24–48 h.
The concentration of E. piscicida before formalin-killed cell (FKC) preparation was calculated by counting colonies, and when the absorbance at 540 nm was 1, it was measured to be 5.3 × 108 CFU/mL. Liposomes were prepared after confirming the initial concentration of the E. piscicida suspension (2.46 × 109 CFU/mL). 5 g of lecithin was mixed into 100 mL PBS buffer and dispersed for 5 min at 6,000 rpm with an agi-homemixer. Then, 2.5 mL of polysorbate 20 (Merck, Darmstadt, Germany) and 1 mL of the E. piscicida FKC suspension were mixed and dispersed in an agi-homomixer at 4,000 ×g for 30 min. The aqueous phase core of the liposome containing E. piscicida FKCs was formed by dispersing and simultaneously dispensing the E. piscicida FKC suspension into a phospholipid aqueous solution dropwise at 1 mL/min using a peristaltic pump to form an emulsion.
The liposomes’ encapsulation efficacy (EE%) was calculated using liposomes prepared with gallic acid. After preparation, the EE% was indirectly measured by determining the gallic acid (junsei) concentration inside and outside the liposomes. After preparing liposomes containing gallic acid, 1 mL was aliquoted and centrifuged at 15,000 rpm for 20 min to separate the upper and lower layers. The suspension was evaporated in a dry oven at 60°C, and 100 μl of triton X-100 was added to each container to decompose the liposome and elute the internal gallic acid. As an internal standard, 900 μl of 0.1% ibuprofen (Merck) was added and analyzed via high-performance liquid chromatography (HPLC; Alliance e2695 Separations Module, Waters, Milford, MA, USA). A C18 column (SunFire, Dresden, Germany, 4.6 × 300 mm, 5 μl) was used as the column. Phosphate buffer (pH 6.8):acetonitrile (Honeywell, Charlotte, NC, USA) = 65:35 was used as the solvent, and the flow rate was 1 mL/min, and the wavelength was measured at 222 nm UV.
The mean diameter of the liposomes was determined using dynamic light scattering with a NanoBrook 90Plus Zeta (Brookhaven Instruments Corporation, Nashua, NY, USA). The polydispersity index (PI) was measured using the same instrument and used to measure liposome size distribution. The FKC liposome morphology was observed by cryogenic transmission electron microscope (Cryo-TEM). The liposomes were loaded onto the Gatan 626 cryoholder (Gatan, Pleasanton, CA, USA) and were examined using the Cryo Field-Emission TEM (Krios G4, Thermo Fisher Scientific, Waltham, MA, USA) at an operating voltage of 120 kV. Low-dose procedures were performed using an electron dose of 8–10 electrons/Å2 for imaging. Images were recorded using an FEI Eagle 4 k × 4 k CCD camera at magnifications ranging from 28,000× to 45,000×.
To determine the degree of FKCs’ elution according to temperature, the liposomes were left at 25, 37, 60, and 80°C for 1 h, and then the dissolution rate was measured via the HPLC method. HPLC analyses was carried out in the same way as the EE% analyses. To determine the degree of antigen dissolution under low pH conditions, artificial gastric juice (pH 1.2) was prepared, and the change in turbidity over time was measured. According to the content of the Korean Pharmacopoeia, this test solution is prepared using hydrochloric acid and sodium chloride, with the concentration of hydrochloric acid being approximately 0.1 mol/L. If the stability of the drug is affected under these conditions, it is necessary to establish test conditions that can maintain the drug’s stability. In practice, many formulations adjust the concentration of hydrochloric acid from 0.001 to 0.1 mol/L when performing dissolution tests under acidic conditions. After reacting for 1 h in 10-minute intervals, absorbance was measured at 695 nm using a UV/Vis spectrophotometer.
