Introduction
Tropical mangroves and estuaries are recognized for their importance as feeding and refuge areas for fish and invertebrates (Alongi, 2015). In tropical regions, those ecosystems are generally associated with estuaries that harbor fish, many of them being important fishing resources such as snappers, catfish, snooks, jacks, crappies (Sandoval et al., 2020), which also represent food security for human populations related to mangroves. Despite its clear importance for fisheries and coastal ecological dynamics and numerous international treaties having been agreed, mangrove forests are being degraded at an alarming rate due to widespread deforestation (Kanniah et al., 2021).
In tropical regions, fish associated with mangroves are categorized according to their use of the ecosystem (Sepúlveda-Lozada et al., 2015), estuarine residents, estuarine dependents, estuarine opportunist, freshwater migrants or marine stragglers, among others. Fish are energy links to other coastal ecosystems such as subtidal hard and soft bottoms, seagrasses, rocky and coral reefs due to their mobility between habitats. In tropical Indo and eastern Pacific mangrove-estuaries, predatory fish feed preferentially on brachyuran crabs (Ramirez-Martínez et al., 2016), that process a large proportion of mangrove leaf-litter (Kristensen et al., 2017). This interrelation mediates the export of organic matter derived from mangroves.
The yellow snapper Lutjanus argentiventris is a marine-estuarine dependent fish of high fishery importance. In the Gulf of California this species shows an approximate production of 8 t/year (Aburto-Oropeza et al., 2009) and in the Pacific of Colombia about 11 t/yr, 80% of which comes from artisanal fishing (de la Hoz et al., 2015). Stomach contents studies indicate that this species is a selective carnivorous-omnivorous predator mainly of crustaceans and fish (Martinez-Pinto & González Leiva, 2017; Ramirez-Martínez et al., 2016; Vega, 2016), which consumes mainly benthic organisms and few pelagic species. There have also been reported differences in the feeding habits between juveniles and adults (Aburto-Oropeza et al., 2009; Vega, 2016). L. argentiventris spends its first life stages in mangrove systems while adults inhabit coral and rocky reefs or caves (Aburto-Oropeza et al., 2009; Martinez-Pinto & González Leiva, 2017; Ramirez-Martínez et al., 2016; Vega, 2016), which highlights the importance of mangroves in sustaining part of the coastal fishery production.
Stable isotope analysis (δ13C, δ15N) has been used as a tool to describe trophic structure, isotopic niche and habitat use of organisms in mangrove ecosystems (Layman et al., 2012; Muller & Strydom, 2017). The δ13C values of the organisms can be used to infer primary sources that support food webs (Tripp-valdez et al., 2015), due to differences in isotopic composition of the primary producers. Moreover, δ15N values are a useful tool for estimating trophic level/position (Post, 2002), as consumer tissues are generally enriched in 15N relative to their food sources. Relatively recent analytical tools also allow the involvement of δ13C to infer consumers’ trophic position (Quezada-Romegialli et al., 2018).
Likewise, using dietary information and modeling tools, it’s possible to describe relationships between species of an ecosystem food web. However, these relationships can vary their intensity level between species, that’s why some links or nodes can perform key roles on the energetic transfer through the system (Zetina-Rejón et al., 2015). In this sense, network analysis generates important information about the food web structure, as well as knowledge about the clusters of species that are more closely interconnected with each other and with other species (Estupiñán-Montaño, 2022). This allows for identifying and comprehending the complexity of the relationships between the species and the relevance of each of them as a link between trophic levels.
Detailed network analyses of the food web supporting L. argentiventris in a mangrove ecosystem context are rare or lacking. Studies that address the trophic ecology of the species have been based on feeding habits (Ramirez-Martínez et al., 2016; Vega, 2016), reproductive biology, diet, fishery (Martinez-Pinto & González Leiva, 2017), recruitment and ontogeny (Aburto-Oropeza et al., 2009). For this purpose, stable isotopes combined with network analysis can be an effective tool to determine species-specific relationships with other species.
In mangroves of the Colombian Pacific, L. argentiventris is a species of high abundance and relative frequency. It has been reported in a size range from 3 to 39 cm indicating that mangroves are inhabited mainly by juvenile and subadult stages (Ramirez-Martínez et al., 2016; Vega, 2016), therefore, this specie is highly dependent on these ecosystems to maintain its population stock. Additionally, L. argentiventris is one of the most valued coastal resources in this region and in the tropical eastern Pacific (Aburto-Oropeza et al., 2009; Martinez-Pinto & González Leiva, 2017; Ramirez-Martínez et al., 2016; Vega, 2016).
In the present study, we analyzed the trophic pathways that sustain the yellow snapper L. argentiventris in a riverine mangrove creek in the central coast of Colombia. Five research objectives were investigated: (1) characterize the isotopic composition (δ13C and δ15N) of L. argentiventris and evaluate if there are significant differences according to size classes, (2) calculate the trophic position for each size class, (3) calculate the average relative contribution from different prey groups to L. argentiventris size classes, (4) estimate the isotopic niche of every size class and the overlap between them and (5) using isotopic data, generate a trophic model to apply network analysis focusing on the role of L. argentiventris in the mangrove ecosystem.
Materials and Methods
The study was conducted in a near pristine riverine mangrove creek (Quebrada Valencia) located in the Panama Bight, central coast of Colombia (Fig. 1). This region is one of the wettest (mean humidity between 88 and 100%) and rainiest (average of 7,236 ± 2,033 mm between 1969 and 2015; station no. 5407003-IDEAM) in America (Medina-Contreras et al., 2018) and has one of the highest annual rainfalls for coastal areas in the western hemisphere (Cantera & Blanco, 2001). The riverine mangrove system (covering 1,240 ha) where the study was developed is in the internal zone of the Bahía Málaga estuary (04°06′35.9″N, 77°15′03.9″W), characterized by its heterogeneity of habitats and declared a Marine Protected Area in 2010. Bahía Málaga has approximately 12,000 inhabitants concentrated mainly in the outer zone, with a small population in the inner part of the bay (< 500 people), with most villager’s dependent on fishing (Palacios & Cantera, 2017). The dominant mangrove species in the mangrove system are Rhizophora mangle, Mora oleifera and to a lesser extent Conocarpus erectus. Relevant characteristics of the area are shown in Table 1.
* Data from Palacios-Peñaranda et al. (2019).
In this study area, L. argentiventris is one of the most common species belonging to the Lutjanidae family that recruit and reside in the mangrove swamp in juvenile stages of their life cycle. Distributed from the Gulf of California to Peru, including all oceanic islands except Clipperton (Robertson & Allen, 2015), the yellow snapper is one of the main species caught in artisanal and industrial fisheries in Colombia, therefore it’s considered a highly important fishery resource for this region (Ramirez-Martínez et al., 2016). The artisanal techniques used for this species capture organisms from 20 cm to 70 cm using hand line, gillnetting and bottom-set gillnetting.
