Introduction
The primary source of stress is environmental disturbances that the fish are unable to adjust to either physically or physiologically. A stressful environment may inhibit fish development by affecting the endocrine system and decreasing the secretion of growth hormones (Haser et al., 2024). As an example, increasing blood glucose levels in fish is a commonly employed technique for animal stress models and evaluating the efficacy of feed additives in reducing blood sugar. Glucose induction (GI) can induce oxidative stress and metabolic problems in fish, adversely affecting their development and health. Disorders in glucose metabolism are frequently observed in fish, with high carbohydrates leading to significant metabolic illnesses (Zhao et al., 2022; Zhou et al., 2023). This significantly affects the health of fish, leading to elevated death rates in stressful and pathogenic factors, and ultimately resulting in substantial economic losses (Dong et al., 2024).
Today 30,000 species have been identified, making fish the most diverse organisms globally (Dong et al., 2024). Consequently, initiatives aimed at enhancing their quality and productivity are crucial. An effective strategy is the incorporation of supplementary feed abundant in nutrients, such as Euglena sp (Maghfiroh et al., 2023). The biomass of Euglena sp. is significant in the defence against abiotic and biotic stress due to its anti-allergic, antibacterial, anti-inflammatory, and antioxidant properties (Suyono et al., 2024a). Euglena sp. is a microalga rich in nutritional components, including β-glucan, essential amino acids, lipids, proteins, vitamins, and minerals, which may serve as supplementary feed to enhance fish health (Maghfiroh et al., 2023). The distinctive fiber composition, specifically paramylon, constitutes 20%–80% of the dry weight of Euglena (Maghfiroh et al., 2023), which is recognized for its beneficial impact on glucose metabolism and reduction of blood sugar levels in test subjects. The incorporation of Euglena sp. into fish feed is anticipated to reduce blood glucose levels and enhance the general health of the fish. Besides that, Euglena sp. also contributes to environmental enhancement by tolerating CO2concentrations of up to 45%, among several health benefits (Suyono et al., 2024b).
R. lateristriata, is recognized as a regional Indonesian consumption fish, with considerable commercial and ecological significance. This species offers several potentials and benefits, including a reduced testing duration, reduced operating costs, and easily regulated experimental circumstances. This species serves as an appropriate animal model for this investigation due to the clear observation of its glucose metabolism response (Retnoaji et al., 2023). This study aimed to examine the impact of feed supplementation with Euglena sp. on glucose-induced R. lateristriata, concentrating on its impacts on growth performance, intestinal histology, and reduced blood sugar levels. The results of this study may potentially be used for other fish species to enhance quality, growth performance, and metabolic efficiency. Cause each fish species exhibits consistent energy intake and expenditure for varied activities, except when influenced by external environmental factors or internal physiological disorders (Haser et al., 2024). The results of this study are important not only for enhancing fish feed quality but also serve as a foundation for developing fiber-based products from Euglena sp. that act as blood sugar stabilizers in both animals and humans in a broader context.
Materials and Methods
The comprehensive investigation occurred in line with the proposal permitted by the Ethical Clearance Commission for preclinical research at the Integrated Research and Testing Laboratory, Universitas Gadjah Mada, Yogyakarta, Indonesia (00064/X/UN1/LPPT/EC/2024).
Euglena sp. was cultivated in Cramer-Myers media for 10 days. The biomass was centrifuged, and the pellets were subsequently washed with distilled water to eliminate the residual medium. The pellets were thereafter kept in the freezer. The pellets were combined with reverse osmosis (RO) water before being introduced into the fish tank as a supplement.
Nine-month-old R. lateristriatawere acclimatized for seven days in five distinct aquariums, each with a capacity of 10 L (utilizing 6 L of RO water), containing nine fish per tank with same size around 2–3 cm. Following acclimatization, preliminary weight assessments were conducted for each group. The subsequent phase involved nourishment and treatments by the experimental framework. Feeding occurred daily, namely in the morning from 8 to 9 Am and in the afternoon from 3 to 4 Pm. Treatment conditions were maintained at a pH range of 6–6.5 and a temperature of 22°C–24°C. The temperature must be maintained because an increase in temperature can cause low dissolved oxygen content.
The immersion process lasted for five days. Specifically, from the first to the fourth day, the fish were immersed in a glucose solution (MilliporeSigma D-(+)-Glucose monohydrate, St Charles, US dissolved in RO water) at a concentration of 50 mM, utilizing 4 L per tank, for four consecutive days with daily water exchanges. The fish were immersed in a 75 mM solution for one day. The fish are prepared for further examination.
The experiment involved inducing R. lateristriata with and without glucose, supplemented with Euglena as a supplementary feed, was compared to the control and negative control groups, which is 5 treatments and 3 replicates as detailed in the Table 1.
Growth parameters are assessed continuously to track fish development. Included are final body weight (FBW), weight gain (WG), specific growth rate (SGR), final length (FL) (dimension was measured: total length), and condition factor (CF) coefficient.
IBW, initial body weight.
