Introduction
Indonesian waters are known for their diverse and economically valuable aquatic resources, including fish, mollusks, algae, and crustaceans. Among these, spiny lobsters of Palinuridae are particularly significant, providing substantial income for local fishermen. However, despite their economic importance, the biology and habitat of these spiny lobster species remain largely unexplored particularly in the waters surrounding Southeast Sulawesi, including the Wakatobi waters, North Konawe, Wawonii Island, Tiworo Strait, Spelman Strait of Central Buton, Bombana, and North Buton. Spiny lobsters, known for their robust bodies, vibrant colors, and large sizes (up to 60 cm), inhabit a variety of habitats and water depths. Studies have found them in the tropical waters of the Indo-West Pacific, ranging from the Red Sea and southeast Africa in the west to Japan and Fiji in the east (Dao et al., 2015), as well as in Indonesia (Wahyudin et al., 2017; Waiho et al., 2021; Waluyo & Arifin, 2021). They are mostly found in coastal waters at depths of 1 to 50 m, or in waters less than 100 m deep (Zhao et al., 2018), with diverse habitat conditions such as sandy substrates, coral reefs, and even coarse substrates.
The biology of spiny lobsters is influenced by oceanographic conditions such as temperature and salinity (Boavida-Portugal et al., 2018; Zhao et al., 2018), and substrate (Butler IV, 2017). Ocean currents are another key factor affecting lobster distribution, especially for lobster seeds. Dao et al. (2015) explained that lobster seeds in Indonesia grow naturally, being moved by ocean currents from Australia, western and southern Papua of Indonesia, and Japan, before returning to Australia. Spiny lobsters thrive in tropical waters where the average temperature of 28°C significantly influence their physiology, behavior, and ecology (Zhao et al., 2018). This temperature also affects their productivity, recruitment (Régnier et al., 2019), and variation in survival rate (Subbey et al., 2014).
The diversity of spiny lobsters’ species in the western Indo-Pacific region is well documented (Anuraj et al., 2017). A total of 11 spiny lobster species have been identified, with 6 species of Panulirus homarus, Panulirus longipes, Panulirus versicolor, Panulirus ornatus, Panulirus penniculatus, and Panulirus polyphagus found in Indonesian waters (Yunus & Parawansa, 2021). All species of Palinuridae in Indonesian coastal waters have significant potential for aquaculture. Indonesian spiny lobsters are highly valued in Southeast Asia, with countries like Vietnam, Malaysia, and Singapore importing both mature and juvenile spiny lobsters from Indonesia (Jones et al., 2019).
Indonesia waters, from the western tip of Sumatra to the southern part of Java, the Arafura Sea, West and East Nusa Tenggara, and Sulawesi, including Southeast Sulawesi, are major spiny lobster fishing locations and serve as major spiny lobster fishing grounds. Species such as P. homarus and P. ornatus are often found in coral reef areas, which provide shelter and feeding grounds. The abundance of spiny lobster species varies by location and season, influenced by habitat suitability and fishing activities. Seasonal changes can affect population dynamics, particularly for berried female spiny lobsters and juveniles, a trend also observed in portunid (Sara et al., 2016). Ecologically, spiny lobsters are present year-round, but the peak seasons of spiny lobster abundance, and consequently the fishing season, vary by location. For example, in southern Java, fishing season occurs from November to February, coinciding with the seasons for octopuses, king mackerels, and prawns (Setyanto et al., 2023). In Southeast Sulawesi, the peak season is from July to September, during which catches often include seed and juvenile stages, necessitating regulation to prevent overfishing. Typically, caught spiny lobsters are juveniles, sometimes held in floating cages until they reach the size that is allowable for harvest. In many coastal waters of Southeast Sulawesi, populations of spiny lobsters, as well as population of crab Portunidae seem to be declining (Sara et al., 2017).
Studies on spiny lobsters in Southeast Sulawesi has been limited, with previous studies focusing primarily on larvae abundance captured using “pocong-pocong” nets (Tasidale et al., 2020) and molecular identification (Yusnaini et al., 2022). The study reveals that the relative growth of among spiny lobster species may differ across geographically distinct locations in Southeast Sulawesi. This study aimed to compare the relative growth spiny lobsters species, covering abundance, variation in sex ratio, relationship between weight and carapace length, growth patterns, and condition factors of spiny lobsters (Panulirus sp.) caught in the fishing grounds of Southeast Sulawesi waters. This study is valuable for providing data on spiny lobster fishing grounds and the biological conditions of spiny lobsters in Southeast Sulawesi.
