Introduction
The Oreochromis niloticus fish species is the most preferred for consumption in Ethiopia, making up 49% of the total captured fish production (Tesfaye & Wolff, 2014). In Lake Hawassa, 90% of the total fish production is attributed to O. niloticus (Reyntjens & Wudneh, 1998). Due to increasing fishing pressure, overfishing of O. niloticus stock from Lake Hawassa has been continuously identified (Tekle-Giorgis, 2018). The ideal fishing effort for O. niloticus was determined to be 700 gill nets/day (Reyntjens & Wudneh, 1998). But overfishing was evident, with 1,954 gill nets/day found in the Lake, more than twice the recommended effort (Tekle-Giorgis et al., 2017). It was seen that 22.7% of O. niloticus stock from Lake Hawassa was harvested before attaining the length at first maturity (TL50) (Muluye et al., 2016). To overcome the threats, another researcher advised that the fishing effort be reduced to 650 gillnets/day to obtain a safe and optimal bio-economical yield of O. niloticus from the lake (Tekle-Giorgis, 2018). Additionally, aggressive fishing with mesh sizes smaller than 7.5 cm is jeopardizing the harvest of small-sized fish species from the lake (Dadebo et al., 2023). Despite these concerns, no detailed study has assessed the current status of O. niloticus’ reproductive biology in Lake Hawassa. Thus, a comprehensive analysis of the reproductive biology is essential to understand the current status of O. niloticus in the lake.
In terms of feeding habits, studies indicate that O. niloticus from Lake Hawassa is an herbivorous trophic category, concentrating on adult sizes ranging from 18–32 cm (Getachew & Fernando, 1989). Juvenile O. niloticus from Lake Hawassa exhibit a preference for zooplankton and some zoobenthos (Tudorancea et al., 1988). However, there is a notable gap in research regarding the dietary overlap between juvenile and adult O. niloticus, hindering the understanding of intraspecific feed resource competition. Therefore, concurrent studies of juvenile and adult O. niloticus are imperative to comprehend intraspecific competitions and ontogenetic dietary shifts in O. niloticus from Lake Hawassa. Furthermore, Lake Hawassa has suffered from pollution-related effects (Tilahun, 2023), leading to a decline in zooplankton abundance and biomass (Beyene et al., 2022), including important species such as Rotifers, Cladocera’s, and Cycloid copepods, previously identified as a dietary source for O. niloticus juveniles (Tudorancea et al., 1988).
There are research reports that certain fish species from Lake Hawassa exhibit a discernible shift in trophic behavior over time, necessitating further investigation into the dietary patterns and feeding habits of O. niloticus. Notably, initial perceptions regarding the carnivorous nature of larger fish species such as the African catfish Clarias gariepinus (Burchell, 1822) indicated insignificant reliance on plankton (Dadebo, 2000). Subsequently, C. gariepinus was reclassified as an omnivorous species, with significant contributions from phytoplankton and zooplankton (Tekle-Giorgis et al., 2016). This transition in feeding behavior underscores the need to reassess the current dietary habits of O. niloticus in the lake. In addition to the previously published information, the first study concerning the levels of microplastic concentration in O. niloticus muscle and gastrointestinal tract was identified (Demsie & Yimer, 2024). All these pollution-related concerns highlight the importance of researching the growth (b) of O. niloticus in Lake Hawassa. Consequently, the current study is designed to elucidate the reproductive biology and feeding habits of O. niloticus from Lake Hawassa. The resultant findings will prove invaluable for the development of stock assessment and modeling, population dynamic studies, Ecopath modeling, and the provision of updated information for management decisions.
Materials and Methods
Lake Hawassa is located within the Central Rift Valley Lakes of Ethiopia at 275 kilometers away from Ethiopia’s capital city, Addis Ababa, in latitudes 6°49′–7°15′N and longitudes 38°17′–38°44′E (Dadebo et al., 2023). Though River Tikurwuha feeds the lake, it lacks a distinct outflow (Admassu, 1996). When the impact of wind is taken into consideration over the dry months, the lake water exhibits a high level of nutrient mixing (Tilahun & Ahlgren, 2010). The lake is characterized as a heaven of hundreds of phytoplankton species (Kebede & Belay, 1994), and 22 known zooplankton species dominated by rotifers (Beyene et al., 2022). Six distinct fish species are available in the lake (Dadebo, 2000). Three fish species are important for commerce: O. niloticus, C. gariepinus, and African big barb Labeobarbus intermedius (Ruppell, 1836). The remaining three species, the straightfine barb Enteromious paludinosis (Peters, 1852), stone lamping minnow Garra quadrimaculata (Ruppel, 1835), and black lampeye Aplocheilichthys antinorii (Vinciguerra, 1883), are ecologically significant and contribute as a food item for large fish species like C. gariepinus (Dadebo, 2000; Fig. 1). Due to the lake’s exposure to several human activities, both the point and non-point pollutant sources are regarded as a threat to aquatic biota (Tilahun, 2023).
Multiple approaches for collecting data have been applied to achieve the stated objectives. The gill net was one of the sampling techniques. The gill net was set at 5:00 PM and retrieved at 7:00 AM following a 14-hour stay. Gillnets with 6, 8, 10, and 12 cm stretched mesh sizes, with 50 × 1.5 m, were used for the reproductive biology study. A randomized sampling was also taken from the two major landing sites, Amoragedel and Tikurwuha. To get a wide size range for understanding the species’ feeding habit and morphometric relationship, additional gillnets with stretched sizes of 2.0 and 2.5 cm were used. Further, hand-net-based sample collection techniques were also employed to get a smaller size of O. niloticus from the shoreline area. A random sample from local fishers who collect by hook was also collected.
Using a portable weighing balance and measuring board, the weight and length of the samples were measured to the nearest 1.0 g and 0.1 cm, respectively. The fish specimens were dissected; the gonads and stomach were removed and preserved in a 5% formaldehyde solution. On the other hand, smaller-sized O. niloticus, measuring less than 10 cm, were measured and dissected at the Hawassa University Fisheries Laboratory using a 0.1g scale sensitive balance and a 0.1 cm measuring board for diet composition analysis. The preserved gonads and stomach samples were taken directly to the fisheries laboratory at Hawassa University for additional examination of their reproductive and feeding biology. Female ripe ovaries were given a specific treatment in the lab. A surgical blade with a 22 mm diameter was used to dissect the ovary longitudinally and preserved until further procedures were applied.