Histological analysis of the fish was conducted using three specimens per treatment group. The initial phase of histology preparation involves tissue processing, which includes dehydration, cleaning, and paraffin infiltration. The second stage involves blocking or embedding, while the third stage entails cutting with a microtome. The sample is sectioned to around 3 microns, after that immersed in a water bath at 50°C, collected using a glass instrument, drained, and tagged with an identifier. Histopathological staining with HE involves deparaffinization and dehydration. Preparation is immersed in running water, followed by core staining with Mayer’s hematoxylin. The stained preparation is washed with water, then subjected to eosin staining, and then rinsed in reservoirs i, ii, and iii, before undergoing dehydration. Transparent tissue, ongoing mounting.
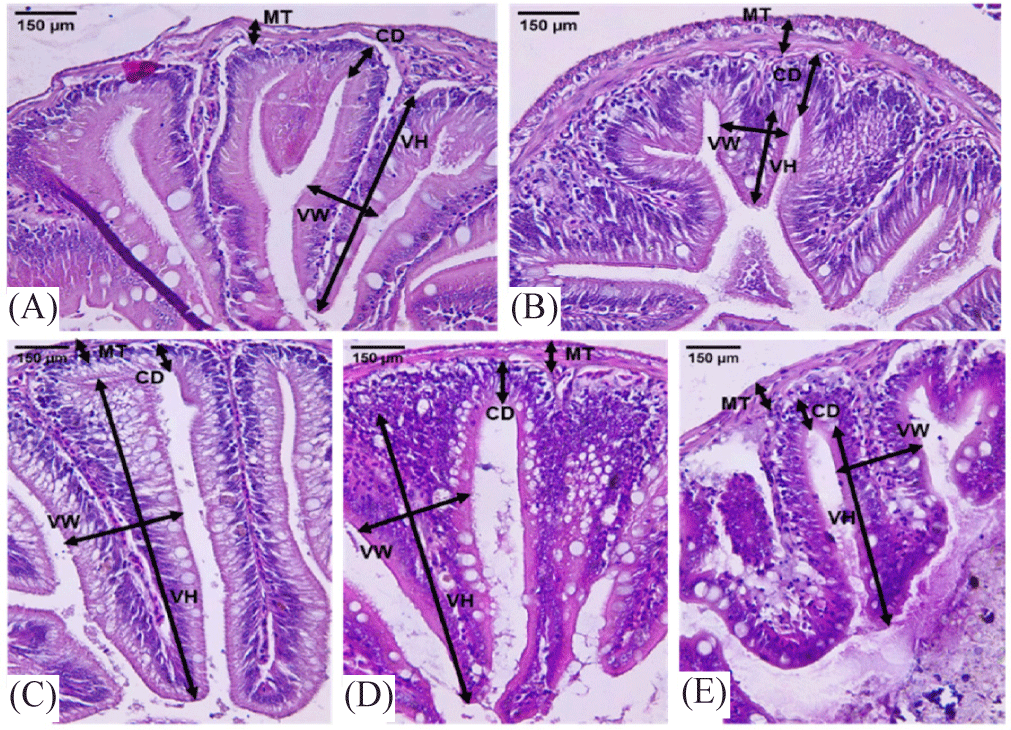
Mucosal structures were examined using a Leica ICC 50 E series, Wetzlar, Germany microscope at ×100 and ×400 magnifications. Visuals of villi and base of intestinal fold were captured with an Opti Lab camera, and measurements were conducted using ImageJ software for life science microscopy. The villus height, defined as the distance from the base of intestinal fold-villus junction to the apex of each villus, and base of intestinal fold, measured from the base of the villus to the deeper mucosa, were assessed for 10 well-oriented villi per section. The mean value for each sampled tissue was derived from three distinct sections prepared from each specimen. Ten villi, and base of intestinal fold were measured per section, with three sections analyzed per sample, and the average values were statistically computed (Mebratu et al., 2023; Yang et al., 2017). The villus height to base of intestinal fold ratio was calculated for each corresponding value and subjected to statistical comparison. The base width of each villus was determined as the distance from the junction to the basement membrane of the epithelial cell located at the bottom of the base of intestinal fold, specifically at the lower third of the villous length. The absorptive surface area of the villus was estimated by modelling the villus as a cylindrical structure and applying the formula provided below (Marchewka et al., 2021).
Following treatment, fish were fasted for one day before blood collection via head excision. A scalpel was used to dissect the fish’s head, blood from the head was then dripped onto the Abbott Freestyle Optium Blood Glucose Test Strips, Witney, UK. which were connected to the Abbott Freestyle Optium Neo Blood Glucose Monitoring System, Witney, UK. The detection tool will conduct an automatic analysis of blood sugar levels.
All analytical data were presented as the mean ± SE (n = 3) and analyzed with origin software. All assays were conducted in triplicate. A one-way analysis of variance (ANOVA) with a Tukey post hoc test was employed to assess significant diffe-rences in multiple comparisons. The results were deemed statistically significant at p < 0.05.