Materials and Methods
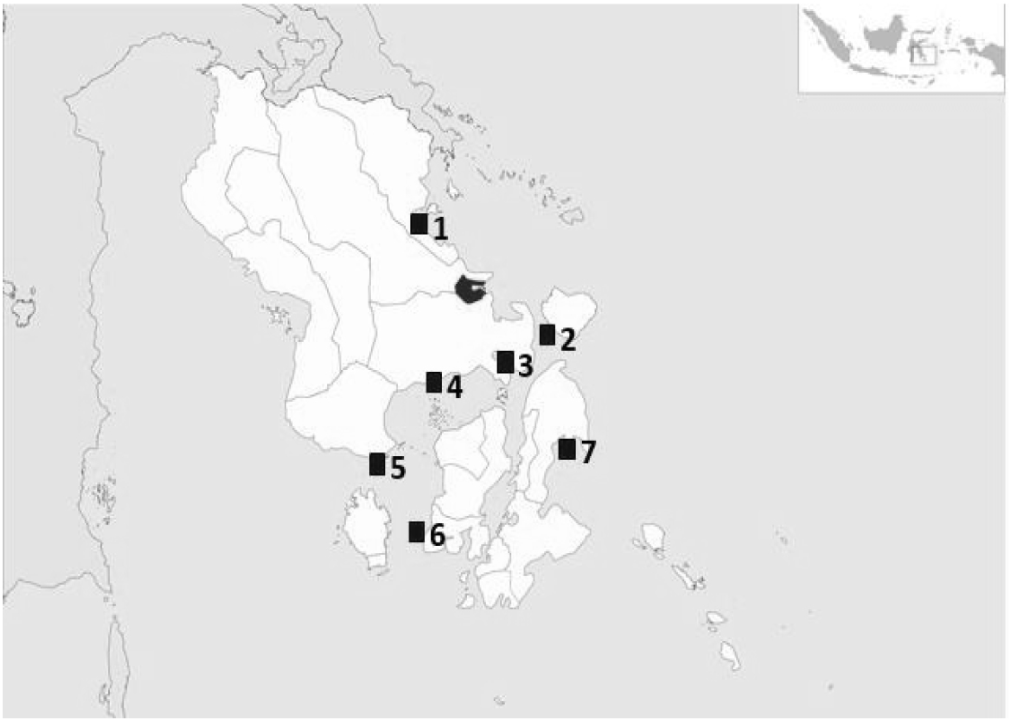
Spiny lobster sampling was conducted across several fishing grounds in the waters of Southeast Sulawesi, including Motui waters of North Konawe, Wawonii Island, Kendari Bay, Tiworo Strait, Bombana, North Buton, and Spelman Strait (Fig. 1). From September to November 2022, spiny lobster samples were collected from all groups of lobster fishers at each location, who used set nets and bottom lobster traps (Fig. 2). The fishing gears were left by the fishermen at each location for approximately 20 h, being set in the afternoon just before high tide, and then retrieved the following morning just before low tide (one spiny lobster fishing trip lasts average around 20 h). The captured spiny lobsters were then collected alive for growth out in a floating cage net.