Eels of 500 ± 20 g (n = 250) were obtained from a fish farm located in Naju, Jeollanam-do, Korea and transported to the clinical trial site (Korea Aquatic Biosecurity Technology, Busan, Korea). A control group, oral vaccine administration groups, and an injection vaccine administration group were designated, with each group containing 50 eels, and the groups was placed in each 500 L tank (28°C). For stability, a screen was installed throughout the tank. Commercially available powdered eel feed was supplied once a day. Also, because the feed was continuously given, changes in the water quality of the tank were continuously observed (water temperature, dissolved oxygen, pH, NO2, NO3). The studies involving animals were reviewed and approved by Silla university committee (SUACAC-2023-003).
Oral vaccine doses were prepared by adjusting the concentration of FKC liposomes to 5, 10, and 20 mg/fish, and the injection vaccine was prepared by diluting FKC in PBS and adjusting the concentration to 10 mg/fish. The control and injection vaccine groups were fed general feed without vaccine added. For the oral vaccine groups, each vaccine dose was quantified using an electric pipette and mixed with a predetermined amount of feed (Fig. 1). In the injection vaccine group, each eel was inoculated intraperitoneally at FKC 10 mg/fish. Anesthesia was used prior to vaccine injection to reduce stress and facilitate the inoculation of test fish. The inoculation concentration and method for each test group are detailed in Table 1.
To analyze antibodies, eel serum was extracted from 8 selected individuals from the 5 experimental groups, beginning two weeks after vaccination. Anesthesia was used to reduce the stress on the test fish and facilitate the test. After anesthetizing the sampled experimental fish, peripheral blood was extracted with a syringe from the tail vains, reacted at 4°C for 1 h, and then centrifuged at 6,000 rpm at 4°C for 15 min to separate the serum. The collected blood was stored in a cryogenic freezer at 80°C until the sample was transported, and the separated serum was packaged and transported together with an ice pack in an insulated container.
E. piscicida, subcultured on brain heart infusion agar, was inoculated into 100 mL of brain heart infusion broth liquid medium and cultured for 24 h in a shaker incubator. The enriched medium was centrifuged at 4,000 rpm for 15 min to separate the medium from the bacteria. The separated medium was discarded, and the E. piscicida were suspended using PBS. After confirming the initial concentration (2.46×109 CFU/mL) of the E. piscicida suspension, 100 μl was injected intraperitoneally into each experimental fish. Anesthesia was administered before injection to reduce the stress of the test fish and facilitate inoculation. Mortality was observed for 15 days after inoculation.
Antibody agglutination titers were measured using the serum samples collected above. To investigate antibody agglutination, 100 μl of PBS was added to each well (except for the last dilution) in a 96-well plate. Serum (100 μl) from each experimental section was put into each well, and a two-fold step dilution was performed. After administering 50 μl of the inactivated E. piscicida suspension (1 × 107 CFU/mL) to each well, the agglutination value over time was checked.
All results of this experiment are indicated in terms of means and standard deviations. The data obtained from this study were first analyzed through a one-way analysis of variance (ANOVA) using SPSS 25 (SPSS, Chicago, IL, USA) and then tested using Duncan’s multiple test at p < 0.05.