Results
The morphological analysis, represented by the final body length in Fig. 1 and Table 2, quantifies R. lateristriata across all treatments. The measurement yielded the highest value in treatment 1% Euglena (E1), with an average length of 53.67 ± 2.17 mm, which was significantly different (p < 0.05) from other treatments. The subsequent longest morphology measured 53.33 ± 2.03 mm and 47.5 ± 2.25 mm, corresponding to the GIE2 and GIE1 treatments, respectively. The control and negative control treatments exhibited the smallest FLs, measuring 46.33 ± 2.20 mm and 45.67 ± 3.30 mm, respectively.
Based on the research, the results of the comparison of the control treatment, and the combination of Euglena feed addition treatments and GI were obtained as shown in Fig. 2 and more in Table 1. The results showed that the WG (%) in the E1 treatment had the highest value of 46.67 ± 0.17%, which was 7 times greater than the control, and was significantly different from other treatments (p < 0.05), then followed by the GIE1 treatment with a WG of 31.25 ± 1.00% which was 5 times greater than the control, the GIE2 treatment produced a WG of 11.76 ± 0.53% which was greater than the control, while the Control was 6.25 ± 0.25% and the smallest was the WG obtained by the negative control treatment of 0.00 ± 0.00%.
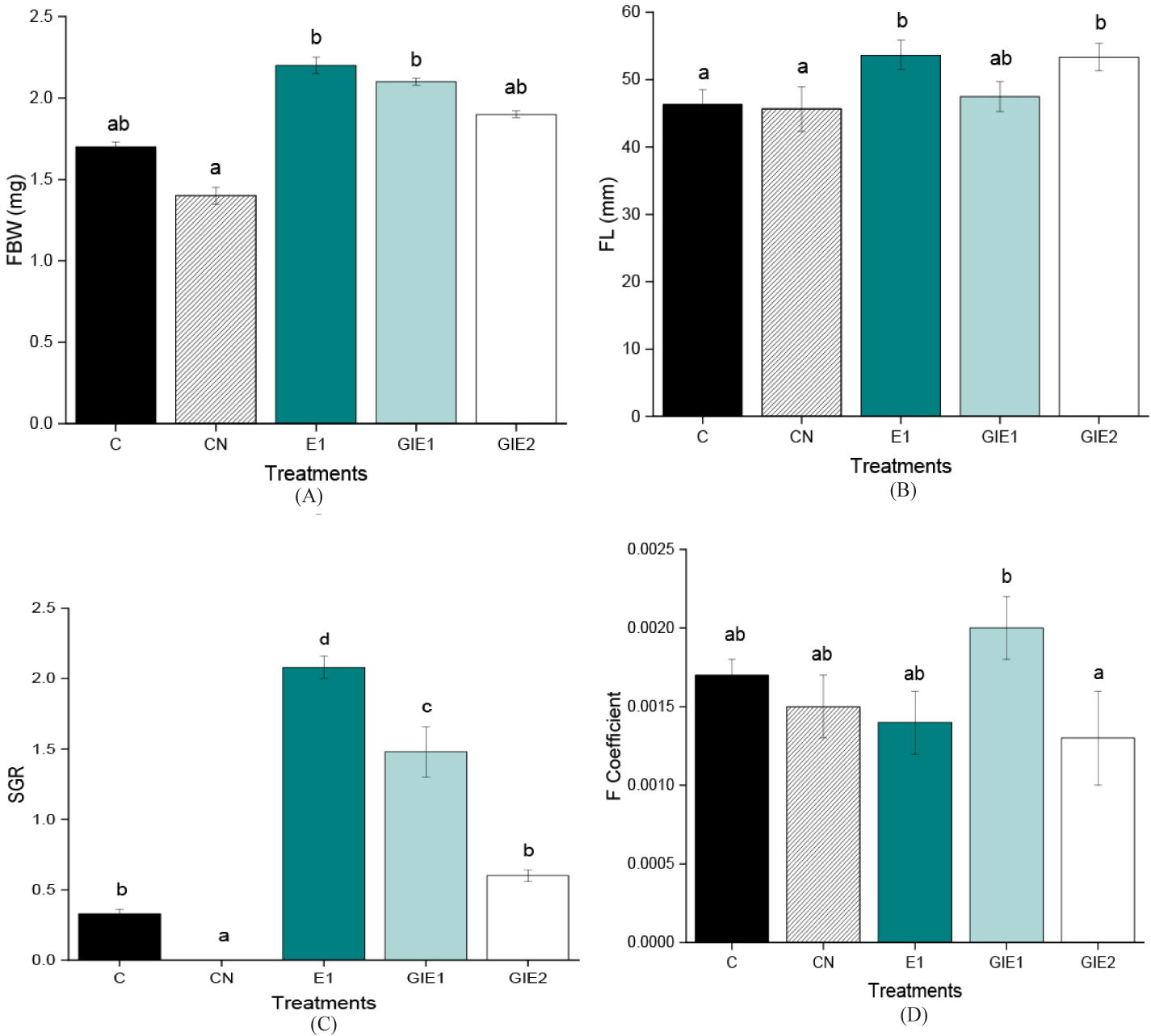
Linear results were also obtained on the SGR value, the highest SGR was obtained by fish in the E1 treatment of 2.08 ± 0.08, the value was 6 times the control treatment, and was significantly different (p < 0.05) from other treatments. The second largest SGR was the GIE1 treatment of 1.48 ± 0.18, which was 4 times greater than the control. The GIE2 treatment had an SGR 2 times the control value of 0.60 ± 0.04, with a control of 0.33 ± 0.03 and the smallest value was in the negative control of 0.00 ± 0.00 or other words there was no WG in the negative control group.