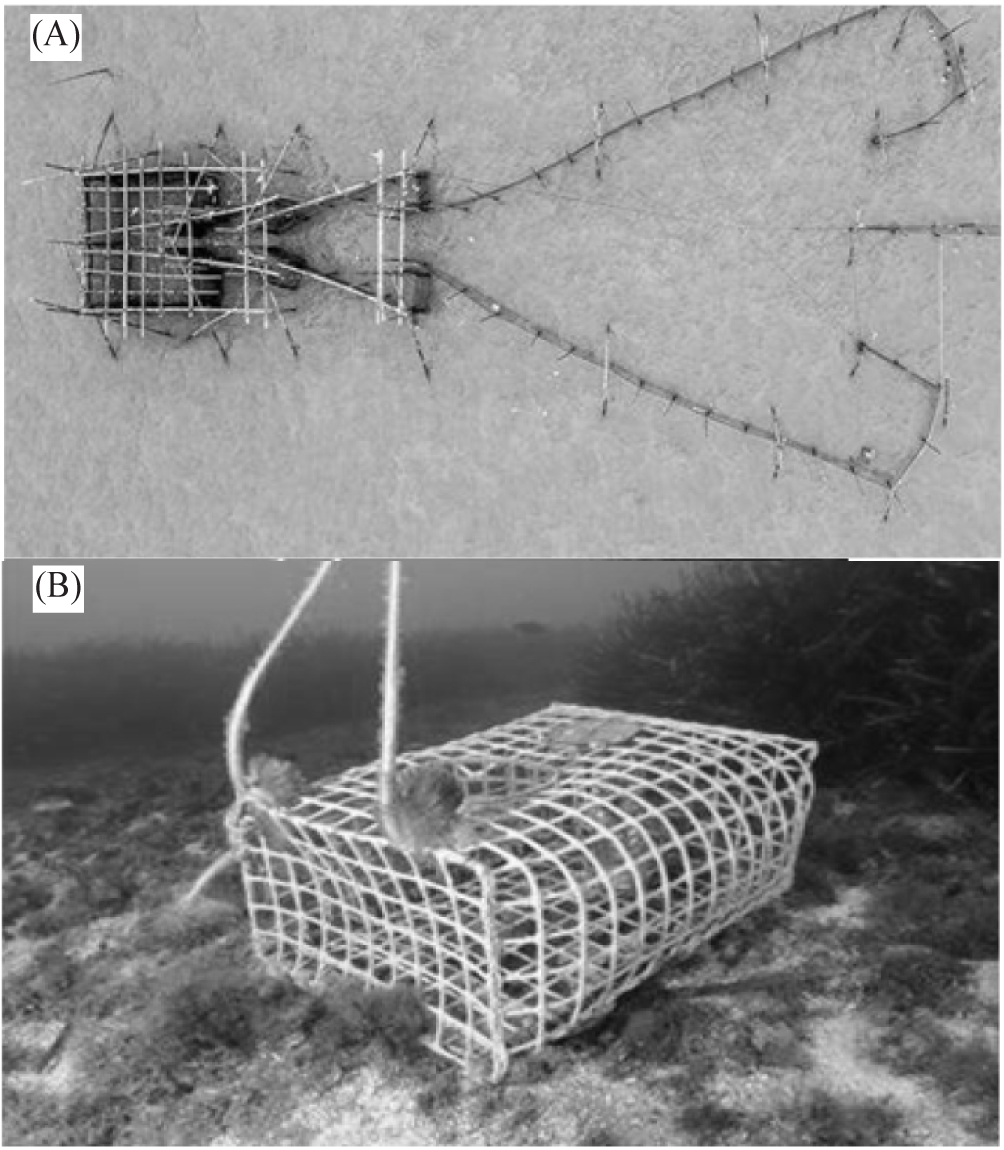
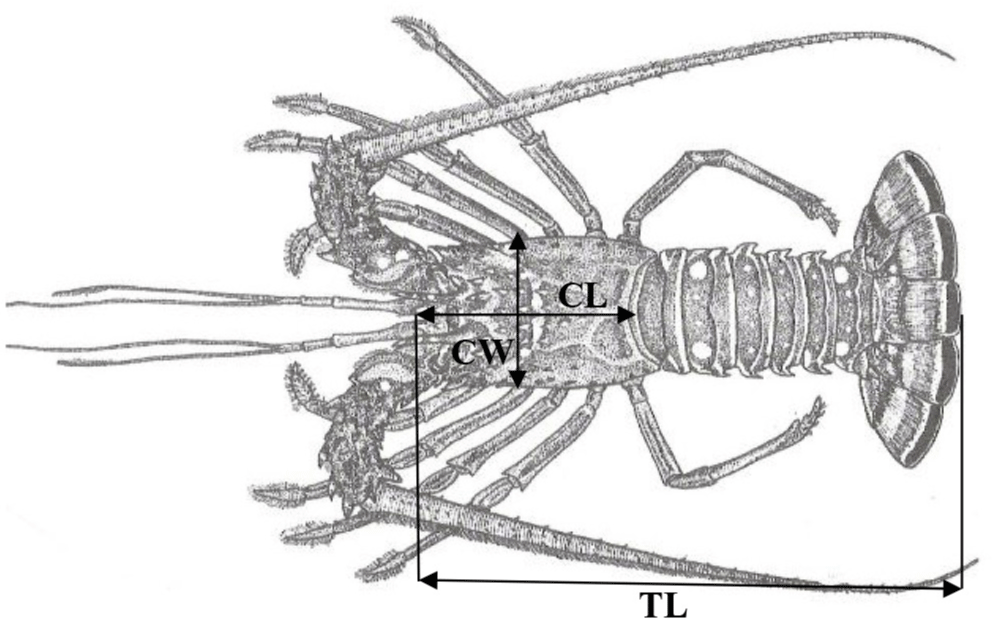
All spiny lobster samples taken from each station in this study were collected alive by fishermen before being placed in floating net cages (thus, IRB/IACUC approval was not required as the samples were returned to the floating net cages alive). These live spiny lobster samples were identified based on their external morphological characteristics, such as walking legs and swimming legs. In this study, samples of juvenile spiny lobster of 54–80 mm total length (TL) and adult spiny lobster of > 80 mm TL caught were combined into a single sample unit. Nevertheless, each sample of juvenile and adult spiny lobster was also individually identified. Juveniles were identified based on the presence or absence of body hair (males have body hair, females do not), while mature spiny lobsters were characterized by their reproductive condition (Yusnaini et al., 2009). Each spiny lobster’s sex was determined by the position of the gonopore, the presence of ovigerous setae on the pleopod, and secondary sexual characteristics, where males have one walking leg and females have two walking legs (Junaidi et al., 2010; Yusnaini et al., 2009). For each sex, spiny lobster samples were recorded, counted, and measured for TL using a vernier caliper to the nearest 0.1 mm (Fig. 3). Their body weight (W) was measured using an electronic balance to the nearest gram (Sara et al., 2016, 2017).
All spiny lobster samples collected from each sampling location were grouped and classified by species and sex. The average catch per unit effort (CPUE) of spiny lobsters collected from each fishing location was counted from all species and sexes per fishing trip (effort), while abundance (number) of each species and sex were counted according to sampling location (Astuti et al., 2022).
The male and female spiny lobsters from each location were counted, and the sex ratio (SR) was analyzed using the following formula (Astuti et al., 2022):
where SRi = sex ratio of species of i (i = 1, 2, 3, … n)
Mi = number of male species of i (i = 1, 2, 3, … n)