Specimen collection was conducted in April 2016; in two diurnal and nocturnal sampling campaigns. Two intertidal mangrove creeks dominated by R. mangle, similar in topography and with an exclusive mouth to the main estuarine channel, were selected. The creeks were blocked at their mouth with block nets (20 × 4 m, 12 mm mesh). Block nets are a common and highly effective method for catching fish that enter intertidal vegetated creeks, representing the composition of fish that consume intertidal mangrove resources, and have been used successfully in mangrove systems around the world and in the eastern Pacific (Ramirez-Martínez et al., 2016). In each creek, at low tide, a block net was deployed. Each net was placed manually on the muddy bottom, covering the creek width. The entire net was then rolled up and attached to the bottom to avoid it ascending during the rising tide. A wooden pole (about 5 m high) was also placed vertically in the center of the creek mouth. Once the tide had risen, the net was extended from the bottom upwards, attached to the wooden pole, and at each side to a mangrove root, covering the entire channel flow. Fish that migrated with the tide into the mangroves were caught as they left the creek at low tide.
Once captured, fish specimens were transported to the research station in the study area and identified using taxonomic guides (Robertson & Allen, 2015). Specimens of L. argentiventris were selected and classified into three size classes based on the reproductive stage identified for this species (Aburto-Oropeza et al., 2009; Martinez-Pinto & González Leiva, 2017; Ramirez-Martínez et al., 2016; Vega, 2016): small juveniles (TL, total length 4– < 10 cm), medium juveniles (TL 10–20 cm) and subadults (TL > 20 cm). For all specimens, TL and weight were measured, and muscle tissue was extracted for isotopic analysis. White muscle tissue from specimens was removed from the trunk below the dorsal fin, carefully washed with distilled water, and preserved on ice during transport to the laboratory, where it was stored in a Thermo Scientific cooler at –21°C; until processing. Baseline specimens for the trophic position estimation were collected manually during low tide, the leaf-eating crab Aratus pacificus as the benthic baseline and the filter feeder crab Petrolisthes zacae as the pelagic baseline. Species comprising the prey groups were also captured manually. Baseline and prey specimens were both preserved whole on ice and transported to the laboratory for processing.
All specimens and samples were carefully washed and thawed before processing. In baseline specimens, the chelae of the crab species were removed. Only white muscle was used for analysis due to its lower δ13C and δ15N variability compared to other types of tissue (Schielke & Post, 2010). All captured specimens of L. argentiventris were used for the isotopic analysis. Samples were dried at 65°C for at least 48 hours in an oven. Then, they were homogenized using mortars until a fine powder was obtained. Between 0.7 and 1 mg of the sample was then weighed and deposited in a tin capsule for subsequent isotopic analysis using a IRMS Delta V Plus (Thermo Scientific, Waltham, MA, USA), coupled to an elemental combustion analyzer, model ECS 4010 (NC Technologies, Milano, Italy), Costech Instruments, in the Instituto Politécnico Nacional, Centro de Interdisciplinario de Ciencias Marinas, La Paz, Baja California Sur, México.
Stable isotope results were expressed in delta notation in (‰) based on the following equation:
where X is the element, h the atomic mass of the isotope, Rsample and Rstandard the isotopic relation between the heavier and lighter isotopes (e.g., 13C/12C and 15N/14N) of the sample and standard respectively. The standard used was Pee Dee Belemnite for carbon, and atmospheric nitrogen for nitrogen. The analytical precision of isotopic measurements was < 0.1‰, implementing the certified primary standards (IAEA-NO–3, IAEA-N, USGS-40, USGS-63) for δ13C and δ15N.
Trophic positions (TP) for all captured specimens and by every fish size class were estimated using the “tRophicPosition” package. The analysis incorporates a Bayesian model using isotopic data of consumers and one or two baselines for the calculation of consumer TP at the population level. It combines Markov Monte Carlo Chain simulations through JAGS and statistical and graphical analyses using R (Quezada-Romegialli et al., 2018). The baseline species incorporated in the two baselines model were the sesarmid crab A. pacificus and the porcelain crab P. zacae. Trophic enrichment factors (TEF) selected were 2.3‰ ± 0.26 for δ15N (Schwamborn & Giarrizzo, 2014) and 1.3‰ ± 0.3 for δ13C (McCutchan et al., 2003). To assess the differences between the three sites size classes, a paired Mann-Whitney analysis was performed. The correlation between weight and length of the individuals with δ13C and δ15N values was evaluated using a Spearman correlation.
Isotopic niche of L. argentiventris size classes were evaluated with the use of the stable isotope Bayesian ellipses (SIBER) routine (Jackson et al., 2011) for the software R. The routine calculates the total area (TA) of the convex hull which encompassing the data points as an indication of niche width; the sample size-corrected standard ellipse area (SEAC) through one data point (used for graphical representations of ellipses) examining the dispersion of the δ13C and δ15N values in the isotopic space and the Bayesian standard ellipse area (SEAB) corresponding to 95% credible intervals to quantify uncertainty in core isotopic niche areas. The Bayesian approximation model considers both mean and variance of each subgroup (size classes) generating estimators. Finally, distributions through 10,000 Monte Carlo simulations and Markov chains were generated (Jackson et al., 2011).
The package NicheRover (Swanson et al., 2015) was used to assess total trophic overlap values for 95% TA between size classes. This package uses a probabilistic method to calculate niche regions and pairwise niche overlap using multidimensional niche indicator data (e.g., stable isotopes). It provides directional estimates of niche overlap, accounts for group-specific distributions in multivariate niche space and produces unique and consistent bivariate projections of the multivariate niche region, while being insensitive to sample size, incorporating statistical uncertainty through Bayesian approach (Swanson et al., 2015).
The relative contributions of prey groups to the diet of L. argentiventris were estimated using isotopic mixing models using the R package “Simmr” (Parnell & Inger, 2016). This package is a potentiated vertion of the package SIAR and although it contains many of its characteristics, the mixing model executed is more robust, based on a simpler interface and has advanced graphics (Parnell & Inger, 2016). Mixing models are sensitive to sources which are not included, potentially generating erroneous results. Additionally, its discriminatory power also decreases with the number of sources and is strongly influenced by the inclusion of a very high number of samples and their differentiation (Phillips et al., 2014). Thus, all possible sources should be included keeping their number as low as possible, this task was achieved by combining isotopically similar sources to utilize the discriminatory power of the model (Phillips et al., 2014). In view of the above, prey items included in the Simmr models were selected based on a study of stomach contents of the species (Ramirez-Martínez et al., 2016). The analysis was developed for all individuals in general and for each size class.
The groups of preys included in the Simmr model were omnivore shrimps (SHRIMPS-OMN), detritivore shrimps (SHRIMPS-DET), herbivore crabs (CRABS-HER), omnivore and carnivore crabs (CRABS-OMN), porcelain crabs (PORCE), fish that were segregated in two groups: benthic (FISH-BEN) subtidal (FISH-SUB) and finally annelids (WORMS).
For the construction and analysis of the trophic relationships between the studied species, a matrix composed of 16 groups was built, three groups correspond to L. argentriventris according to the size classes, and the remaining 13 groups were composed by the main dietary items of the studied fish and the potential food sources for these items. This matrix was created with weighted data resulting from the diet contribution estimated by mixing models (Medina-Contreras et al., 2022a) and stomach contents information (Ramirez-Martínez et al., 2016). The network analysis was performed using igraph package ver. 1.2.11 built for the R programming environment (Csardi & Nepusz, 2006).