Results
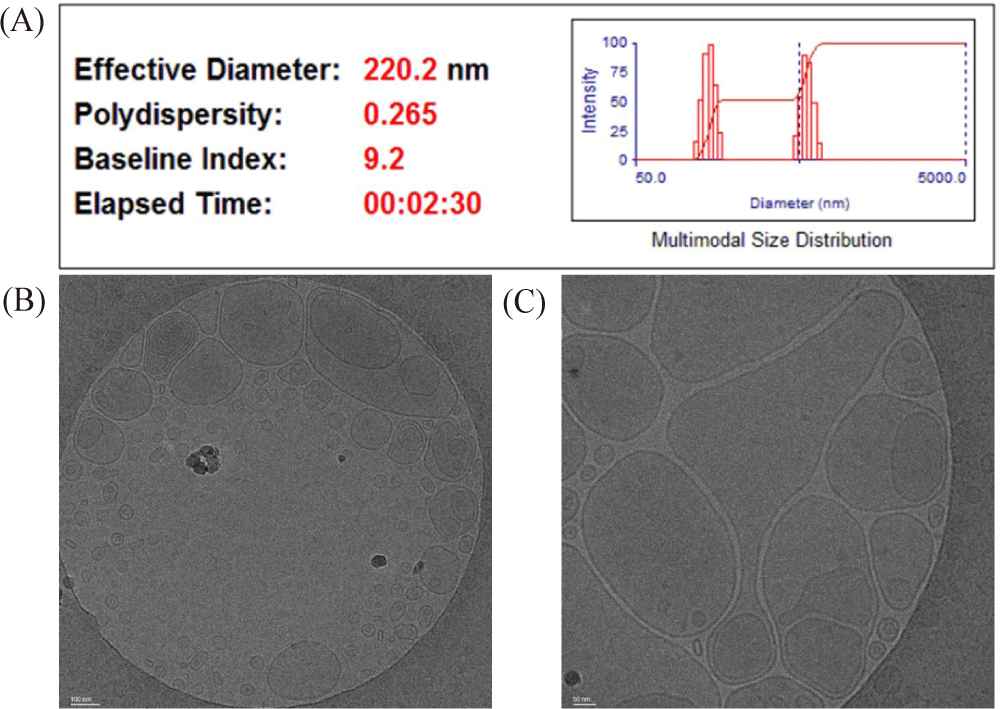
The EE% of the prepared liposome oral vaccine was determined using HPLC, by calculating the concentration of gallic acid remaining inside (38.36%) and outside (61.64%) the liposomes after preparation. From this, the concentration of FKCs inside the liposome was calculated, and then the eels were inoculated. The average particle size of the liposome was 220.2 nm, and the PI was measured to be 0.265, which means that the size of the prepared liposomes was constant (Fig. 2A). The Cryo-Transmission Electron Microscope measurement result confirmed that the phosphatidylserine liposome formed a stable double membrane structure (Fig. 2B).
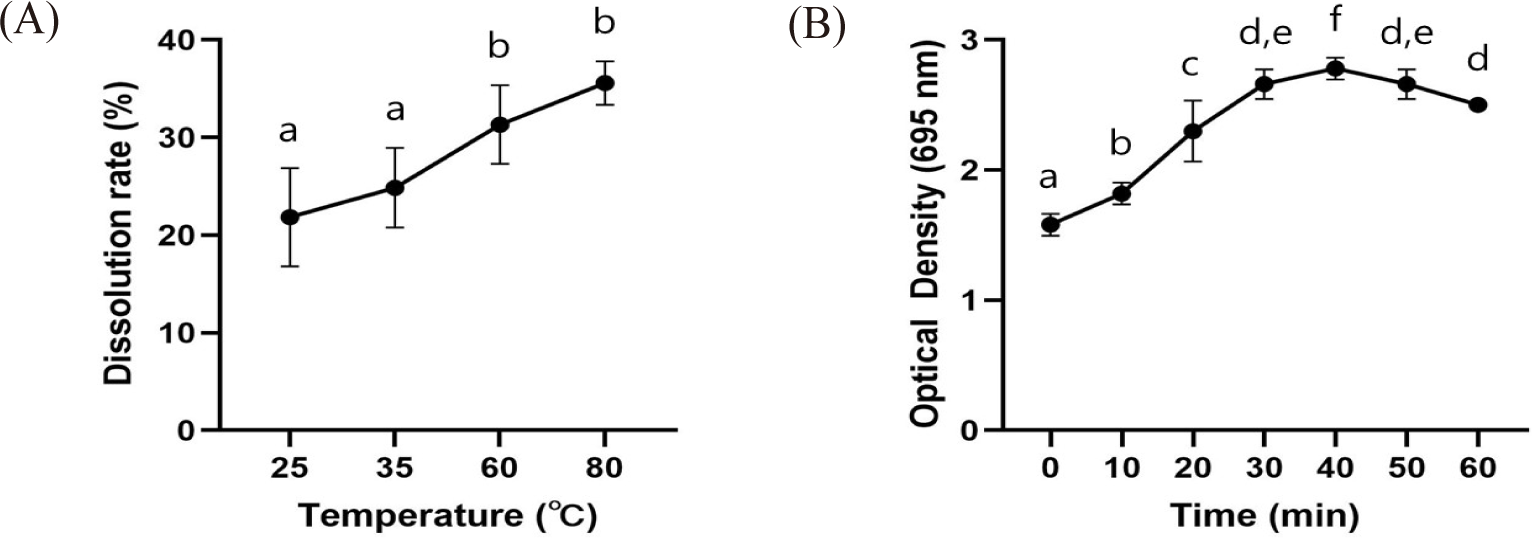
The liposome dissolution rate at room temperature (25°C) was 21.85 ± 7.11%. As the temperature increased, so did the dissolution rate; however, at 37°C, it was 24.86 ± 5.77%, at 60°C it was 31.33 ± 5.68%, and at 80°C it was 35.59 ± 3.15%, which meant that there was no significant difference (Fig. 3A). It was found that the prepared liposome was stable, even in artificial gastric juice (pH 1.2; Fig. 3B).