Gonad stage identification: The maturity stage of each sample was grouped into five points of maturity (Holden & Raitt, 1974). Finally, the data were expressed in percentages after categorizing them as non-breeding (I and II) and breeding (III, IV, and V).
where, MSi%= the percent of O. niloticus at maturity sge i, MSi = the number of fishes at maturity stage i, and ∑MSi, is the total number of O. niloticus from maturity stage I to V.
Sex ratio: The sex ratio was calculated after categorizing the data into sampling months, size class, and total population. Finally, the sex ratio was compared using the chi-square test (Tadesse, 1997).
x2 is the chi-square value, Oi is the observed value, and is the expected value.
Length at 50% maturity: The length at 50% sexual maturity (TL50) was calculated based on the logistic regression model (King, 2013). The numbers of mature and immature O. niloticus were identified after grouping into 2 size classes. The modal class (mid-length) was plotted as an X variable. Whereas the natural logarithm of the proportion was represented as a “Y” variable, which was calculated as , where LN represents the natural logarithm and p represents the proportions of matured fish per total sample in each size class. After identifying the value of X (mid-length) and Y (LN transformed proportion), X-Y scatterplots were run using the Excel 10 sheet. The slope (b) and intercept (a) were extracted from the X-Y scatter plot graph which represents the mid-length and the proportions respectively. After this step, the specific value of TL50 was calculated using the equation , where b represents slope and a represents intercept. To get the probability of being mature in each modal class, the equation was run as
where pm is the probability of being mature. Finally, the graph of the sigmoid curve was set with MATLAB software (MathWorks, Natick, MA, USA).
Breeding season: The ripe ovaries were extracted from the total sample for each sampling month to observe the specific frequency of ripe fish. The frequency of males and females of O. niloticus with ripe ovaries was plotted against the sampling month in percentage to show their peak and non-peak breeding seasons (Admassu, 1996).
Fecundity: For estimating the fecundity potentials, only ripe females (gonad stage V) were considered. The longitudinally preserved samples of each ovary were gently shaken and washed with tap water continuously to remove the ovarian membrane. Finally, total counting was applied for each egg from each ovary to know its fecundity potential.
Diet composition analysis: The preserved prey items were transferred with a known volume of a graduated cylinder test tube. Once the overall volume was known, the samples were shaken to homogenize the stomach contents. Larger specimens were identified using a dissecting microscope (LEICA MS), and smaller prey items were identified using a compound microscope (LEICA DME) with a magnification power of 100X. Finally, the results were interpreted based on the frequency of occurrences, volumetric contributions, and index of preponderances.
Frequency of occurrence: The number of specific preys per non-empty stomach was counted to understand how much it occurred in the total non-empty sample. The occurrences of each prey were expressed as a percentage (Hyslop, 1980).
where, FO = frequency of occurrences of specific prey from non-empty O. niloticus stomach, TNSPi= total number of O. niloticus stomach with specific prey item i, and TNSi = total number of nun-empty O. niloticus considered for feeding habit analysis.
Volumetric contribution: The volumetric contributions of each food item per total volume of the non-empty stomach sample were expressed as percentages (Bowen, 1996).
where Vi% indicates the volumetric contribution of prey item i in percentage, VSPiFS indicates the volume of a specific prey item found in all specimens, and VFiFS indicates the volume of all food items found in all non-empty stomachs.
Index of preponderance: The index of preponderance was calculated based on the frequency of occurrences and volumetric contribution. It was expressed as a percentage (Natarajan & Jhingran, 1961).
where Fi is the frequency of occurrence of food item i;IoP% is the index of preponderance of food item i in percentage and Vi is the percentage composition by volume of food item; i;n is the number of prey types, to facilitate comparisons among species.
Seasonal variations of diet compositions: The diet com-positions were once again grouped and categorized based on rainy and dry months. Frequency of occurrences, volumetric contributions, and index of preponderance methods were used to compare the diet composition variations among seasons. Furthermore, the major prey item was statistically cross-checked using a chi-square test for the frequency of occurrences and an independent sample t-test for volumetric contributions, respectively (Dadebo et al., 2014).
Ontogenetic dietary shift and dietary overlap analysis: To determine the size-based ontogenetic dietary shift, the samples were grouped into 4 different size classes depending on their total length, and the percentage of volumetric contribution was calculated. For the first group, samples < 10 cm, the second group ranged from 10–19.9 cm, the third group was 20–29.9 cm, and the fourth group was considered above 29.9 cm. The volumetric contributions of major prey items were calculated in each size class, plotted with a stacked chart to indicate their contributions, and expressed as percentages. The dietary overlap was also considered to crosscheck the intra- and interspecific competitions. For the present study, intraspecific competitions based on size class comparisons were cross-checked depending on the major prey items contributed for each size class. The comparisons of dietary overlap of major prey items were compared between < 10 cm and 10–19.9 cm, < 10 cm and 20–29.9 cm, < 10 cm and ≥ 29.9 cm, 10–19.9 cm and 20–29.9 cm, 10–19.9 cm and ≥ 29.9 cm, 20–29.9 cm, and ≥ 29.9 cm size class were considered. Mathematically, dietary overlap was calculated based on the Shooner dietary overlap index (Schoener, 1970).
where C is Schoener’s similarity index metric, Pxi and Pxy and are the proportions of diet item i in the stomach of species x and y, respectively.