To describe the basic properties of the network under study (i.e., complexity, size, centralize degree and diversity), the following global indicators were estimated: 1) Diameter, 2) Average path length, 3) Density, and 4) Clustering (Table 2).
| Index | Definition | Reference |
|---|---|---|
| Global indicators | ||
| Diameter | Longest distance between nodes | Csardi & Nepusz (2006) |
| Average path length | The average distance between any pair of nodes | Travers & Milgram (1969) |
| Density | Ratio of the number of edges and the number of possible edges, which indicates the network complexity | Wasserman & Faust (1994) |
| Clustering | Probability that nodes tend to group together in a networks | Barrat et al. (2004) |
| Local indicators | ||
| Degree | Indicates the number of connections between pairs of nodes and is the sum of the connections between predators and prey. | Estupiñán-Montaño (2022) |
| Closeness | Indicates the speed transmission of information through the food web. The lower Ci indicates quicker transmission through the network than those with higher values. | Wasserman & Faust (1994) |
| Betweenness | This intermediation index represents the capacity of the network to control the exchange of information. | Wasserman & Faust (1994) |
| Eigenvector centrality | Measures the influence of a node in a network, considering not only the total number of adjacent nodes but also considering their importance. | Bihari & Pandia (2015) |
The identification of key trophic groups (trophogroups) was based on the estimation of local indicators of the positional importance of each node in the network. For this purpose, the following indicators were estimated: 1) degree index (prey-predator), 2) closeness index, 3) betweenness index, and 4) eigenvector centrality index (Table 2).
In addition, to find food web compartments between the prey groups described before, we used the “fast greedy community finding” algorithm, which is included in “igraph” package for the R-project. This algorithm maximization function based on modularity that measures network divisions in modules (compartments) based on the concept that there should be more intense connections within modules than between them (Zetina-Rejón et al., 2015).
Results