After immunizing the eels with oral and injection vaccines, the immunity against E. piscicida was investigated. After aseptically separating the kidneys and spleen of dead individuals and preparing a cross-section of the tissue on salmonella-shigella medium, it was confirmed that hydrogen sulfide generated by E. piscicida was reacting with iron citrate to form black colonies.
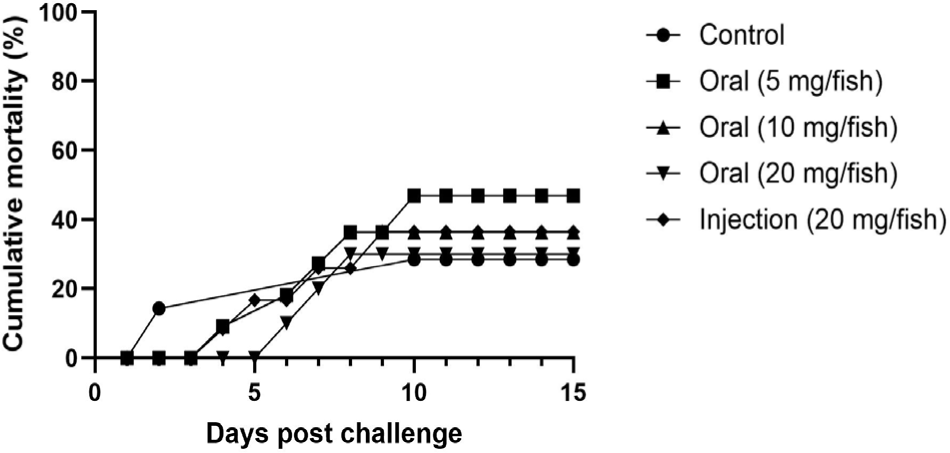
The relative percent survival rate was highest in the oral administration 20 mg group, and decreased as vaccine concentrations decreased: oral administration 20 mg > injection (10 mg) > oral administration 10 mg = oral administration 5 mg > control. Specifically, in the unvaccinated control group, the cumulative mortality rate was 46.0%, 4.18 times higher than the average mortality rate (11.0%) of the vaccinated experimental groups. In addition, in the case of the group that received the highest concentration oral vaccine (20 mg/fish), the cumulative mortality rate was only 6.0%, the lowest cumulative mortality rate among all vaccinated groups (Fig. 4).
Two weeks after eels were treated with either the oral or injected E. piscicida inactivated preventive vaccine, it was determined whether they had generated immunity by examining the serum agglutination antibody titer. These analyses confirmed that antibody agglutination values were highest in the injected eels, and decreased in order of concentration in those that received oral vaccines: IP injection 10 mg > oral 20 mg > oral 10 mg > oral 5 mg > control. Unsurprisingly, the vaccine-administered experimental groups all showed higher antibody agglutination values than the control group. Among the experimental groups, the injection-administered eels had the highest antibody agglutination value at 28.25, followed by the 20 mg oral administration group at 25.125 (Table 2).