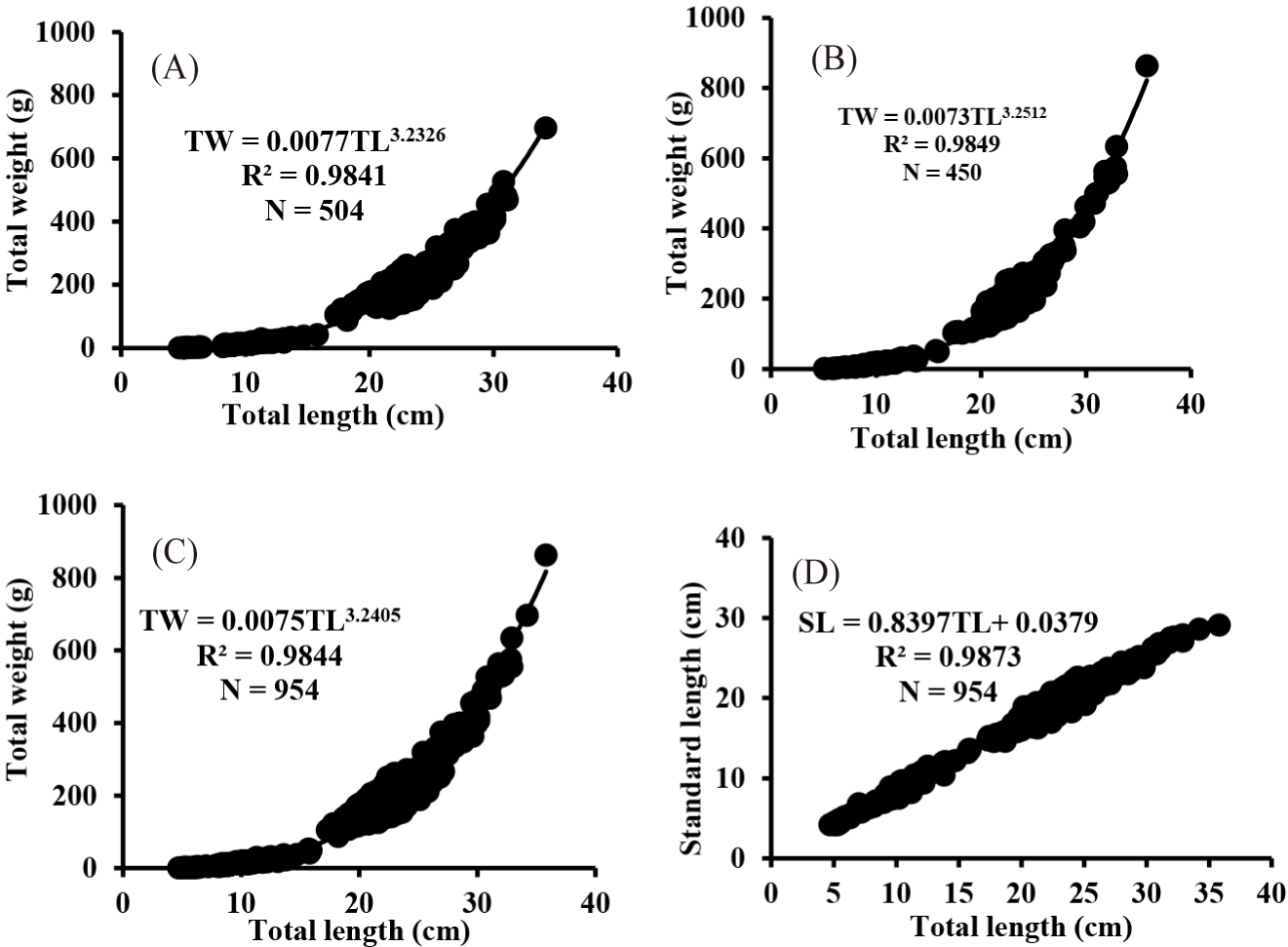
For length-weight relationship analysis, the data was sorted and run using the power function after removing the outliers as follows: TW = aTLb (Pauly, 1984), where TW= total weight of fish (g), TL = total length of fish (cm), a = the intercept of the regression line, and b= the exponent (slope) of the regression line. Based on the value of the exponent (b), the fish growth is categorized into three groups (Pauly, 1984). When the slope value b = 3, the species has isometric growth; b < 3, the species follows a negative allometric growth; and b > 3, positive allometric growth. Considering their length-length relationship, linear regression comparisons were applied (Zar, 1999). The value was set as Y = aX + b, where Y and X represent the standard length (SL) and TL of O. niloticus, a is the intercept, and b is considered as the slope of a regression.
The interpretations of the O. niloticus health condition depend on the exponent (b) value generated from the length-weight relationship. Fulton’s condition is considered for isometric growth (b = 3), whereas, for non-isometric growth (b ≠ 3), and statistically significant difference from 3, the relative condition factor is considered as a benchmark for understanding the health of fish species (Jones et al., 1999). The comparisons were run both within the sampling month and sex-based comparisons as indicated below.
(Zar, 1999),
where RCF indicates the relative condition factor, w represents total body weight, and a, and b describe constants and slopes of the length-weight relationship, respectively.
The data was analyzed with descriptive statistics using IBM SPSSv27 (SPSS, Chicago, IL, USA) and MS-Excel 10 (Microsoft, Redmond, WA, USA). For sex ratio comparison, the chi-square test was applied. The exponents (b) of the length-weight relationship were taken from the logarithmic transformed data and tested using a student’s t-test with a 95% confidence interval to know whether the value is significantly different from the expected cube value (3). The equation used for t-test comparisons of the exponent (b) is calculated as:
(Zar, 1999),
where ts is the t-calculated value, b is the slope, and sdb is the SE, obtained from the log-transformed data’s regression analysis. MATLAB software (MathWorks) was used to draw the graph of TL50. Regarding the diet compositions, descriptive statistics using Microsoft Excel 10 (Microsoft) were used. The chi-square test for frequency of occurrences and independent sample t-test for volumetric contributions were applied to test whether the diet composition shows a significant variation between rainy and dry months. The dietary overlap was tested using Schoener’s dietary overlap index to compare the intraspecific competition. The index was concluded as strong dietary overlap if the value is above 0.6 and considered as biological significance.
Result
Sex ratio: From the total 816 samples collected for reproductive investigation, about 386 (47.3%) were females, and 430 (52.7%) were males. The result revealed that more males than females were caught. Nevertheless, comparisons of their sex ratio (1:0.93) were not statistically significant from the hypothetical 1:1 ratio (χ2, p > 0.05; Table 1). However, based on size-based comparisons, the result indicates that the sex ratio demonstrates a significant difference in a size range of 25.8 to 27.7 cm and from 27.8 to 30.7 cm (χ2, p < 0.05; Table 2).