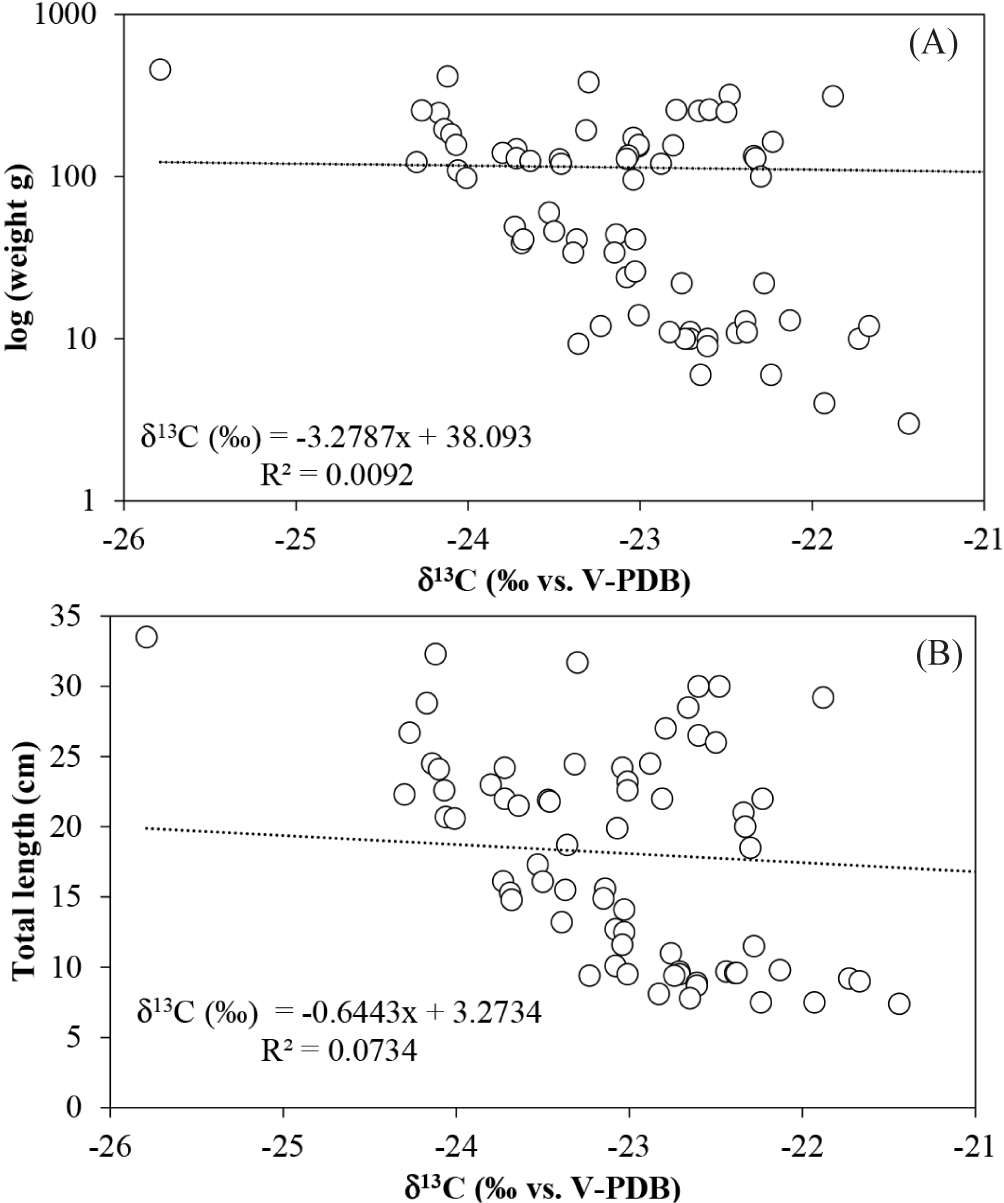
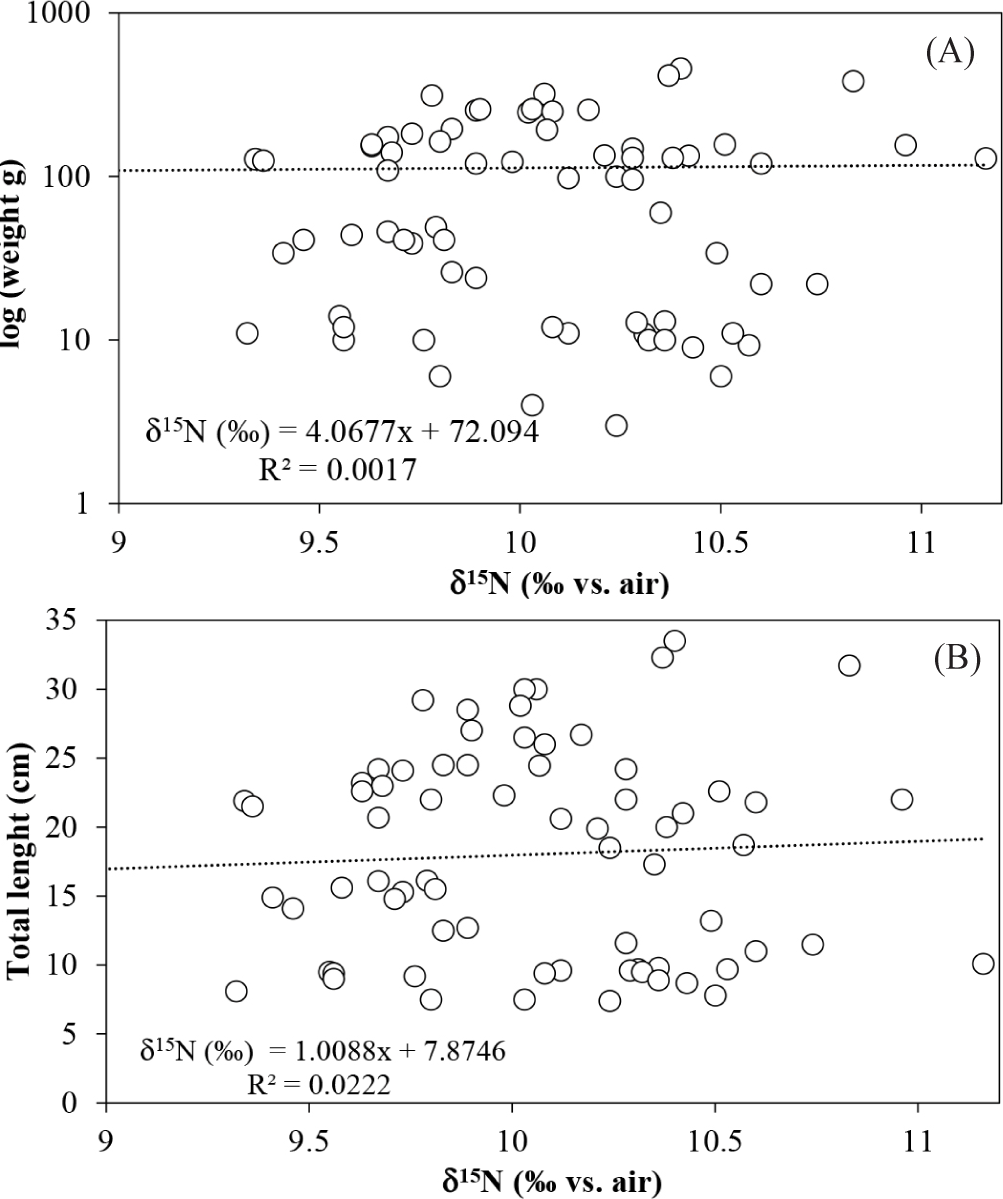
Stable isotope data were obtained from 75 individuals of L. argentiventris ranging in size from 7.4 to 33.5 cm (average 18. 0 cm) and weights between 3 to 458 g (average 111 g). The δ13C values were between –25.8 to –21.4‰ (average –23.1 ± 0.8‰) and δ15N between 9.3 to 11.2‰ (average 10.1 ± 0.4‰; Fig. 2). No correlation was found between δ13C or δ15N values with length or weight of the L. argentiventris individuals (Spearman: R2 < 0.1; Figs. 3 and 4). The group of small juveniles was different (Mann-Whitney p < 0.05) in δ13C compared with the groups of medium juveniles and subadults; these last two groups did not show differences between them (Mann-Whitney p > 0.05). For δ15N, no significant differences were found between any of the three size classes (Mann-Whitney p > 0.05).
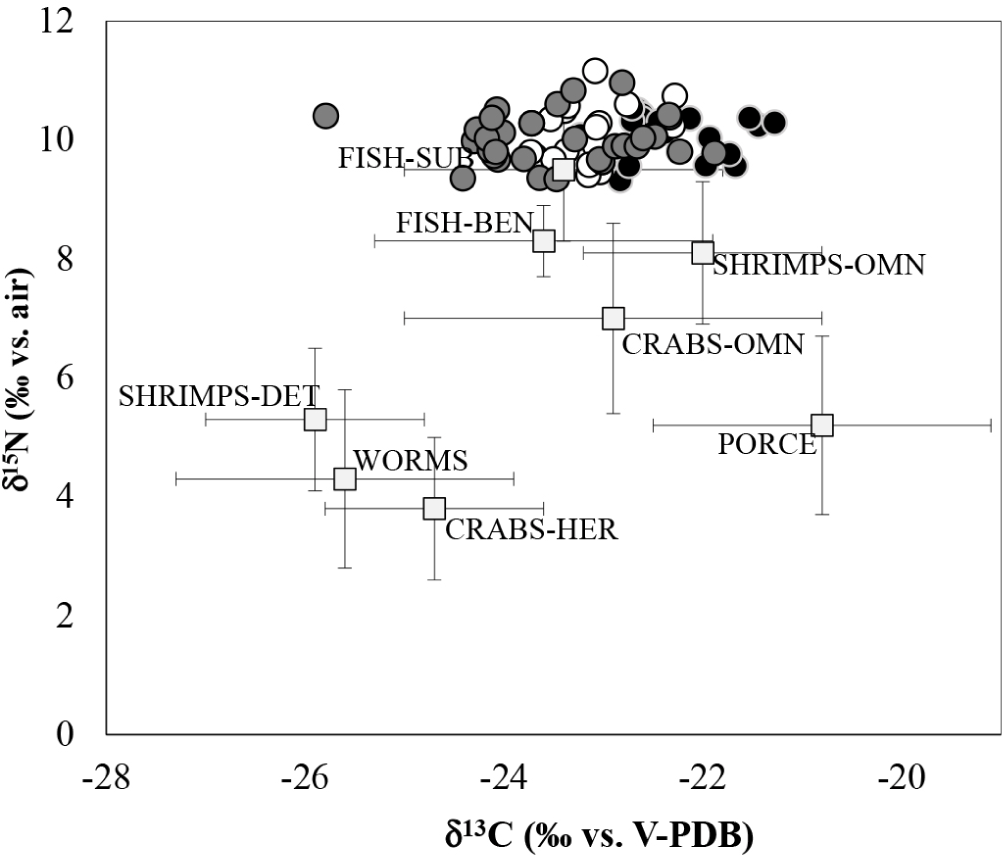
We analyzed 226 isotopic data (C and N) from the tissue of potential prey (Table 3) obtained directly from species collected during sampling (> 20 species) present in the study area and belonging to the preys reported in the stomachs from previous studies (Ramirez-Martínez et al., 2016). The isotopic values of all preys found were δ13C values between –27.5 to –19.1‰ and δ15N values between 2.1 to 10.0‰. SHRIMPS-OMN showed an average δ13C of –22.0 ± 1.2‰ and δ15N of 8.1 ± 1.2‰, this group was composed of four species. SHRIMPS-DET showed average δ13C values of –25.9 ± 2.0‰ and δ15N of 5.3 ± 1. 0‰, group composed of three species. CRABS-HER an average of –24.7 ± 1.1‰ for δ13C and 3.8 ± 1.2‰ for δ15N, group with two species. CRABS-OMN average of –22.9 ± 2.1‰ for δ13C and 7.0 ± 1. 6‰ for δ15N, group with three species. PORCE was composed of one species with δ13C average of –20.8 ± 1.2‰ and δ15N of 5.2 ± 0.6‰. FISH-BEN with δ13C average of –23. 6 ± 1.7‰ and δ15N of 8.6 ± 0.6‰ and FISH-SUB with average δ13C of –23.4 ± 1.6‰ and δ15N of 9.5 ± 1.2‰, these groups were composed of five intertidal benthic fish species and seven subtidal fish species. WORMS showed a δ13C average of –25.6 ± 1.7‰ and δ15N of 4.3 ± 1.5‰. In general, all prey groups were found between the second and third trophic position.
* Data from Ramirez-Martínez et al. (2016).