The next parameter, namely CF, which is the conditional factor value between mass and the power of 3 of fish length, got the highest value in the GIE1 treatment of 0.0020 ± 0.0002, then continued by 0.0017 ± 0.0001; 0.0015 ± 0.0002; 0.0014 ± 0.0002; and 0.0013 ± 0.0003 which are the treatments for control, negative control, E1, and the smallest for GIE2 treatment.
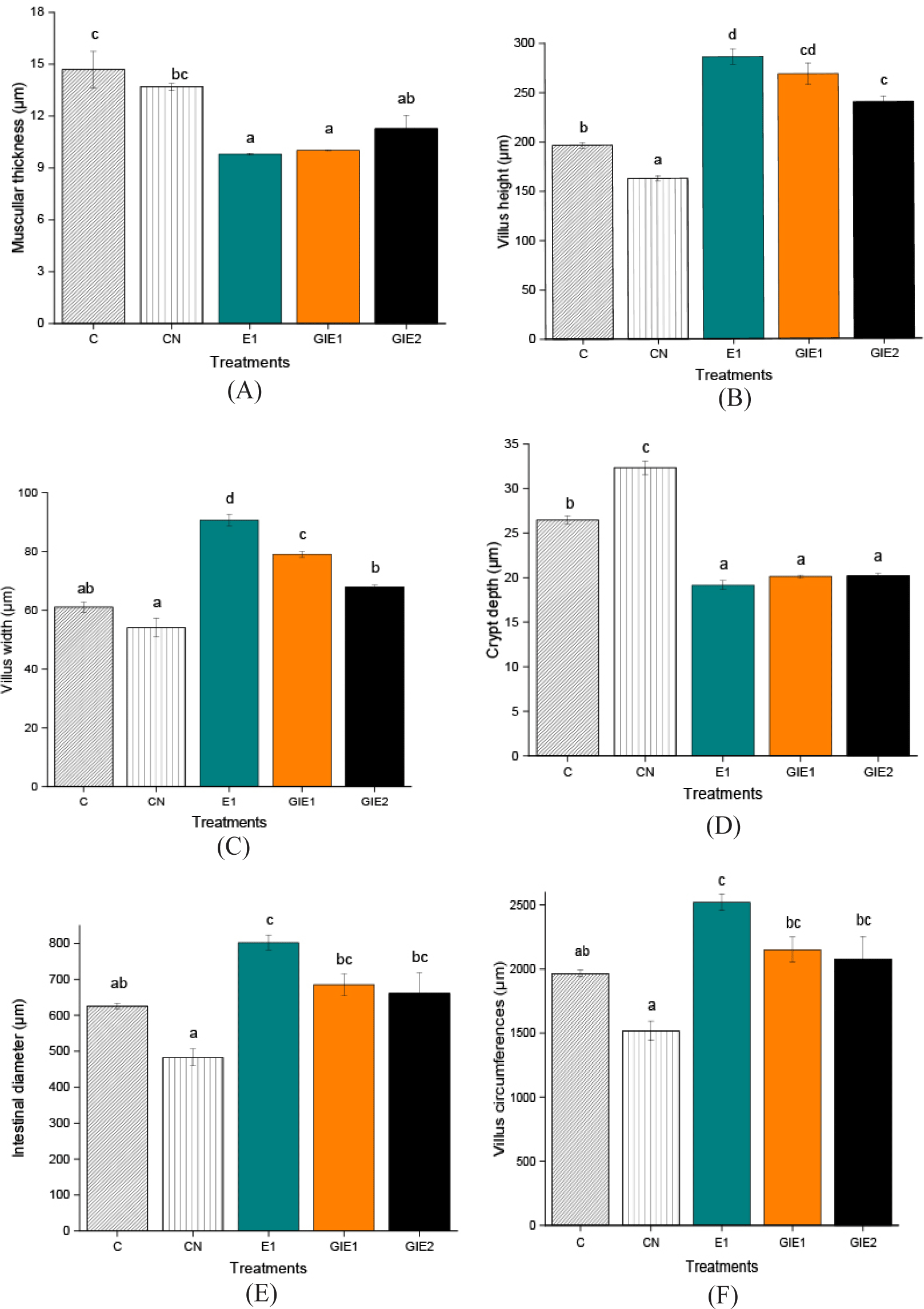
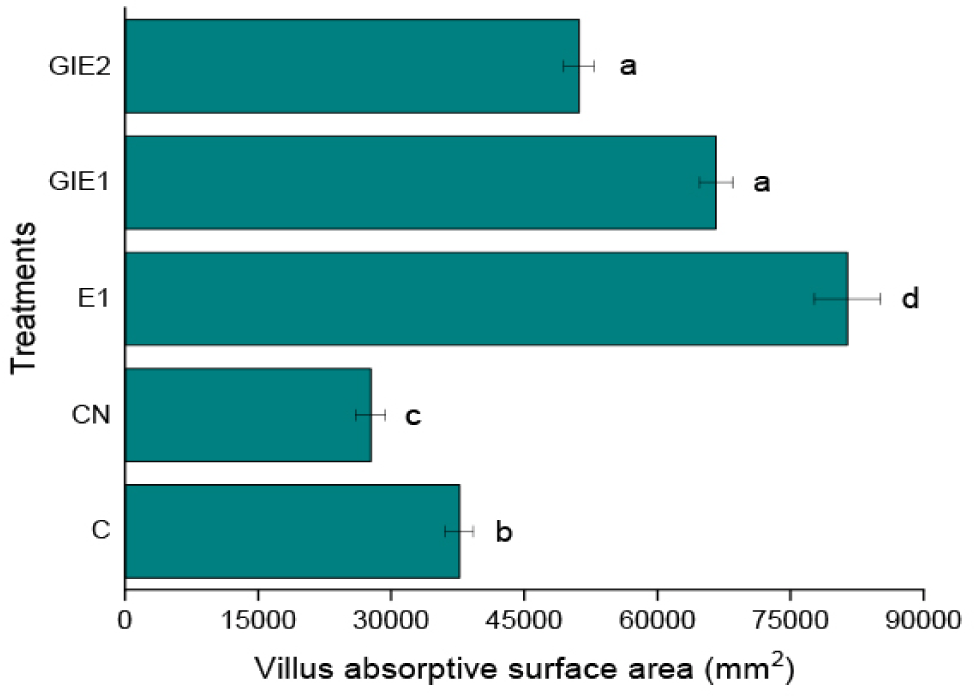
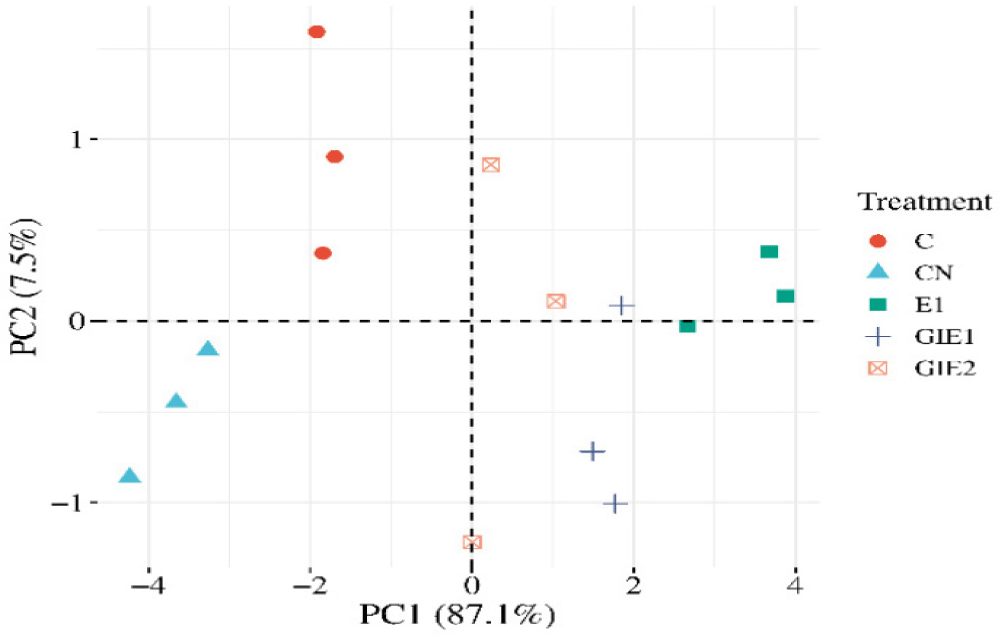
Intestinal histology examines variations in histological cross-sections relevant to the investigation of intestinal function, particularly in the villi region. The data presented in Table 3 and visualization in Figs. 3 and 4 indicate that the E1 treatment resulted in the largest measurements for villus height, villus width, intestinal diameter, and villus circumference, with statistically significant differences observed compared to all other treatments (p < 0.05). The GIE1, GIE2, and Control treatments yielded the next results, while the Negative Control exhibited the lowest value (Fig. 5). The E1 treatment exhibited the lowest values for muscular thickness and base of intestinal fold parameters (p < 0.05). Subsequently, higher values were recorded in the GIE1, GIE2, and Control treatments, with the highest values observed in the negative control group. According to the stated features, it can be determined among Fig. 6 that the maximum absorption area of the greatest villus is located in the gut of the E1 treatment, which is significant different from the other treatments (p < 0.05). The following biggest area is observed in GIE1 and GIE2, which are greater than the control and negative control, both of which exhibit the smallest villus surface area. The principal component analysis (PCA) graph on Fig. 7 illustrates the sample distribution according to two principal components (PC1 and PC2), which together account for 94.6% of the overall data variability. PC1 represents 87.1% of the variability, whereas PC2 represents 7.5%. PC1 denotes the variability in the dimensions and complexity of villi. Factors including villus height, villus width, and villus surface area substantially influence PC1. Elevated PC1 readings signify enhanced villi growth, characterized by increased height and surface area, which correlates directly with improved nutrient absorption capacity. PC2 denotes the impact of metabolic stress (GI) on villus architecture. Factors include the ratio of villus height to base of intestinal fold and intestinal diameter influencing PC2. Elevated PC2 levels signify structural adaptation of the villi in reaction to metabolic stress, exemplified by alterations in the ratio of villus height to base of intestinal fold to enhance glucose absorption efficiency.