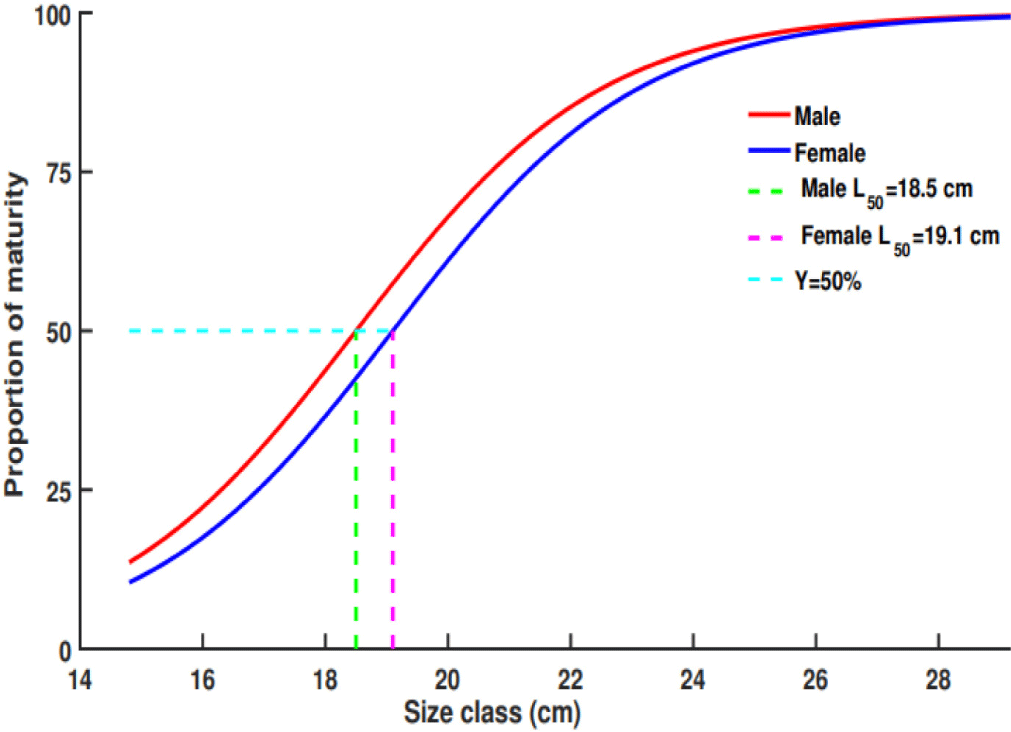
Stage of maturity and length at first maturity: from the total sample size, 78.43% of O. niloticus was mature (breeding), whereas 21.57% was immature (non-breeding). Males’ O. niloticus revealed that 23.95% were immature (non-breeding) and 76.05% were mature (breeding). Of the females’ O. niloticus, 18.65% and 78.53% were immature and mature, respectively. The findings showed that in Lake Hawassa, more immature male O. niloticus were caught than female O. niloticus. Mature male O. niloticus was recorded at 17.8 cm in length and 123 gm in weight. Whereas female O. niloticus, was starting to mature at 17.4 cm in length and 103 gm in weight, respectively. In the current investigation, the TL50% maturity for male and female O. niloticus was 18.5 cm and 19.1 cm, respectively. The result indicates male O. niloticus start to mature faster compared to females (Fig. 2).
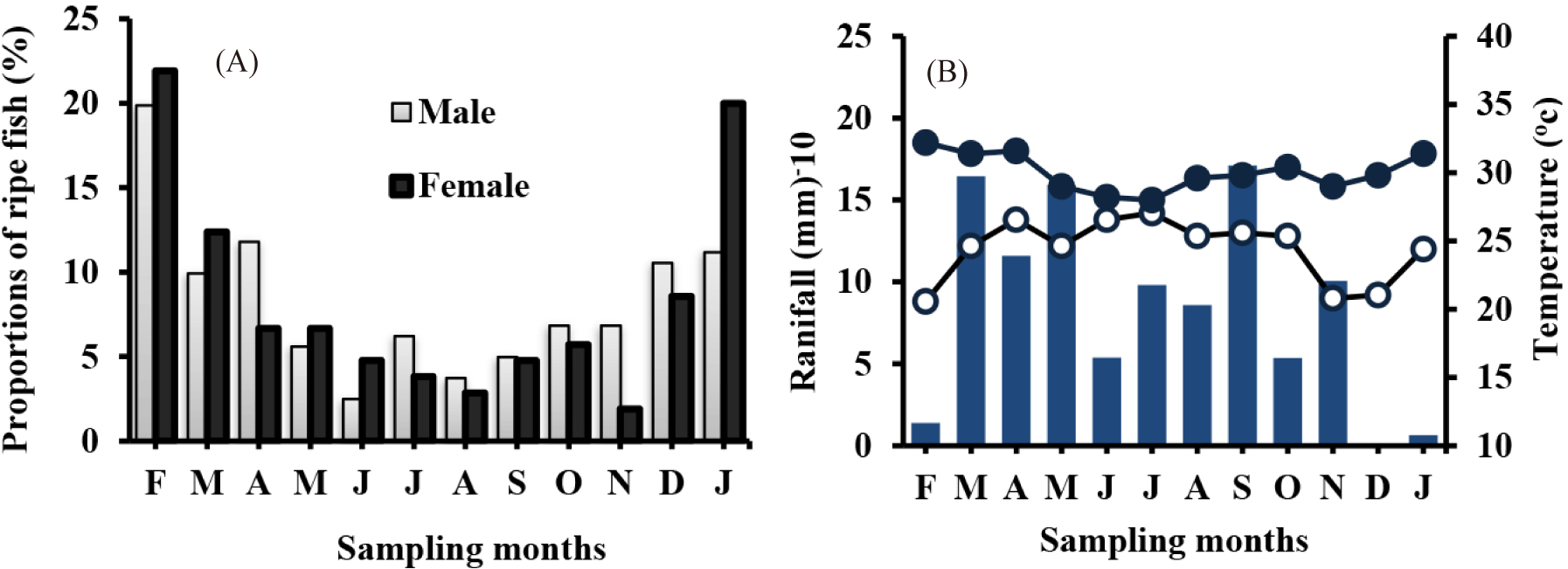
Breeding season: In Lake Hawassa during the present study, the O. niloticus breeding month indicates the species has the potential to breed throughout the year in both sexes. In both sexes of O. niloticus, the peak breeding seasons extended from February to April (Fig. 3). The peak breeding seasons for Females’ O. niloticus were seen by January (20.0%) and February (21.9%). February (19.9%), April (11.8%), and January (11.2%) respectively were also the peak breeding seasons of males’ O. niloticus.