Fi = number of female species of i (i = 1, 2, 3, … n).
The significance of the sex ratio was tested using the chi-square test (α = 0.05) (Gomez & Gomez, 1976; Sara et al., 2016) as follows:
where X2 = chi-square
Oi = frequency number of observed male and female of each species of i (i = 1, 2, 3, … n)
E = frequency number of expected male and female of each species.
The relationship between spiny lobster W and TL was analyzed using the equation W = a × TLb, which can be transformed into LogW = Log a + b × LogTL, where W = body weight, TL = total length, a = the intercept on the TL axis or the initial coefficient of growth, and b = the slope or growth coefficient that indicates the growth patterns of spiny lobsters (Vigo et al., 2024). Condition factor can be determined by measuring the length and weight of spiny lobster.
Organisms typically follow an isometric growth pattern (b = 3), meaning that increases in W is proportional to increases in TL. When growth patterns are allometric, they can be classified as either positive allometric (b > 3), where W increases faster than TL, or negative allometric (b < 3), where W increases slower than TL (Vigo et al., 2024; Waiho et al., 2021). If the growth pattern is isometric that the health index of spiny lobster was analysed using “condition factor” CF = [105 W/TL3], while allometric growth pattern (b ≠ 3) that condition factor (CF) = W / [a × TLb] (Paramo et al., 2024), where W is the average body weight of the spiny lobster (g), TL is the average total length of the spiny lobster (cm), a is the constant or intercept, and b is the length exponent or slope. The W = a × TLb formula is a parabolic equation and then transformed into the linear using a logarithmic approach: Log W = Log a + b Log TL (Astuti et al., 2022; Noori et al., 2015; Paramo et al., 2024; Sara et al., 2016). A good growth state of the species was indicated by a CF ≥ 1.0, confirming a healthy condition, while a species in poor growth conditions when the CF <1.0. The ideal CF occurs when total length increases proportionally to body weight (isometric growth) (Jisr et al., 2018; Paramo et al., 2024).