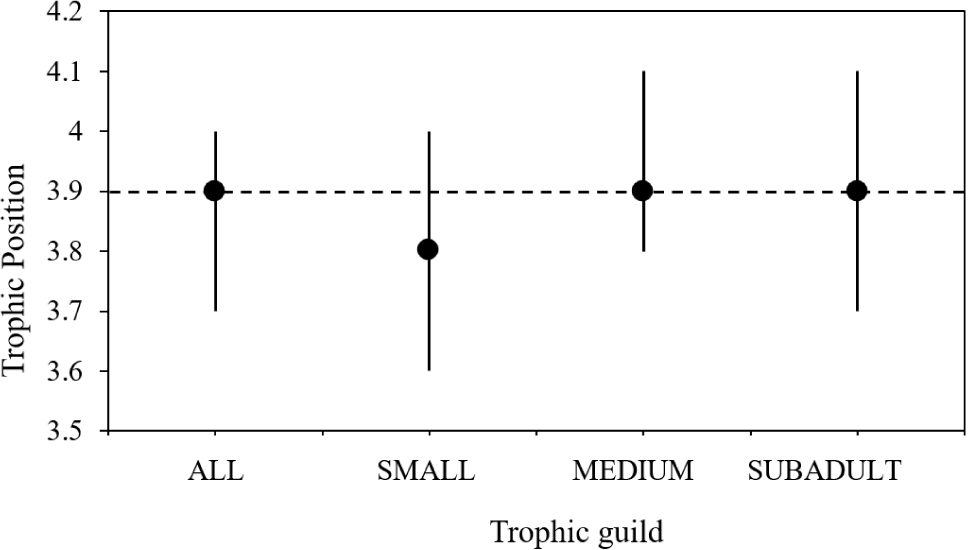
TP of for all specimens of L. argentiventris was estimated between 3.6 and 4.1 (average 3.9 ± 0.1), with few variations between size classes, indicating that the yellow snapper is a predator moving between the third and fourth TP (Fig. 5).
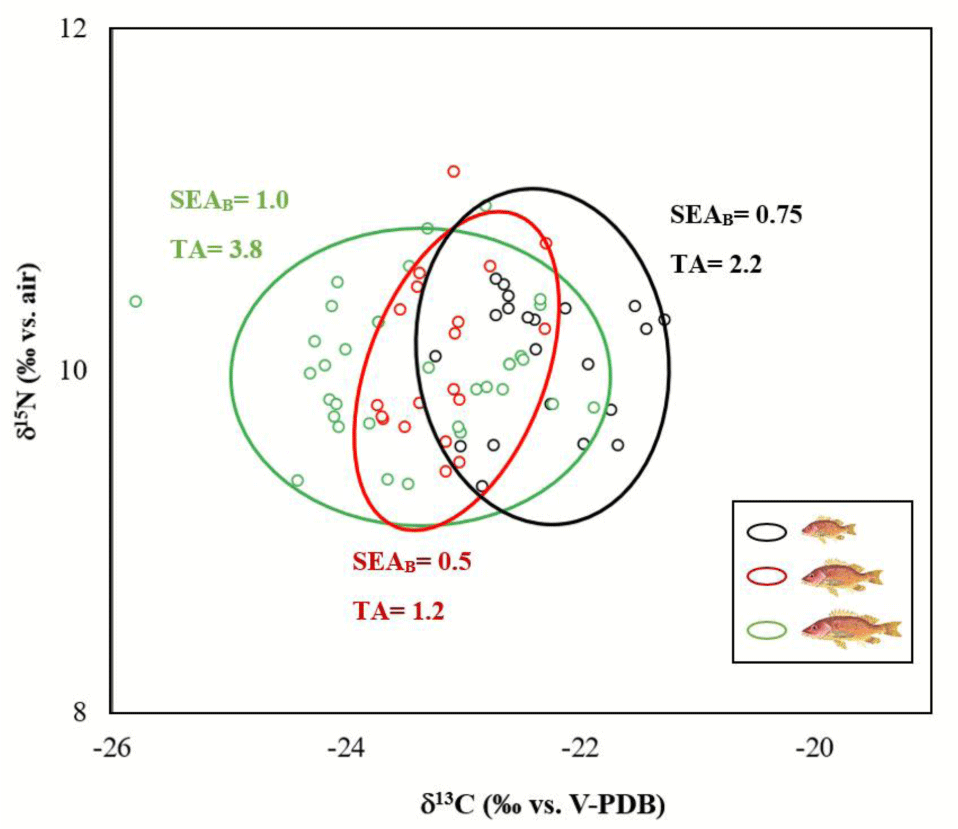
The SIAR analysis showed that subadults specimens (mean δ13C: –23.4‰ – δ15N: 10.0‰) has a wider isotopic niche (TA = 3.8) than small (mean δ13C: –22.3‰ – δ15N: 10.0‰ – TA: 2.2) and medium juveniles (mean δ13C: –23.1‰ – δ15N: 10.1‰ – TA: 1.2). The subadults specimen’s niche tends to grow more through the δ13C axis while the isotopic niche of small and medium size classes tends to grow more in the δ15N axis. The mean probability of niche overlap between the size classes was high, especially between medium vs subadults (94.6%), small vs subadults (80.4%) and medium vs small juveniles (75%). Other possible combination probabilities of niche overlap were between 52 and 64% (Fig. 6).
The results of the Simmr analysis for all individuals (Fig. 7) indicate that the prey groups with the highest probability of contribution were FISH-BEN (Mean: 28% BCI: 12–42%) followed by SHRIMPS-DET (Mean: 25% BCI: 13–35%) and FISH-SUB (Mean: 12% BCI: 2–24%). The other groups presented an average probability of contribution lower than 11%.