Fecundity: The smallest length of a female O. niloticus with a ripe gonad was 17.4 cm and 102 g, respectively. The fecundity potential ranged from a minimum value of 105 eggs/ripe fish to a highest value of 1,541 eggs/ripe fish, with a mean fecundity of 556 eggs/ripe fish. Based on the regression analysis, there is a significant interaction (ANOVA, p < 0.001) and a linear association between gonad weight and fecundity. The correlation analysis also showed a strong interaction between gonad weight and fecundity (r = 0.881). The equation was F = 57.635GW+105.88, n = 108, where F indicates fecundity, and GW indicates the gonad weight of O. niloticus.
Diet composition: From the 987 total samples collected for food and feeding habit analysis, 28 samples (2.84%) were empty, and the remaining 959 specimens (97.16%) stomachs were non-empty, which consists of several food items. Seven major food items were identified: phytoplankton, detritus, macrophytes, zooplankton, nematodes, fish eggs, and digested muscle (Table 3). Phytoplankton occurred to 95.72%; volumetrically, contributed to 88.20%, and the index of preponderance also shows 96.60% compared to the total food items. Compared to different types of phytoplankton, the non-filamentous types like blue-green algae and diatoms were the most preferred food item by O. niloticus. Detritus and macrophytes are also the second and third most preferred food items occurrence in 31.07% and 18.98%, with volumetric contributions to 7.16% and 2.53%, and index of preponderance 2.54% and 0.55%, respectively. Zooplankton is also the intermediate preferred food item, which occurred in 15.85%, volumetrically contributed to 1.68%, and the index of preponderance recorded 0.30%. However, nematodes, fish eggs, and digested muscles were the least contributors compared to the other preferred food items.
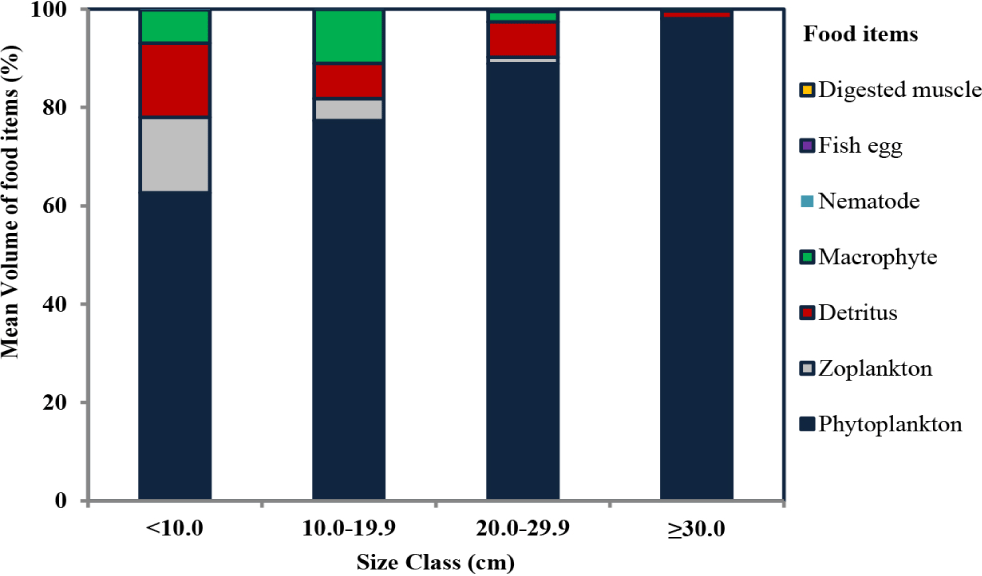
Ontogenetic dietary shift and dietary overlap: The diet composition of O. niloticus from Lake Hawassa indicated a slight ontogenetic dietary shift throughout their lifetime (Fig. 4). However, those slight shifts of feed resources have no significant change in all size classes which showed a strong dietary overlap (C > 0.6; Table 4). The contributions of phytoplankton increase when their size class increases. At smaller sizes below 10.0 cm, phytoplankton contributed to 62.63%; whereas in the size class10.0–19.9 cm, the phytoplankton contributed to 77.32%; at 20.0–29.9 cm, it contributed to 88.90%; and the larger size above 30.0 cm contributed to 98.05%. In a contradictory way, the contributions of zooplanktons at smaller sizes below 10.0 cm contributed more, and their contributions slightly decreased when their sizes increased. On larger size classes of O. niloticus above 30.0 cm, zooplanktons have no contributions, and their size class increases.
| Size class (cm) | Shooner dietary overlap index (C) |
|---|---|
| < 10 and 10–19.9 | 0.81 |
| < 10 and 20–29.9 | 0.73 |
| < 10 and > 29.9 | 0.65 |
| 10–19.9 and 20–29.9 | 0.88 |
| 10–19.9 and > 29.9 | 0.79 |
| 20–29.9 and > 29.9 | 0.91 |
Seasonal variations: O. niloticus from Lake Hawassa showed slight diet variations in rainy and dry seasons. During the rainy season, the species preferred phytoplankton > detritus > macrophyte > zooplankton > nematode > fish egg > digested muscles (Table 5). However, except for macrophytes, prey occurrences in the rainy season didn’t significantly differ from the dry season (χ2, p > 0.05). Similarly, except for detritus, the volumetric contributions of prey items didn’t show a significant difference from the dry season contributions (t-test, p > 0.05). In the rainy season, phytoplankton occurred in 96.20%, volumetrically contributing to 87.70% and 96.36% on the index of preponderance. Blue-green algae were the major contributing prey item, compared to other phytoplankton taxa. Detritus was the second most preferred prey item in the rainy season, which recorded 32.07%, 8.25%, and 3.02% on the frequency of occurrences, volumetric contributions, and index of preponderance, respectively. Macrophyte and zooplanktons are intermediate contributors, which represent 15.33%, 1.91%, 0.33%; and 15.05%, 1.65%, and 0.82% in frequency of occurrences, volumetric contributions, and index of preponderance, respectively. Nematodes, fish eggs, and digested muscles are the least contributors compared to the major food items in the rainy season.
a, b Values of respective food items under the same category given different superscript letters are significantly different (α < 0.05).