Results
Fishermen capture spiny lobsters an average of 12 times (12 d) per month. Spiny lobster samples collected from each fishing location (without separating juveniles and adults) in the waters of Southeast Sulawesi were counted for CPUE. The highest average CPUE was found in Southeast Sulawesi waters with 6–7 individuals per trip, followed by CPUE in the Wakatobi waters and North Buton waters with 4–5 individuals per trip and 1 individual per trip, respectively (Table 1). These spiny lobster samples collected from each fishing locations were identified based on their physical characteristics and classified into four species: P. ornatus, P. homarus, P. longipes, and P. versicolor. P. longipes (Table 1). The most dominant species found throughout the research from all sampling locations were P. longipes (51.87%) and P. ornatus (24.77%), while P. homarus and P. versicolor were 8.88% and 10.49%, respectively. Sampling at each station revealed that spiny lobsters showed preferences for specific locations, as indicated by the abundance of each species in different areas. Although all species were present in all fishing locations of Southeast Sulawesi waters, P. longipes exhibited the broadest habitat range, being found in all fishing locations. This species was particularly dominant in Wakatobi waters and North Buton, where other species were rarely found.
The species abundance of spiny lobsters by sex at each location (Table 1) indicated that the sex ratio of each species also varied by location (Table 2).
The results of the analysis of the spiny lobster TL – W relationship for each species, collected from various locations during the study in Southeast Sulawesi waters, were presented in Table 3. These data illustrated the W – TL relationship for each spiny lobster species and sex, sampled from each location during the study period. The data showed a negative allometric growth pattern (b < 3) for all spiny lobsters species. Consequently, their health indices were calculated using the “CF” derived from the W – TL relationship, to determine the CF index for spiny lobsters (Table 4).
Discussion
During the study conducted from September to November 2022, spiny lobster samples were collected from various locations serving as spiny lobster fishing grounds across Southeast Sulawesi waters. The data on spiny lobster catches (all species and sexes combined) by fishermen from each fishing location indicated that the highest CPUE was in Southeast Sulawesi waters, followed by Wakatobi waters and North Buton waters (Table 1). Although the CPUE for spiny lobsters in Southeast Sulawesi waters is higher than other fishing locations, when separated by species, then P. longipes exhibited a high abundance in Wakatobi waters compared to the same species sampled from other locations. The other three species, P. ornatus, P. homarus, dan P. versicolor were found limited only in Southeast Sulawesi waters. The abundance of P. ornatus in the fishing locations of Southeast Sulawesi waters was higher than that of the other three species (P. homarus, P. longipes, and P. versicolor) within the same fishing locations (Table 1). This contrasts with the spiny lobster catch in Kupang waters, which predominantly comprised P. versicolor (60% of the total catch), while P. ornatus and P. homarus accounted for only around 1% of the total catch (Triharyuni & Wiadnyana, 2017). In Palabuhanratu waters of West Java, spiny lobster catches were dominated by P. homarus (Kintani et al., 2020) which is known to favor coral habitats (Mukhtar et al., 2021). Southeast Sulawesi waters and Wakatobi waters are recognized as part of “the triangle coral reef” in Indonesia, characterized by high coral biodiversity and serving as habitats for spiny lobsters (for shelter, spawning, nursery, feeding, and mating). According to Subbey et al. (2014), the deterioration of coral reefs can reduce juvenile spiny lobsters’ recruitment. Protecting coral reefs is crucial for maintaining juvenile spiny lobster survival and determining biological reference points in fisheries management (van Poorten et al., 2018). This indicates that abundance and distribution of spiny lobsters are influenced by the extent of coral reef areas, such as found in Wakatobi waters.
Typically, spiny lobsters in Wakatobi waters primarily inhabit coral reef areas at depths exceeding 20 m, with high water transparency, and minimal domestic pollution. The region is renowned for its coral reefs with high biodiversity, leading to its designation as the Wakatobi National Park. Consequently, these waters are well-maintained, protected from pollution, and safeguarded from activities that could harm the coral reef ecosystem.
The well-preserved condition of the coral reefs and waters provides a suitable habitat for various organisms, especially P. longipes. Similarly, in Kili-kili Bay of East Java, spiny lobster fishing grounds are situated at a water depth of approximately 19.25 m, with water transparency around 5.74 m and a sandy bottom substrate. Direct observations of spiny lobster habitats across all fishing locations in Southeast Sulawesi waters indicated their presence in shallow waters with depths less than 9 m, characterized by sandy and rocky substrates.