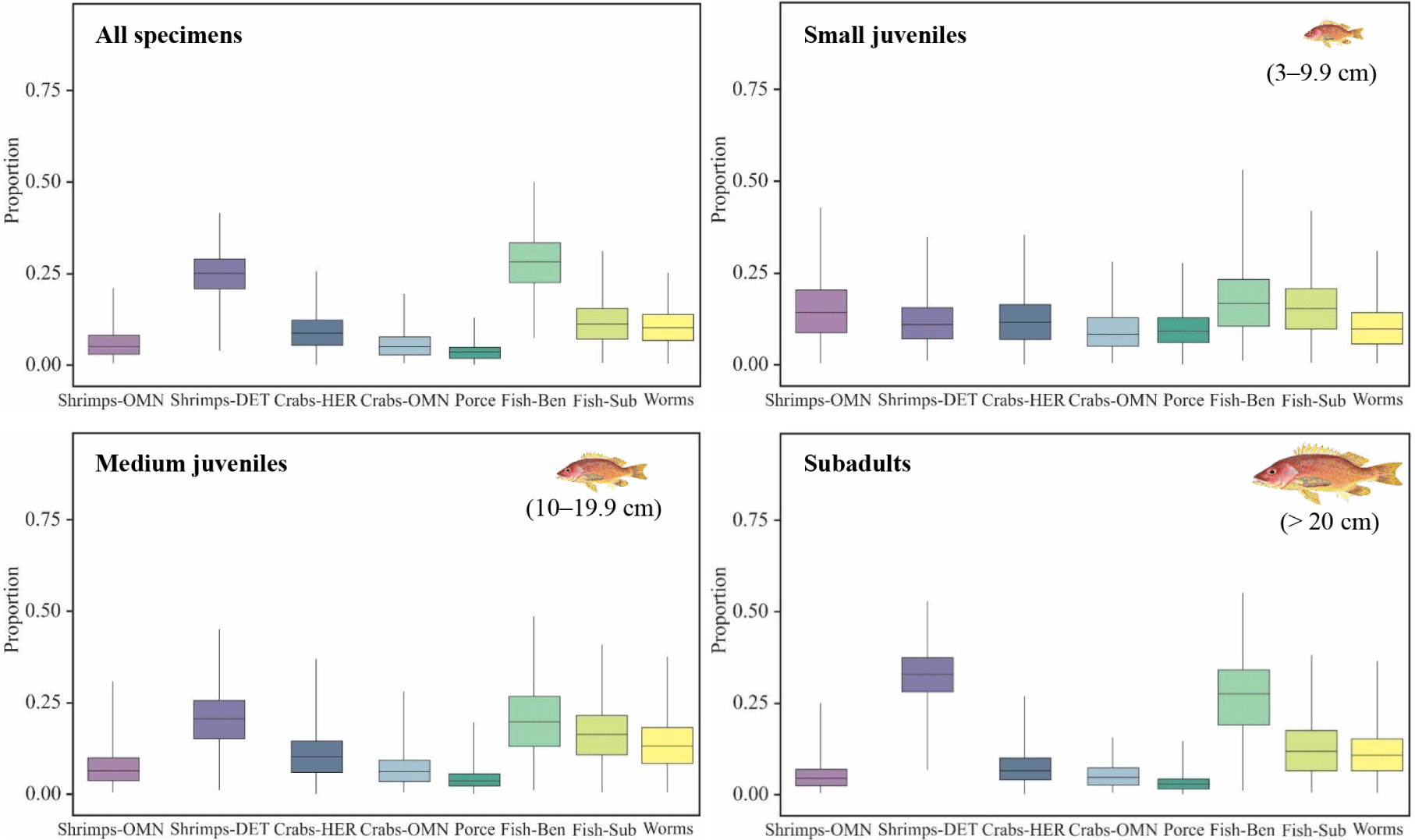
The analysis Simmr for every size class (Fig. 7) showed that for the group of small juveniles (TL 4 < 10 cm), FISH-BEN are the prey with higher relative contribution probability (Mean: 17% BCI: 3–35%), following by two prey groups with similar contribution probabilities FISH-SUB (Mean: 15% BCI: 3–30%) and SHRIMPS-OMN (Mean: 14% BCI: 2–32%), the groups SHRIMPS-DET and CRABS-HER showed the same contribution probabilities (Mean: 12% BCI: 2–25%). The least contribution probabilities were found in WORMS (Mean: 10% BCI: 2–22%), PORCE (Mean: 10% BCI: 2–20%) and CRABS-OMN (Mean: 9% BCI: 2–22%).
The 40% of the mean probabilities for medium juveniles (TL 10–19.9 cm) was from FISH-BEN and SHRIMPS-DET (Mean: 20% each), another 40% of the mean probabilities was distributed between FISH-SUB (Mean: 16% BCI: 3–31%), WORMS (Mean: 13% BCI: 2–27%) and CRABS-HER (Mean: 11% BCI: 2–24%). The last 20% was granted by SHRIMPS-OMN, CRABS-OMN and PORCE. In subadults (TL > 20 – 33.5 cm), is evident that there’s a preference for SHRIMPS-DET (Mean: 31% BCI: 16–44%) followed by FISH-BEN (Mean: 26% BCI: 6–42%), FISH-SUB and WORMS showed similar relative contribution probabilities whit means of 12% and 11% respectively. The last four groups (CRABS-HER, CRABS-OMN, SHRIMPS-OMN and PORCE) contributed less than 10% each (Fig. 7).
Comparing with the other size classes, the small juveniles showed similar mean values (> 20%) of the potential relative contribution percentages between almost all prey groups, which may explain a more generalist diet with a slight preference for fish FISH-BEN which appear to be important prey items for all size classes. PORCE were the group with lower contribution probabilities for all size classes (< 10%; Fig. 7).
Based on the network analysis, the food web supporting L. argentiventris in the mangrove system studied consists of 16 trophogroups and a total of 72 connections (Fig. 8). This food web has a complexity of 0.3 based on the density, a mean distance of 1.46, and a diameter of 3. The clustering coefficient was 71.1% whereas the centralization indicates 0.284, which means that this food network tends to be homogeneous.
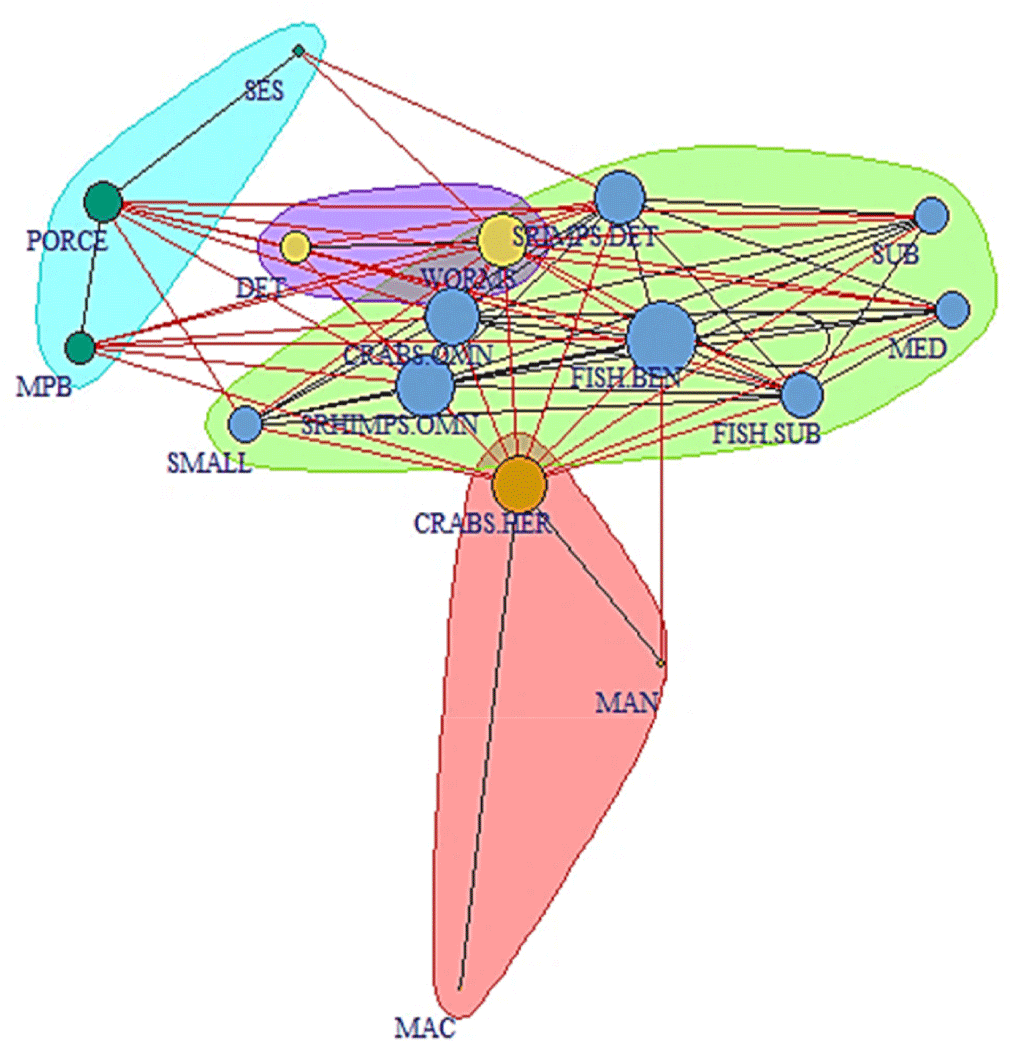
According to the local indicators (Table 4); the groups that have more trophic connections indicated by the degree index were FISH-BEN (1.13), SHRIMPS-OMN (0.93), CRABS-OMN (0.87), and CRABS-HER (0.87), meanwhile the groups with less connections were SES (0.20), MAN (0.13), and MAC (0.07). Based on the closeness index, the groups that have a higher influence on the others were FISH-SUB (2.32), PORCE (1.35) and FISH-BEN (1.20). According to the betweenness index, the most critical groups were FISH-BEN (16.5), SHRIMPS-OMN (13.3), and CRABS-HER (13.3), in comparison with the eigenvector centrality which indicated that WORMS (1), DET (0.82), FISH-BEN (0.77), and SHRIMPS-DET (0.77) were the groups with the greatest influence considering the number of connections and their importance.