During the dry season, the contributions of diet com-positions indicate phytoplankton > macrophytes > detritus > zooplankton > fish eggs. Unlike the rainy season, during the dry season, macrophyte is considered the second most important food item, contributing to 29.44%, 4.20%, and 1.42% with the frequency of occurrences, volumetric contributions, and index of preponderance respectively. Detritus is also an intermediate contributor during dry season, which occurred in 28.23% and accounted for 4.18%, and 1.35% on the index of preponderance. Regarding the zooplankton, their occurrence (18.15%), contributions (1.77%), and preponderance (0.37%) showed a slight increase in the dry season compared to the rainy season. Unlike the rainy season, fish eggs contribute a very small amount, and there were no contributions from nematodes and digested muscles in dry seasons.
The length-weight relationship of O. niloticus in the present study showed a curvilinear relationship (Fig. 5A–5C), and the regression parameters generated from the power function of the length-weight relationship are statistically significant (ANOVA, p < 0.05). The slope of the regression line (b) for males, females, and mixed sexes of O. niloticus were 3.23, 3.25, and 3.24, respectively. The growth of both sexes followed a positive allometric growth pattern (b > 3). The p-value of the exponent (b) was significantly different from 3 for males, females, and both sexes (t-test, p < 0.001). The length-length relationship of O. niloticus showed a strong relationship (r2 = 0.99), and is highly correlated (r = 0.99; Fig 5D).
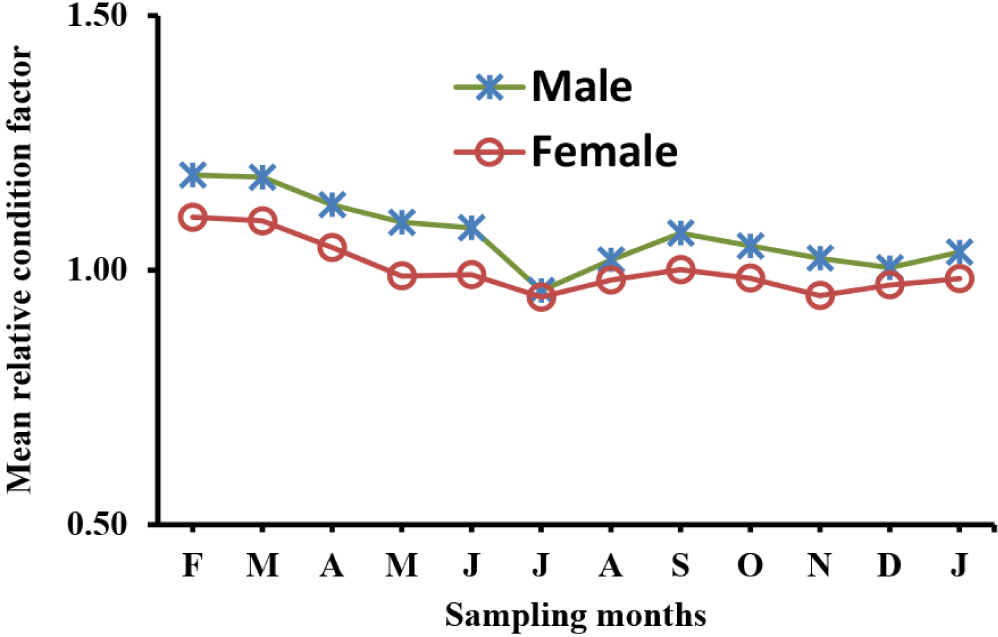
The relative condition factors of O. niloticus from Lake Hawassa ranged from a minimum value of 0.52 to a maximum value of 1.58 for females with a mean ± SE value of 1.01 ± 0.0063, while that of the males ranged from 0.63 to 1.87 with a mean value of 1.08 ± 0.0074. The largest relative condition factor was recorded in February and March for both sexes, while the smallest value was recorded in July for both sexes. The value of the relative condition factor indicates a significant variation between sampling months (ANOVA, p < 0.05). However, the inte-raction between months by sex shows an insignificant variation (ANOVA, p > 0.05), meaning the trend for the O. niloticus relative condition factor shows a similar fluctuation pattern between sexes (Fig. 6).
Discussion
The overall sex ratio (M:F) for O. niloticus in the present investigation was 1:0.93, test = 2.37, suggesting that, there was no significant difference from the theoretical 1:1 sex ratio. The current results are consistent with other research, which found no significant differences in the O. niloticus sex ratios such as from Lake Boyo as reported by Handago et al. (2024), and Lake Awassa as reported by Admassu (1994). However, the current result showed contrary to certain other studies that found significant differences in the sex ratios of the same species such as: from Lake Tana as reported by Tadesse (1997), and from Lake Hayq as reported by Tessema et al. (2019). Sex ratio variations might be related to different reasons such as the method of fishing (Admassu, 1994), fishing pressure (Tekle-Giorgis et al., 2017), and sexual segregation (Dadebo et al., 2011).