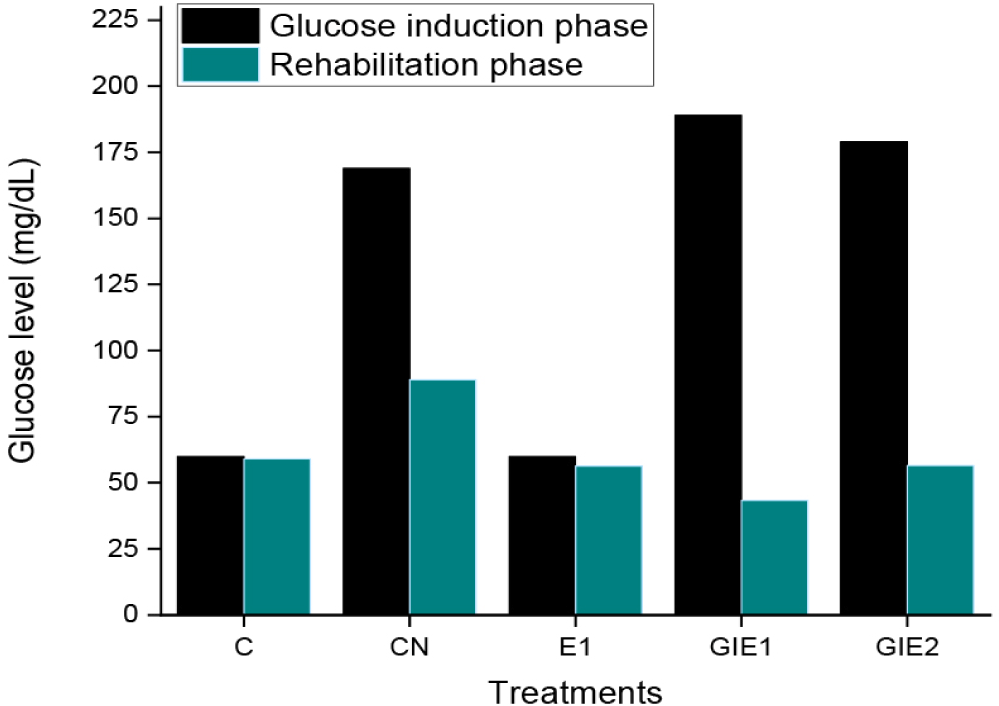
Blood sugar measurements conducted before and following the healing process yielded results presented in Fig. 6. The data indicate that the GIE1 treatment resulted in the most significant reduction in blood sugar levels when compared to both the GIE2 treatment and the negative control. The negative control exhibited the least reduction in blood glucose levels, as it solely depended on the fish’s intrinsic physiological processes.
Discussion
The parameter list in Table 2 demonstrates that the treatment with 1% Euglena sp. exhibits the highest growth performance value, significantly differing from other treatments (p < 0.05). The body of this group is in optimal condition, specifically devoid of GI, which induces metabolic stress, and supplemented with Euglena species represent a noteworthy group of microalgae, characterized by their ability to synthesize diverse bioactive compounds applicable as food supplements. Euglena contains several substances, including carbohydrates, lipids, proteins, β-1,3-glucans, antioxidants, phytotoxins, wax esters, and polyunsaturated (Maghfiroh et al., 2023). Beta glucan is one of the ecologically friendly prebiotics that can enhance the immune system, digestive system, and growth performance of target animals (Hoang et al., 2024; Suyono et al., 2024b). Recent studies indicate that the incorporation of β-glucan into the diet may enhance various aspects of feed utilization in pompano fish, such as feed conversion ratio (FCR), feed conversion efficiency (FCE), protein efficiency ratio (PER), and protein retention value (PPV) (Hoang et al., 2024). Moreover, increased growth rates, reduced FCR, and FCE and protein efficiency PER can lead to decreased feed costs, a shortened aquaculture cycle due to faster attainment of commercial size by fish, minimized waste per culture unit, and heightened production and profits for farmers (Hoang et al., 2024).
The treatment of GI also had a greater growth value than the control, which shows that Euglena also has the potential to be an efficient pharmaceutical product even though the fish are experiencing metabolic stress plus the process of its cultivation is quite simple (Maghfiroh et al., 2023). Beta glucan has been demonstrated to be an eco-friendly antibiotic that is harmless for organisms, particularly fish, and the environment (Das et al., 2017). β-glucan supplementation enhances immunological function in pompano Trachinotus ovatus (Do-Huu et al., 2022) and promotes growth, immunity, and feed efficiency in pompano (Do-Huu et al., 2019; Hoang et al., 2018). The incorporation of β-glucan into the diet elevates stress-induced ammonia levels in pompano (Do-Huu et al., 2022). Prior research has demonstrated that β-glucan in feed enhances growth rate and immunity in pompano fish, T. ovatus (Do-Huu et al., 2019, 2022), and improves disease resistance in both pompano fish (T. ovatus) and golden trevally fish, Gnathanodon speciosus (Hoang et al., 2023), against Streptococcus iniae (Do-Huu et al., 2019).