SMALL , Small juvenile specimens of the yellow snapper; MED, Medium juvenile specimens of the yellow snapper; SUB, Subadult specimen of the yellow snapper; FISH-BEN, Benthonic fish; FISH-SUB, Subtidal fish; SRIMPS-OMN, Omnivore shrimps; SRIMPS-DET, Detritivore shrimps; CRABS-OMN, Omnivore crabs; CRABS-HER, Herbivore crabs; PORCE, Porcelain crabs; WORMS, annelids; MAN, Mangrove; DET, detritus; MAC, Macroalgae; SES, Seston; MPB, Microphytobenthos.
Discussion
Given the importance of snappers specifically, L. argentiventris as a valuable resource for artisanal fisheries and for food security in the tropical eastern Pacific (de la Hoz et al., 2015; Martinez-Pinto & González Leiva, 2017; Vega, 2016) and, due to its high abundance and frequency in the mangrove creek under study. The analysis of the trophic relationships encompassing this species is an adequate approach to understand the functional role of mangroves and their macroinvertebrate assemblages in sustaining the population of marine-estuarine-dependent fish (Marguillier et al., 1997; Medina-Contreras et al., 2018).
In the present study, several factors were relevant to analyze this issue: firstly, specimens captured had a size range between 3.0 and 35.5 cm, which correspond entirely to juvenile stages including the subadults which are consider a juvenile stage (Robertson & Allen, 2015; Ramirez-Martínez et al., 2016; Vázquez et al., 2008; Vega, 2016). Our results are similar to what has been recorded in other mangrove systems of America, where just juvenile stages of L. argentiventris were collected; for example in La Paz Bay, Mexico (max 10 cm; López-Rasgado, 2016), Luisico creek, Colombia (max 36 cm) (Ramirez-Martínez et al., 2016), and Gulfs of Montijo and Chiriquí, Panamá (max 40 cm; Vega, 2016). This finding is an example of how the mangroves of the study area operate as nurseries for fishery species and, provides evidence of the use of these systems as a fundamental part of the ecosystem mosaics that fish use during their lifetime (Nagelkerken et al., 2015).
On the other hand, we proposed the hypothesis that smaller individuals should showed more depleted 13C values related with mangroves sources and, larger individuals should showed enriched 13C related with external sources, the above, based on results of previously similar studies whit other mangrove fish species (Sandoval et al., 2020) but, also in the fact that we found a wide range of snapper sizes so we would expect to find a change or pattern in the δ13C related to the length and weight. Also, in the fact that L. argentiventris is usually mentioned as a well-known example which shifts its ontogenetic habitat between mangroves and nearby rocky ecosystems (Aburto-Oropeza et al., 2009; Vega, 2016). However, no relationship was found between δ13C and length or weight, although significant differences in δ13C values of small specimens from medium and subadults were found and a slight tendency for larger animals to have depleted 13C, which is contrary to the expected pattern showed for another mangrove-fish dependent (Sandoval et al., 2020). The relative narrower δ13C range compared with other species in the study area (see section two) may be associated with that most studies of the species’ feeding habits has demonstrated that L. argentiventris has a low prey spectrum and is considered a specialist consumer, in the present study specially on medium juvenile and subadult stages (Martinez-Pinto & González Leiva, 2017; Vázquez et al., 2008; Vega, 2016).
According to the results, the degree of specialization of the species could vary with the environmental characteristics of the mangrove. For example, in the lagoonal mangrove of the California Gulf, a greater range of δ13C (5‰) was obtained (López-Rasgado, 2016) and the size of specimens was distributed from 3 to 10 cm, apparently presenting high variability in their diet although they belong to the same size class. In this system there is a connection with rocky and coral reefs, therefore it is possible that snappers move between the mangrove and the reef to feed or that within the same system they find a diversity of food sources, with different trophic bases. To conclude this issue, further analysis is required.
Regarding δ15N, we hypothesized that animals of greater weight and size would show higher 15N values and, therefore, high TP because of an ontogenetic change in the diet. However, no relationship was found between δ15N with length and weight, nor significant differences in δ15N values between size classes. Based on the above and on the TP estimation, which was similar among size classes, it is possible to identify the continuous functional role of the species as a top predator across all size classes, feeding mainly on smaller fish and crustaceans (Aburto-Oropeza et al., 2009; Ramirez-Martínez et al., 2016; Vega, 2016). This result is contrary to the found for another mangrove-fish dependent (Ariopsis canteri) in the Colombian Caribbean, where the δ15N increased with the size class of the specimens (Sandoval et al., 2020). The above is a clear example of how the functional relationship with mangroves could be species dependent, knowledge which is highly relevant for the adequate management of fishery resources and mangroves conservation. In addition, it is important to mention that, for oceanic and marine systems where food webs are longer, TP values usually found in top consumers are higher than those found in this study, because they contain predators such as sharks that are not found in mangroves (Estupiñán-Montaño, 2022). The TP values shown in this study correspond to typical top predator values previously recorded in mangrove ecosystems (Medina-Contreras et al., 2018, 2020).
SIAR analysis shows that sub-adult specimens have a broader niche than small and medium juveniles; research on other marine-estuarine-dependent fish species has also found this pattern (Muller & Strydom, 2017). Most studies on the diet of L. argentiventris agree that it could present low niche breadth due to a low number of preys in the stomachs (Aburto-Oropeza et al., 2009; Vega, 2016) or the dominance of few preys (Martinez-Pinto & González Leiva, 2017; Ramirez-Martínez et al., 2016). In the current study, the greater niche width of the subadults is due to a tendency of the larger sizes to exploit 13C depleted sources than the other two sizes.
Studies based on stomach contents of L. argentiventris in mangroves of El Salvador suggest high trophic overlap with other fishes of different sizes (Martinez-Pinto & González Leiva, 2017), which is evident in the results obtained here with niche overlap probability percentages higher than 55%. In the present study, the overlap occurred especially in the mid-range of the carbon axes as the small juveniles tend to be 13C enriched, this is contrary to our hypothesis and to results found in mangroves of the Colombian Caribbean for an estuarine resident fish (Sandoval et al., 2020). Stable isotope studies have also shown high niche overlap between small and large juveniles with larger juveniles of other marine-estuarine-dependent species (Muller & Strydom, 2017). Our results also agree that the niches of small and medium juveniles overlap in a higher proportion (> 77%) with that of subadults and not the opposite (Muller & Strydom, 2017).