In the current study, TL50 was 18.5 cm for males and 19.1 cm for females, respectively. The TL50 from the present study showed a larger value compared to other studies from the same species such as: from Lake Hayq where the TL50 value was 12.8 cm for males and 12.9 cm for females (Tessema et al., 2019), and 10.0 cm for males and 7.7 cm for females from Lake Boyo (Handago et al., 2024). However, compared to certain different lakes that were reported from the same species, the TL50% maturity was smaller in Lake Hawassa. These included from the same study area Lake Hawassa 20.8 for males, and 20.3 for females O. niloticus (Muluye et al., 2016), and 20.5 cm for males and 19.6 cm for females from Lake Tana (Degsera et al., 2021). The variations of TL50% maturity might be fishing pressure, mortality, and food availability (Admassu, 1994). For example, Muluye et al. (2016) reported 20.8 for females and 20.3 for males O. niloticus in the same study area, which indicates a higher value than the current research. However, studies from Lake Hawassa that focused entirely on dealing with problems related to fish stock assessments reveal a trend toward fishing that is becoming more than the safe level (Tekle-Giorgis, 2018). Harvesting of immature fish, rising fish mortality, and exploitation are negatively correlated with TL50 (Tekle-Giorgis et al., 2017). Fish try to adapt to new environmental conditions and shift to sustain their recruits when the fishing pressure increases (Dadebo et al., 2011).
In the current investigation, O. niloticus breeds throughout the year in Lake Hawassa, it was seen February through April is the first peak breeding season, and January is the second peak breeding season. The present finding agrees with different studies on the same species which were identified from different water bodies with the exceptions of some peak breeding months. For instance, O. niloticus breeds throughout the year in the same study area, with January to March and August to October representing the peak breeding seasons (Admassu, 1994). February to March, and July to September from Lake Boyo (Handago et al., 2024), between February and April in Lake Hayq (Tessema et al., 2019) were seen as the peak breeding months of O. niloticus. The variations of ripe fish proportions might be a result of temporal variation (Tadesse, 1997), and food availability (Admassu, 1996). O. niloticus prefers to reproduce where their offspring survive more especially during the rainy months (Admassu, 1996). Based on the present study, O. niloticus showed intensive breeding in both rainy and dry months. This might be the effect of limnological-related aspects. The diverse compositions of phytoplankton and zooplankton from Lake Hawassa help to continue their breeding at any time of the year when they get favorable situations (Admassu, 1996). Additionally, the dominant food resource of O. niloticus like blue-green algae specifically microsistis from Lake Hawassa didn’t show a significant change in rainy and dry months (Kebede & Belay, 1994). The above-mentioned taxa of blue-green algae are protein-rich (de la Fuente et al., 1977), and might create a favorable situation for O. niloticus breeding at any time of the year in Lake Hawassa.
With a mean value of 556 eggs per fish, the fecundity O. niloticus in Lake Hawassa ranged from 105 eggs per fish to the highest value of 1,541. O. niloticus from Lake Hawassa have good fecundity potentials in contrast to Lake Hayq, where 217 eggs per fish were reported (Tessema et al., 2019). However, O. niloticus from Lake Hawassa shows very poor fecundity potential than Lake Tana, where 730 eggs/fish on average (Tadesse, 1997). The individual size difference, ovary size, and quality of food resources might be the reason for variations in the fecundity potential of O. niloticus. For instance, the digestive efficiency of Lake Hawassa O. niloticus is higher than Lake Langano (Tadesse, 1999). Therefore, the quality of prey that they eat may limit their fecundity potential.
The O. niloticus prey items found in Lake Hawassa are similar to those seen in other lakes of the same species such as from Lake Tinishu Abaya (Anteneh et al., 2023), Lake Langano (Temesgen et al., 2022), Koka reservoir (Engdaw et al., 2013), and from the same study area (Tudorancea et al., 1988), and from the Gulf of Gorgora (Lake Tana) as reported by Engdaw (2023). Among the identified prey items of O. niloticus from Lake Hawassa, phytoplankton are the dominant (primary) prey item, which occurred at 95.72% of non-empty O. niloticus. This result agrees with some other Ethiopian Rift Valley Lakes which reported that phytoplankton were the primary prey for O. niloticus. For instance, the occurrences of phytoplankton from O. niloticus from Tinishu Abaya showed 94.1% (Anteneh et al., 2023), 100.0% from Lake Langano (Temesgen et al., 2022), 77% from Lake Tana (Engdaw, 2023), and 80.1% from Koka reservoir (Engdaw et al., 2013). Unlike the present finding, Handago et al. (2024) from Lake Boyo reported detritus was the most frequently occurring prey item, which occurred 99.2%.
Compared to the different taxa of phytoplankton, blue-green algae are the most frequently occurring prey item from O. niloticus of Lake Hawassa based on the present finding (Table 3). Similar results have been reported from Lake Langano (Temesgen et al., 2022), from Tinishu Abaya (Anteneh et al., 2023), Gulf of Gorgora, (Engdaw, 2023), and from Koka Reservoir (Engdaw et al., 2013). Contradictory to the present finding, Tadesse (1999) from Lake Langano, reported that diatoms are the most frequently occurring prey. This variation might be related to the individual preference of fish, morphometry of Lake, biomass, and abundance of phytoplankton.
Based on volumetric contributions, phytoplankton contributed 88.2% of O. niloticus from Lake Hawassa and it is considered a primary prey item. The present finding agrees with other reports which indicate phytoplankton from non-empty O. niloticus contributed 39.5% from Tinishu Abaya (Anteneh et al., 2023), 47.5% from the Koka reservoir (Engdaw et al., 2013), 44.3% from the Gorgora Gulf of Lake Tana (Engdaw, 2023), and 64.3% from Lake Langano (Temesgen et al., 2022). Unlike the present finding, the volumetric contributions of macrophytes from Lake Boyo (73.6%) were the primary prey items for O. niloticus (Handago et al., 2024). Detritus is the second most occurred prey item of O. nilotics in both frequency of occurrence and volumetric contribution accounting for 31.1% and 7.2% from Lake Hawassa. This finding agrees with other Ethiopian Rift Valley Lakes of O. niloticus prey items, such as O. niloticus from Lake Tinishu Abaya detritus where the second important prey item occurred at 74.3% (Anteneh et al., 2023). Contradictory from the present result, macrophyte was the second contributing prey (25.8%) in volumetric contribution from Lake Tinishu Abaya (Anteneh et al., 2023).