Milton et al. (2014) noted that spiny lobsters tend to inhabit sandy and coral habitats at depths of 5 to 10 m, with calm currents and murky conditions. Fishers in all fishing locations often capture small-sized spiny lobsters (in the juvenile stage) and place them in floating cages, nurturing them until they reach sizes ranging from 15 to 25 cm. These floating cages are typically constructed in coastal waters with fluctuating water depths of 4 to 8 m, high water transparency, and protection from strong winds and waves.
The sex of a lobster can be identified morphologically by examining its leg. In fisheries management, understanding the sex ratio of each species is crucial information. Spiny lobsters and other crustaceans have an approximate 1:1 male-to-female which affect mating, reproduction and population dynamics (Koepper et al., 2021; Ogburn, 2019). It has been reported that in female crustaceans, sperm-limitation can occur if there are not enough reproductive males to fertilize all the eggs in the population (Pardo et al., 2017). This issue should be considered, especially when the lobster population is subject to sex-biased harvesting. Fisheries regulations, such as minimum legal sizes and protection of ovigerous (egg bearing) and larger females, have been implemented in Indonesia waters to ensure the sustainability of the spiny lobster fishery (Regulation of the Minister of Marine Affairs and Fisheries of the Indonesia No.7 of 2024).
Our current study revealed that in the Wakatobi waters, male specimens of P. ornatus, P. homarus, and P. longipes predominated over females, contrary to findings in Southeast Sulawesi waters for P. longipes and P. versicolor, while the sex ratio of P. longipes in North Buton waters was balanced. However, across different fishing locations, the sex ratios of these spiny lobsters did not significantly differ (p < 0.05), except for P. longipes in Wakatobi waters (Table 2). Astuti et al. (2022) observed that sex ratios can deviate from the expected 1:1 ratio both between species and within the same population over time.
Discrepancies in the abundance of males and females likely contributed to variations in sex ratios. These variations are influenced by spiny lobster behavior, habitat variability (including shelter availability, food resources, predators, and oceanic dynamics), fishing seasons throughout the year (temporal effects), fishing pressure, geographic location, sampling timing (e.g., during flood or ebb tides, day or night), and sampling methods. Bias in sex ratios has been previously explained in other crustaceans, such as P. pelagicus (Sara et al., 2016), which can be caused by factors like seasons (migration, mating and spawning season), diseases, predation, food availability, location conditions, migration patterns, capture methods, and growth and mortality rates. The peak and low seasons of spiny lobster fishing may also influence capture activities.
Previous research indicated that environmental factors or fisheries management practices could skew the approximate 1:1 sex ratio of Homarus americanus, potentially impacting mating behaviors and reproductive rates with significant ecological and economic implications (Koepper et al., 2021). The authors also suggested a strong correlation between seabed temperature and sex ratio, which was dependent on spiny lobster size. Generally, higher seabed temperatures increased the likelihood of sampling male spiny lobsters.
Local fishermen in Southeast Sulawesi commonly catch spiny lobsters in shallow waters (10–20 m) at night using spears, often capturing more male spiny lobsters. This aligns with findings by Koepper et al. (2021) who noted that larger male spiny lobsters are often found in shallower and warmer waters, while larger females tend to inhabit deeper and colder waters. A study on Portunidae (blue swimming crab) showed a trend where male crabs outnumbered females with increasing water depth. Astuti et al. (2022) emphasized that differences in sex ratios between males and females are not attributed to a single parameter but result from complex interactions among various factors.
In the fisheries management for various aquatic organisms, including spiny lobster populations, growth pattern, habitat condition, species morphometric characteristics can be assessed using W – TL relationships (Falsone et al., 2022; Kampouris et al., 2020), derived from weight-length frequency data. This method is valuable for estimating biomass and comparing species life history across different regions (Paramo et al., 2024), aiding stock assessment, informing exploitation strategies, determining growth patterns (Afzaal et al., 2018), facilitating spiny lobster population management, and contributing to the knowledge of crustacean biology (Paramo et al., 2024).
The condition factor parameters can be derived from a simple regression analysis of the W – TL relationship, resulting in the growth pattern (b) (Sara et al., 2016), which can indicate either an isometric growth pattern (b = 3) or an allometric growth pattern (b ≠ 3). Spiny lobster and other crustaceans grow through molting several times during their life circle, with total length increasing and followed by an increase in body weight.