This raises the question of whether L. argentiventris specimens at a higher developmental stage are migrating to nearby rocky habitats. Previous studies using stable isotopes have questioned if juveniles within mangroves successfully recruit to the adult populations on reefs (McMahon et al., 2011). Much of the research assumes that there is a migration between nursery habitats (mangroves) to rocky or coral environments assuming fish movements based on size (Aburto-Oropeza et al., 2009). A smaller portion of studies searches for evidence of these ontogenetic movements using tagging or capture-recapture (McMahon et al., 2011; Muller & Strydom, 2017). Results about other marine estuarine-dependent species using mark-recapture and stable isotopes have shown that mangrove residence rates (on the scale of days and weeks) are high in small and high juveniles (Muller & Strydom, 2017).
Tracking movement of fishes between juvenile and adult habitats requires the ability to either follow individuals between habitats over long time scales or identify juvenile habitat associations in adult fishes (McMahon et al., 2011). However, there are authors who suggest the effectiveness of stable isotopes to elucidate this issue (Layman, 2007). The ontogenetic patterns previously described for L. argentriventris are not clear in the present study since it was not found the expected pattern of values closer to the mangrove in small specimens and further distant in larger specimens (Aburto-Oropeza et al., 2009; Ramirez-Martínez et al., 2016; Vega, 2016). But there is evidence that L. argentiventris uses the studied mangrove creek from early life to near maturity and nearby systems with similar characteristics (Ramirez-Martínez et al., 2016).
The mixing models’ results highlight the relative importance of cryptobenthic fishes as prey for L. argentiventris for all three size classes. Cryptobenthic fishes are a poorly studied group that is generally excluded from general and functional ecology studies (Castellanos-Galindo et al., 2020). Due to the difficulty in sampling, their small size to obtain stomach contents and its close relationship with substrate is usually assumed that those fishes are mainly detritivores (Robertson & Allen, 2015). Stable isotopes offer a useful tool to involve this group in functional ecology studies, demonstrating that most species have high nitrogen values characteristic of high protein diets and therefore prey on an inconspicuous and uncharacterized food web based on endofauna and microfauna (Medina-Contreras et al., 2018). Our work is one of the first to involve it as part of a trophic analysis in the study area, opening a new research perspective.
The information on the prey found in the stomachs of L. argentiventris (Ramirez-Martínez et al., 2016) was an adequate approximation to select the prey groups for the mixing models. Since it was possible to collect them in the study area to obtain their isotopic signature (see section two) and stablish their feeding habits (Medina-Contreras et al., 2022a), it is considered that the models obtained here, adequately reflect the trophic status of L. argentiventris in the mangrove creek under study.
Stable isotopes (present study) show different results from stomach contents (Ramirez-Martínez et al., 2016) in terms of prey assimilation ratios. For example, porcelain crabs are not as important in the snapper diet as suggested by stomach contents, but instead polychaetes appear to be contributing more than what was recorded in the stomachs. This is because chitinous exoskeletons take longer to be digested or excreted, while soft tissue is more easily digested, which favors the overestimation of crustaceans and the underestimation of worms (Bagnato et al., 2020). In the case of fish, scales and spines indicate predation on this group but it is not possible to know with certainty the species (Ramirez-Martínez et al., 2016; Vázquez et al., 2008; Vega, 2016). Our field observations validated that some of the fish in the stomachs of L. argentiventris especially small and medium juveniles, belong to the families Gobiidae and Eleotridae which are residents of the estuary of Bahía Málaga and their mangrove creeks (Castellanos-Galindo et al., 2020; Medina-Contreras et al., 2022b).
A key property of flow networks is their degree of connectivity, also known as link density (average links flowing into or out of a typical node; Ulanowicz, 2005), being an index valid for weighted networks. The results of this model show that L. argentiventris food web presents a link density of 0.3, suggesting that the yellow snapper food web is relatively simple (Zetina-Rejón et al., 2015). However, this result does not establish that the mangrove creek isn’t a complex ecosystem as this index depends on the number of model components included and ecosystem size (Pimm, 1979), and since this model only focuses on one species food web, the lack of complexity is justified.
The trophic dynamic of the food web of L. argentiventris is dominated by 4 trophogroups (benthic fish, omnivore shrimps, herbivore crabs, worms), which were selected based on the highest local and centrality index values obtained. These indices quantify the importance of each group in the system (Gouveia et al., 2021), showing their importance in the maintenance of trophic connectivity and cohesion of the links and the stability and ruggedness of the L. argentiventris surrounding trophic network (Estupiñán-Montaño, 2022).
As from the index degree, it is highlighted that the most important predators in this network (excluding the yellow snapper) are FISH-BEN and SHRIMPS-OMN, meanwhile the most important prey groups are CRABS-HER and WORMS. In the case of predators, few studies about cryptobenthic fish ecology in estuarine systems on the tropical eastern Pacific indicates the lack of information about ecology of this group and the role they could play in the food webs of mangrove systems (Castellanos-Galindo et al., 2020). Stable isotope studies for another mangrove creek nearby to the study area showed that cryptobenthic fish have an important role as predators in the mangrove food web (Medina-Contreras et al., 2018). Another stable isotope study in the study area had reported omnivorous macroinvertebrates with high trophic levels (Medina-Contreras et al., 2022a) validating the role as a predator of these groups.
The eigenvector centrality values showed that WORMS have the highest influence and importance level among all the groups, a result contrary to what was previously concluded based on stomach contents of the species (Ramirez-Martínez et al., 2016; Vega, 2016). The relevance of CRAB-HER and WORMS also highlights the valuable role of mangroves and detritus in sustaining the food web of L. argentiventris in the study area. It is possible that CRABS-HER and WORMS represent the most important preys on this food web given their high abundance and frequency in the mangroves studied (Jamie et al., 1999). Also, this group is energetically sustained mainly by mangroves and detritus (Medina-Contreras et al., 2022a) which confers on them the functional role of transferring energy from the mangroves to higher trophic levels as demonstrated in the present study.
Conclusions
This work provides evidence that the mangrove food web under study is highly complex by obtaining a network of four communities despite only modeling a single top predator species; also there are no ontogeny effects on the trophic position of the L. argentriventris. The trophic pathways that support L. argentriventris in the riverine mangrove under study derive from several primary sources: (1) MPB through porcelain crabs that are specialists in the consumption of benthic microalgae. (2) seston related mainly with shrimp that are intermediaries in the food webs acting as predators of small fauna (Medina-Contreras et al., 2018, 2020). (3) detritus connecting to polychaetes as a primary food source for invertebrate predators and crypto benthic fish (Willems et al., 2016). (4) mangroves through herbivorous crabs that as has been demonstrated consume and assimilate mangrove leaves being almost exclusive in connecting mangrove-derived carbon with other levels of the web (Medina-Contreras et al., 2022). This pathway involves bacteria for processing. (5) macroalgae, whose function is less clear and requires future study, although it is suggested that they may be entering the food web via herbivorous crabs. Cryptobenthic fish are predators mainly of these groups, being one of the most important intermediaries between the middle and lower trophic levels to the upper trophic levels. Ultimately this energy ends up in the top predator (L. argentriventris) that would later be depredated or exploited as a fishery resource. Analyses such as this one provides a detailed view of the complexity and dynamism of mangrove food webs. Would ontogenetic migration of adult individuals to rocky ecosystems occur after the sub-adult stage recorded in the present study? A study on the movements of the species between nearby rocky systems will allow a better understanding of this issue, to integrate the trophic dynamics of the mangroves into a mosaic of ecosystems in the study area.