Cladocera is a major prey item, compared to other texa of zooplankton which occurred at 7.9%. Unlike the present finding, rotifers were a major prey item compared to other zooplanktons texa from other Rift Valley Lakes such as O. niloticus from Lake Langano (Temesgen et al., 2022), and Tinishu Abaya (Anteneh et al., 2023). This might be the effect of pollution where the biomass of zooplankton including Rotifera from Lake Hawassa shows a decreasing trend as a result of anthropogenic effects (Beyene et al., 2022). The occurrence and contributions of nematode from Lake Hawassa are too low compared to Lake Tana which reported 28.1%, and 0.1% respectively (Engdaw, 2023), and from Lake Langano which indicates 7.8% and 0.3% respectively (Temesgen et al., 2022). These variations might be related to the protein source of prey items (Tadesse, 1998). In the case of Lake Hawassa, there are different optional planktons contributed as protein sources. Additionally, some algae contributed as a prey item for O. niloticus in the present study such as blue-green algae specifically microsists are the most frequent prey item compared to different blue-green algae. Microsists are protein-rich (de la Fuente et al., 1977), and O. niloticus concentrates on protein-rich prey (Tadesse, 1998).
Based on the index of preponderance methods of comparison, the major prey item for O. niloticus from Lake Hawassa showed 96.6% phytoplankton (Table 3). A similar finding is reported from other inland water bodies of Ethiopia. For example, Temesgen et al. (2022) from Lake Langano reported 74.5%, and 52.3% were reported from Lake Tana (Engdaw, 2023). Contradictory to the present finding, detritus denotes 79.1% from Lake Boyo (Handago et al., 2024).
Unlike the present finding, it was reported that aquatic insects are considered important prey items for O. niloticus. For instance, from Lake Tana (Engdaw, 2023), Lake Langano (Temesgen et al., 2022), and the same study area Lake Hawassa, zoobenthos also has a contribution as a prey for O. niloticus (Tudorancea et al., 1988). These variations might be the effect of interspecific competitions. Aquatic insects from Lake Hawassa have a role as a diet of larger fish species of Lake Hawassa like C. gariepinus (Dadebo, 2000). The present finding indicates there was a slight ontogenetic dietary shift of O. niloticus from Lake Hawassa (Fig. 4) but not significance shift (C > 0.6). In all size classes, the contributions of phytoplankton were identified from the stomach of O. niloticus (Fig. 4). The contributions of animal-origin sources are inversely related to the size class of O. niloticus from Lake Hawassa. A similar finding was reported from Koka Reservoir (Engdaw et al., 2013). This indicates that in lower-size classes, fish require a high protein source for survival and overall growth activities (Temesgen et al., 2022). On the other hand, O. niloticus in the lower size class the size of their stomach is small, and have not much space to load and digest larger food items like plant origins (Engdaw et al., 2013).
At all, except for the volumetric contributions of detritus and occurrences of macrophyte, there was no statistical significance diet composition of O. niloticus in Lake Hawassa from the present study on rainy and dry seasons comparisons (Table 5). The present finding contradicts other studies that were carried out at Lake Langano (Temesgen et al., 2022). The above-mentioned researchers identified a seasonal variation in phytoplankton occurrences from the stomach contents of O. niloticus. The present non-significance phytoplankton variations might be the effect of dietary overlap. The present study indicates a strong overlap index (Table 4) and the preference of prey might be limited.
Based on the current study, O. niloticus has a curvilinear length-weight relationship and is highly significant (r2 > 0.98, p < 0.05). The finding agrees with other studies on the same species such as from Lake Boyo (Handago et al., 2024), and Lake Tana (Engdaw, 2023). The finding also confirms some former works which were identified as allometric growth such as in Lake Boyo with the value of regression coefficient 3.18 for females, and 3.12 for combined sexes exhibited slight positive allometric growth pattern as reported by (Handago et al., 2024). The present results are in disagree with some findings that were identified as isometric, and negative allometric growth, such as those from Lake Hayq, where b = 2.50 for females and 2.33 for males, respectively (Tessema et al., 2019), and b = 2.8 from the Gulf of Gorgora in Lake Tana (Engdaw, 2023). These variations of slope (b) of O. niloticus depend on several external and internal factors. During the present study, the dry months were very short. This might affect the total biomass production in the lake which might create good conditions for growth. There is a contradictory idea of the primary productions of Lake Hawassa. for instance, primary production increases in wet months compared to rainy months (Gebre-Mariam & Taylor, 1989). In a contradictory way, the nutrient mixing and primary production from Lake Hawassa increase during the dry month (Tilahun & Ahlgren, 2010). This situation might be helping the growth of O. niloticus to get good food resources in both dry and rainy months.
The mean relative condition factors of O. niloticus for the present study were found to be 1.08 ± 0.0074 for males and 1.01 ± 0.0063 for females. The mean relative condition factors for both sexes showed Kn > 1, indicating that the health status of O. niloticus from Lake Hawassa is currently safe (Jones et al., 1999). These findings align with comparable values reported for O. niloticus populations in Lake Victoria (Njiru et al., 2006). The slight differences in relative condition factors between males and females may reflect physiological demands related to reproduction and environmental influences during the sampling months (Jones et al, 1999).
Conclusion
The present study shows that O. niloticus male-to-female sex ratio (1:0.93) is almost equal to the theoretical 1:1 ratio. The breeding seasons of O. niloticus from Lake Hawassa were bimodal, intensively breeding from February to April, and by January. O. niloticus under 19.1 cm total length should be protected to save the non-breeding fish from unwise fishing. It is also recommended to close at the peak breeding season for continuing their recruits. Based on the stomach contents of O. niloticus from Lake Hawassa, it is seen that both plant and animal origin are ingested. Strong dietary overlaps are observed in the present finding and are predominantly focused on phytoplankton. Insignificant dietary shift and phytoplankton dominance in the diet of O. niloticus suggest that a parallel study on phytoplankton biomass and primary production, as a proxy of yield and management of Tilapia stock in Lake Hawassa, should be considered for future studies.