The other parameter is the CF coefficient. High CF correlates with increased intestinal weight. CF also indicates the body shape index of fish over a specific time frame (Yu et al., 2023). The augmentation of fish intestine weight correlates with an elevated rate of intestinal peristalsis, so shortening meal retention time in the intestine, which subsequently reduces nutrient absorption and leads to reduced growth performance (Liu et al., 2018). Treatments E1 and GIE2 have the lowest CF values so they have the best nutrient absorption performance compared to other treatments.
In response to stress, the body releases antioxidant enzymes to mitigate its effects and prevent mortality. Under this condition, the body expends significant energy and impacts its processes to inhibit the secretion of growth hormones, which stops the process of growth. The next occurrences in the negative control group are described as follows. Within the GI group receiving Euglena, both subjects exhibited superior growth compared to the control group. Body mass and length demonstrated significant growth. The increase in Euglena as a nutritional supplement, beside basal feed, explains this phenomenon. Lipid ranged from 0.52 ± 0.03 g/L is a component of Euglena sp., primarily serves as a source for the formation and repair of damaged body tissue, as well as a food reserve (Maghfiroh et al., 2023; Pratama et al., 2018). The protein component of Euglena sp. ranged from 3.10 ± 0.2 × 10–2 g/L primarily serves as a source of essential amino acids, which are necessary for the synthesis of nonessential amino acids and proteins in the body (Hou et al., 2015; Maghfiroh et al., 2023). In organisms experiencing stressful conditions, cortisol levels are typically elevated in absent of Euglena. Research by Pfalzgraff et al. (2021) demonstrated that lipids and proteins are stored in greater quantities in non-stressed fish, whereas stressed fish exhibit reduced levels of lipid and protein, as these nutrients are utilized as energy sources to mitigate stress.
The intestines are the initial component of the digestive system exposed to dietary constituents, hence influencing the development of the local mucosa and its immune response (da Silva et al., 2024). Several research has concentrated on creating morphophysiological and histopathological techniques for the diagnosis and prevention of gastrointestinal disorders induced by plant-based diets, thereby enhancing dietary quality (da Silva et al., 2024). In this study, researchers demonstrated Euglena pellets as nutrition and supplements for R. lateristriata in normal conditions and glucose-stressed conditions. The greater height of villi, the wider the surface area of the intestinal wall and mature epithelial cells, this will increase absorption and improve digestion. Villi are protruding and evaginated structures in the gut that contain microvilli to enhance the surface area for nutrition absorption. The villi develop from mucosal folds, which are enveloped by a single-layer columnar epithelium including microvilli on the apical surface (Kiela & Ghishan, 2016). Inversely proportional base of intestinal fold, the greater the depth, the higher the cell replacement process, resulting in excess energy consumption. Unlike villi, base of intestinal fold are structures in the gut that exhibit invagination (Kiela & Ghishan, 2016). Thus, a smaller base of intestinal fold and a larger villi height will both increase nutrient utilization and digestion so as resulting in better organism growth performance (Lee et al., 2023). Another objective indicator of digestive effectiveness is the ratio of villi height to base of intestinal fold. Better nutrient absorption is indicated by a greater ratio of villi height to base of intestinal fold (Shirani et al., 2019). Additionally, research has highlighted the impact of diet on the structural features of the intestinal base. Gewida et al. observed that dietary supplementation resulted in enhanced villus height and modifications at the base of the intestinal folds, demonstrated by variations in crypt depth (Gewida et al., 2024). In addition to the structural aspects, the functional implications of the base of intestinal folds are evident in the work of Verdile et al., who confirmed that the stem/progenitor cell zone is consistently located within the intestinal folds across different ages of fish, indicating a critical role in growth and regeneration (Verdile et al., 2020). In addition, thinner muscular thickness can reduce digestion time and increase the absorption of available nutrients (Lee et al., 2023). The treatments E1, GIE1, and GIE2 progressively reveal signs of better growth performance in comparison to the control and negative control. E1 had the most optimum results compared to metabolic stress treatment supplemented with Euglena feed. The elongated villi in the fish gut can increase the number of enterocyte cells involved in nutrient digestion and absorption. The diabetic condition results in deficient absorption, leading to an increase in the number of acidic goblet cells in fish, which subsequently acidifies the gastrointestinal tract environment Mohammadi et al. (2020).