| Group (n = 8) | Average agglutination titer (Log2) |
|---|---|
| Control | 1.375 ± 0.48 |
| 5 mg | 4 ± 1.11 |
| 10 mg | 4.25 ± 0.82 |
| 20 mg | 5.125 ± 1.05 |
| I.P | ± 0.43 |
Discussion
Developing an effective vaccine would be the best way to reduce this misuse of antibiotics and increase production in the aquaculture industry (Priya & Kappalli, 2022). Unfortunately, to date, most effective fish vaccines are injection vaccines, and in practical terms it is very difficult to effectively treat a large number of fish in the field. In addition, there was a case in which an experimental vaccine was mixed with fish feed, but the effect was insufficient. Therefore, it is necessary to develop an effective oral vaccine that can improve the efficiency and efficacy of inoculation. An oral vaccine would be very useful in the field due to the nature of the aquaculture industry, where inoculation costs are closely related to the economic feasibility of the industry.
There are several methods for vaccinating fish, one of which is oral vaccine administration. In this study, an oral vaccine was produced by mixing liposome-encapsulated antigens into feed. Administering antigens in fish feed has the advantage of being stress-free and easy to administer to many fish at once (Plant & LaPatra, 2011). Mucosal immunity provides more effective protection than injection immunity against mucosal infection by pathogenic microorganisms (IiJima et al., 2001). Mucosal vaccines have advantages over systemic vaccines from a production and regulatory perspective. They are also practical for mass vaccination and do not pose the risk of bloodborne infections due to contaminated needles. Ease of administration and the possibility of non-medical personnel administering them are also considered advantages of the mucosal vaccine strategy (Lycke, 2012). Despite recent developments in and studies of mucosal vaccines by many researchers, mucosal vaccines are having difficulties in commercialization due to the efficiency, safety, and absence of mucosal immune enhancers (Venu et al., 2023).
This study evaluated the efficacy of an oral liposomal vaccine for the prevention of Edwardsiellosis in eels, including the preparation method, administration via feed, vaccine concentrations, and efficacy (measured by both mortality rates and antibody agglutination levels). With oral administration, destruction of antigens by digestive enzymes is inevitable, unless a method can be established to safely deliver antigens to the intestine and induce an effective immune response locally. Liposome oral vaccine is a technology that can deliver immunogens to the intestinal mucosa without being degraded or denatured in the stomach (Huang et al., 2022). With oral administration, destruction of antigens by digestive enzymes is inevitable, unless a method can be established to safely deliver antigens to the intestine and induce an effective immune response locally. Liposome oral vaccine is a technology that can deliver immunogens to the intestinal mucosa without being degraded or denatured in the stomach (Huang et al., 2022). While the oral vaccines gave a good protection against mortality, there is a discrepancy between the observed agglutinating antibody titer following mucosal versus injection vaccination and the level of protection obtained after challenge. There is also need to perform additional studies to explain why the oral vaccine provide higher level of protection against mortality following injection challenge than the injection vaccine, although the difference is marginal.
The liposome-based fish vaccine developed in this study was orally administered to eels, and successfully induced an immune response that elicited an antibody response against E. piscicida, and gave a good protection against lethal challenge. In the artificial infection experiment after oral vaccine administration, the 20 mg/fish administration group showed the lowest mortality rate at 6%, and the I.P. administration group showed the second lowest mortality rate at 10%. In addition, the oral vaccine administration groups showed an average mortality rate that was 4.18 times lower than the control group, confirming the efficacy of the oral vaccine. In the antibody agglutination titer measurement results performed on the serum extracted after oral vaccine administration, I.P. showed the highest antibody agglutination titer, followed by the 20 mg/fish administration group.
When the mortality rate and antibody agglutination titer results in the artificial infection experiment were confirmed, the oral vaccine coated with liposomes of the inactivated strain of E. piscicida, a major pathogen of eels, appears to be able to function as a vaccine by inducing a sufficient immune response. Therefore, the liposome vaccine created and tested in this study can be used for the prevention of Edwardsiellosis in eels.