In our study, the W – TL relationships data for both male and female spiny lobsters exhibited variations in correlation coefficients (Table 3). However, these generally showed strong correlation coefficients (r ≥ 0.75), despite the growth patterns of spiny lobsters from fishing grounds around Southeast Sulawesi being consistently negative allometric (b < 3). This suggests that the growth rate in TL surpasses the increase in W. This indicates that the food consumed by spiny lobsters contributes more to the growth of their TL than to their W.
Similarly, a study on the reproductive biology of scalloped spiny lobsters (P. homarus) in Palabuhanratu Bay showed strong correlation coefficients (0.8154 for males and 0.8961 for females) and negative allometric growth patterns (2.5510 for males and 2.4926 for females) (Kintani et al., 2020). The spiny lobster found in Sebatik Island waters also exhibited negative allometric, with coefficient of 2.7747 for females and 2.8573 for males (Muzammil & Kurniadi, 2021). This is possible due to abiotic, including high level of pressure from the marine environment, dominant human activities, functional gonad maturity, and oceanographic processes (Setyanto et al., 2023). Previous studies conducted in various locations, such as Palabuhanratu Bay in South Java (Wahyudin et al., 2017), and Nabire in Papua (Pranata et al., 2017), also demonstrated similar growth patterns (b < 3). These consistent growth patterns may be attributed to similar habitat characteristics supporting food availability, water quality, and substrate conditions conducive to spiny lobster growth. Other factors affecting growth patterns include sexual, gonad maturity level, and habitat well-being (Setyanto et al., 2023).
It’s widely acknowledged that growth in crustaceans is not continuous, and size increases primarily occur after molting. Crustacean growth is influenced by molt increment and molt frequency. Observations on spiny lobster and mud crab molting have shown the physiological and morphological changes involved in preparing for and recovering from ecdysis, where the old carapace is replaced with a new one just after an increase in somatic mass. The duration between molts in crustaceans is affected by factors such as food availability, substrate texture, organism age, genetic factors, and seasonal variations, while molting is influenced by water temperature (Sara et al., 2016). spiny lobsters generally exhibit thermoregulatory behavior, and their growth rate may directly correlate with environmental temperature up to a certain point, beyond which mortality rates may increase. Studies on mud crabs have shown that factors like sex, maturity level, and organ disappearance contribute to correlation coefficients and growth coefficients.
Despite showing negative allometric growth in all fishing locations in our study, the relative CF of spiny lobsters were relatively similar (Table 4), indicating overall healthy indexes. However, a study conducted in Tanjung Kasuari waters of West Papua showed relatively lower CF for both male (1.0049) and female (0.9865) (Situmorang et al., 2021) compared to data in Table 4. Similarly, the CF of spiny lobsters found in Palabuhanratu Bay, West Java exhibited lower values (0.0908 – 0.1436 for males and 0.0935 – 0.1241 for females) (Kintani et al., 2020). This data (CF < 1) implies that the spiny lobster is poor growth condition. This variation in CF of present study and others may be influenced by the reproductive period of these organisms, which depends on seasonal and habitat characteristics. Generally, high CF values in spiny lobsters indicate increased reproductive activity. These CF data are strongly influenced by the growth coefficient (b), where a higher the growth coefficient (b) value corresponds to a higher (healthier) CF.
CF is used to evaluate overall fish health, productivity, and the physiological condition of fish populations. Thus, the CF values of the spiny lobster obtained in this study (Table 4) serve as an indicator of the health of the aquatic ecosystem, which is the habitat of the spiny lobster in Southeast Sulawesi. The spiny lobster, which is highly consumed both domestically and internationally, commanding a premium price. Due to this, the challenge in managing spiny lobster in this region lies in controlling intensive exploitation to ensure the population remains sustainable. The government has issued regulations governing the capture and trade of spiny lobster, from juvenile to adult sizes (Ministry of Marine Affairs and Fisheries Regulation of the Republic of Indonesia Number 7 of 2024 on the Management of Lobster (Panulirus sp.), Mangrove Crab (Scylla sp.), and Blue Swimming Crab (Portunus sp.). However, field observations indicate that not all lobster fishers and collectors (local lobster traders) comply with this regulation, raising concerns about the sustainability of spiny lobster populations in the region. Therefore, maintaining the health of the aquatic ecosystem, which is influenced by factors such as the intensity of fishing and other factors such as pollutants entering the ecosystem, the physical and chemical conditions of the aquatic environment, and climate change (particularly increases in temperature, acidity, and dissolved oxygen, among others) is crucial to sustaining the health of the spiny lobster population in these waters.