Augmenting the inclusion of Euglena sp. in the R. lateristriata diet has demonstrated an enhancement in villi length and absorption surface area, resulting in an elevated nutrient absorption capability. The gut epithelium layer and villi are adaptive tissues that continuously respond to variations in dietary content. An increased surface area of the villi enhances nutrient absorption, hence positively influencing development (Alyileili et al., 2020; Noleto-Mendonça et al., 2021). Two treatments with comparable dosages Euglena exist; the distinction is in E1, where R. lateristriata is not stimulated by glucose, but GIE1 is stimulated by glucose. GIE1 ranks second in growth performance due to metabolic stress, such as a major increase in glucose levels leading to diabetes, occurs, the body prioritizes stabilizing its state and may reduce or suspend nutrient absorption necessary for cellular development. Furthermore, the GIE2 treatment with 2% Euglena sp. ranked third in terms of growth performance, although containing a higher nutrient concentration. The level of dose in the following analysis requires attention due to certain effects exhibiting a nonlinear pattern and showing the existence of an optimum dosage for Euglena consumption. given that the dose is excessive, its benefits will be reduced. Excessive fiber intake can lead to fat storage in the colon and resulting stress (Apajalahti & Vienola, 2016), increasing protein fermentation, which may trigger inflammation via the formation of biogenic amines (Mebratu et al., 2023). Therefore, it is essential to optimize nutritional quantities utilized as feed supplements. The objective is to identify optimal levels that can maximize target development without any reduction or accumulation of undesirable chemicals.
Overall, dietary probiotic β-glucan enhances fish growth and improves feed and nutrient utilization by optimizing the gastrointestinal system of fishBeta glucan which is a component of Euglena has a lot of influence on the growth process starting from the efficiency of the digestive system. Previous references state β-glucan enhances the intestinal absorption surface area in pompano fish (Hoang et al., 2023, 2024) and lobsters, while also increasing VL and VW in pompano fish (Hoang et al., 2024). Furthermore, enhancing gut morphology can booster the immune system of fish, activate pathogen defence mechanisms, promote health, and elevate growth rates.
PCA was performed to reduce the dimensionality of the histological data and identify the main patterns underlying changes in villus structure in response to Euglena treatment and metabolic stress. The PCA results showed that the first PC1 explained most of the variance in the data and likely represented variation in villi size and complexity. Group E1, which received 1% Euglena supplementation without stress, tended to cluster at higher PC1 values, indicating better villi development compared to the control group. The second PC2 showed the effect of metabolic stress on villus structure. Groups GIE1 and GIE2, which experienced GI, tended to cluster separately from groups E1 and control on the PC2 axis. This indicated structural adaptation of the villus in response to metabolic stress. Although groups GIE1 and GIE2 also showed increased PC1 values, this increase may not be as large as group E1, indicating that metabolic stress may inhibit optimal villus development. The PCA results are in line with the research hypothesis, namely that the addition of Euglena can increase the surface area of villus absorption. Group E1, which received 1% Euglena supplementation without stress, showed the best villus development, consistent with the hypothesis that non-stress conditions are optimal for villus growth. However, these results also indicate that metabolic stress can affect villi structure, although Euglena supplementation still has a positive effect. The difference between the GIE1 and GIE2 groups indicates that stress levels and Euglena doses can interact in affecting villi structure.
Blood glucose serves a critical function as the primary energy source in cellular metabolism, particularly for neuronal cells. Glucose serves as an indicator of stress in fish, with normal blood glucose levels ranging from 40 to 90 mg/dL, as reported by Haser et al. (2024) Fish subjected to stress exhibit primary and secondary responses, with elevated glucose levels serving as a secondary response following the primary response characterized by increased stress hormones, including catecholamines and cortisol. Stressed fish necessitate increased energy, which is allocated to catecholamine secretion, cortisol regulation, and enzyme activation for protein catabolism. These proteins enhance the concentration of amino acids in the bloodstream, thereby activating insulin, which facilitates the transport of glucose to maintain normal physiological conditions (Haser et al., 2024). The natural recovery process of the R. lateristriata group CN is generally longer than that of GIE1 or GIE2. The limited protein intake in basal feed results in metabolic stress, which can be decreased by the addition of Euglena. This organism enhances insulin activation due to its protein content, aiding in the recovery of bodily conditions. Furthermore, the flavonoids present in Euglena contribute to improved growth conditions. This aligns with research by Mohammadi et al. (2020) which indicates that silymarin, a flavonoid derived from various phytonutrients, has demonstrated effectiveness in patients with type 2 diabetes. According to the study conducted by Uren Webster et al. (2018), Environmental stressors induce alterations in the epigenome, resulting in permanent impacts on transcription regulation. However, the addition of Euglena resulted in a more rapid decrease in the blood sugar levels of fish in this study. and demonstrated greater growth relative to controls. This demonstrates that the incorporation of Euglena as feed can promote stress relief and positively impact gene transcription regulation. The subsequent analysis concerning gene regulation requires further investigation.
Conclusion
This study demonstrates that morphology and intestinal histology, particularly the villus, can adapt to increased quality feed intake. Euglena has been shown to significantly enhance growth in terms of morphology, body mass, and efficiency. The incorporation of Euglena supplements may treat healthy body conditions and metabolic stress. Enhancing nutrient absorption and blood glucose metabolism is a critical consideration in the application of Euglena as a functional feed. Facilitating the subsequent assessment of Euglena ingredients as potential components of growth supplements for aquaculture and as candidates for blood sugar reduction for researchers employing advanced methodologies.








