Introduction
Abalone is a primary aquaculture resource in the seafood industry in several countries, including China, Korea, and Australia, due to its high market value. Beyond its high market price, abalone aquaculture plays an important role in the economy by creating employment opportunities, supporting business development, and promoting tourism (Wagner & Engel, 2021). Additionally, abalone is highly valued for its high nutritional benefits. Scientific research has confirmed that abalone extract exhibits anti-inflammatory, anti-thrombotic, antioxidant, anticarcinogenic, and antimicrobial activities (Suleria et al., 2017).
With the development of abalone cultivation systems, transitioning from land-based to sea cage-based systems has significantly increased global abalone aquaculture production (Sim et al., 2021). However, sea-based cultivation systems face considerable challenges as they depend on coastal water input, which is influenced by several uncontrollable abiotic factors (Morash & Alter, 2016). Environmental factors such as extreme temperature, salinity, O2 levels, pH, CO2 concentration, and other aquaculture conditions can significantly impact abalone physiology and growth, potentially resulting in mass mortality (Morash & Alter, 2016). Apart from environmental factors, mass mortality can result from infections by disease pathogens, which are exacerbated by poor environmental quality (Sim et al., 2021). In addition to bacterial infections, high mortality in many-colored abalone (Haliotis diversicolor supertexta) has been reported in Taiwan due to abalone herpesvirus (AbHV) infection (Corbeil et al., 2020).
The aquaculture ecosystem is a complex system with multiple stressors that can amplify the negative impacts on organisms (Morash & Alter, 2016). Kim et al. (2023) reported that high temperatures and low pH can promote oxidative stress in Pacific abalone (Haliotis discus hannai), specifically by increasing levels of hydrogen peroxide (H2O2), Malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT), and caspase-3. Pacific abalone also experiences heightened stress when cultured in environments with low salinity and low water temperatures (Yang & Min, 2019). In the hemolymph of Pacific abalone cultured at 4°C and 6°C, the levels of SOD and glutathione were significantly higher compared to the control group at 26, 30, and 34 psu. The survival rate of Pacific abalone also decreased to 25%–55% at 4°C in 26, 30, and 34 psu. Juvenile red abalone (Haliotis rufescens) exposed to low PH and low oxygen levels exhibited significantly reduced growth rates compared to controls (Kim et al., 2013a). Not only abiotic factors but also biotic factors, such as exposure to Vibrio harveyi for 24 hours in European abalone (Haliotis tuberculata), are known to cause a decrease in cellular hemocyte parameters, immune response gene expressions, and enzymatic activities (Cardinaud et al., 2015).
Environmental stress and disease can negatively impact abalone, potentially influencing global abalone aquaculture production. Understanding the effects of stress and disease on abalone physiology and growth can aid in developing effective abalone farming management techniques. This study aims to summarize scientific literature on the impact of abiotic stress (temperature, salinity, hypoxia, and pH) and diseases on the physiology and growth of abalone across different life stages. This information can be used as a resource for researchers who want to develop studies on the effects of environmental changes on abalone.
Stress Factors in Abalone Aquaculture Farms
The stress factors affecting abalone in aquaculture systems are highly diverse. Abalone are sensitive to environmental changes. Although mild environmental fluctuations act as stress factors that abalone can overcome, drastic or critical environmental changes can exceed their adaptive capacity, potentially resulting in mortality (Morash & Alter, 2016). In particular, exposure to high temperatures during summer and low-salinity seawater caused by heavy rainfall or typhoons, which are frequent occurrences in abalone farms in Korea, have been linked to mass mortality events. For instance, in 2007 and 2011, summer rainstorms and the influx of low-salinity seawater discharged from the Three Gorges Dam in China resulted in mass mortality of abalone at a Korean abalone farm (Lim et al., 2014). In July 2021, approximately 29 million farmed abalone in the southern waters of Korea died following several days of summer monsoon rainfall. The mass mortality was attributed to freshwater runoff from severe rainfall, which inundated the coastal marine areas where the farms were located (Dong-hwan, 2021). Summer mortality is not confined to Korea but has also been observed in Australian abalone farms. Mortality patterns during summer are often irregular and vary between tanks within farms, with cumulative mortality in individual tanks ranging from 15% to 50% (Bansemer et al., 2023).
Furthermore, various stress factors arise during the abalone cultivation process. During the sorting and transportation process, abalones are typically separated and detached from shelters by submerging them in exfoliating chemicals (e.g., commercial oxytetracycline or organic acids with a pH of 2.1–2.6) for approximately 10 to 30 s (Kim et al., 2013c). When abalones are exposed to severe stress from exfoliating chemicals, they experience additional stress, including hypoxia, air exposure, and physical stress during sorting and selection procedures (Choi et al., 2024; Kim et al., 2013c). Abalones detached after prolonged exfoliation procedures or exposed to lower pH levels exhibit slower recovery rates. Even in small numbers, the exfoliation process can result in abalone mortality (Kim et al., 2013b).
High-density culture may also adversely affect the survival rate and specific growth of abalone. In Korea, most abalones are raised in high-density tanks due to economic constraints and to facilitate ease of management. However, abalones in these tanks are subject to chronic stress due to the heightened competition for resources and space at high stocking densities. Despite an increase in food quantity, certain abalones continue to struggle with food acquisition due to restricted mobility. Furthermore, increased feeding under high stocking density conditions may block the mesh and hinder gas exchange (Pang et al., 2022). High-density cultivation, combined with elevated feeding rates, insufficient waste disposal, and inadequate water exchange, can result in ammonia accumulation in the water and a heightened incidence of disease (Morash & Alter, 2016).
Additionally, stress caused by transportation during shipment can be highly detrimental to abalone. During live transport, abalone are confined in insulated containers for around 48 h before being unloaded at their destination (Venter et al., 2025). Reduced oxygen levels during transportation, particularly during live export, can impair aerobic energy metabolism, induce osmotic stress, and cause cellular and tissue damage through oxidative stress and apoptosis (Alfaro et al., 2021). Additionally, Sawangwong et al. (2019) reported that 30 h of air exposure at 5°C and 10°C could lead to a decrease in total hemocyte count (THC), an increase in hemocyte mortality, and elevated reactive oxygen species (ROS) levels.
All of the stress factors that occur on aquaculture farms might cause a physiological stress response in abalone. The next sections describe major stressors, as well as how abalone respond to these factors.
Physiological Stress Responses of Abalone to Various Stressors
Living organisms maintain the equilibrium of their biological systems in response to external stimuli, a process termed homeostasis (Billman, 2020). Environmental stimuli can disrupt homeostasis if they exceed the organism’s adaptive capacity, resulting in a state known as stress. Stress is defined as a state of threatened homeostasis caused by the actions of stressors. The stress response involves a series of coordinated physiological systems that enhance an organism’s ability to maintain homeostasis under undesirable stimuli (Eissa & Wang, 2016).
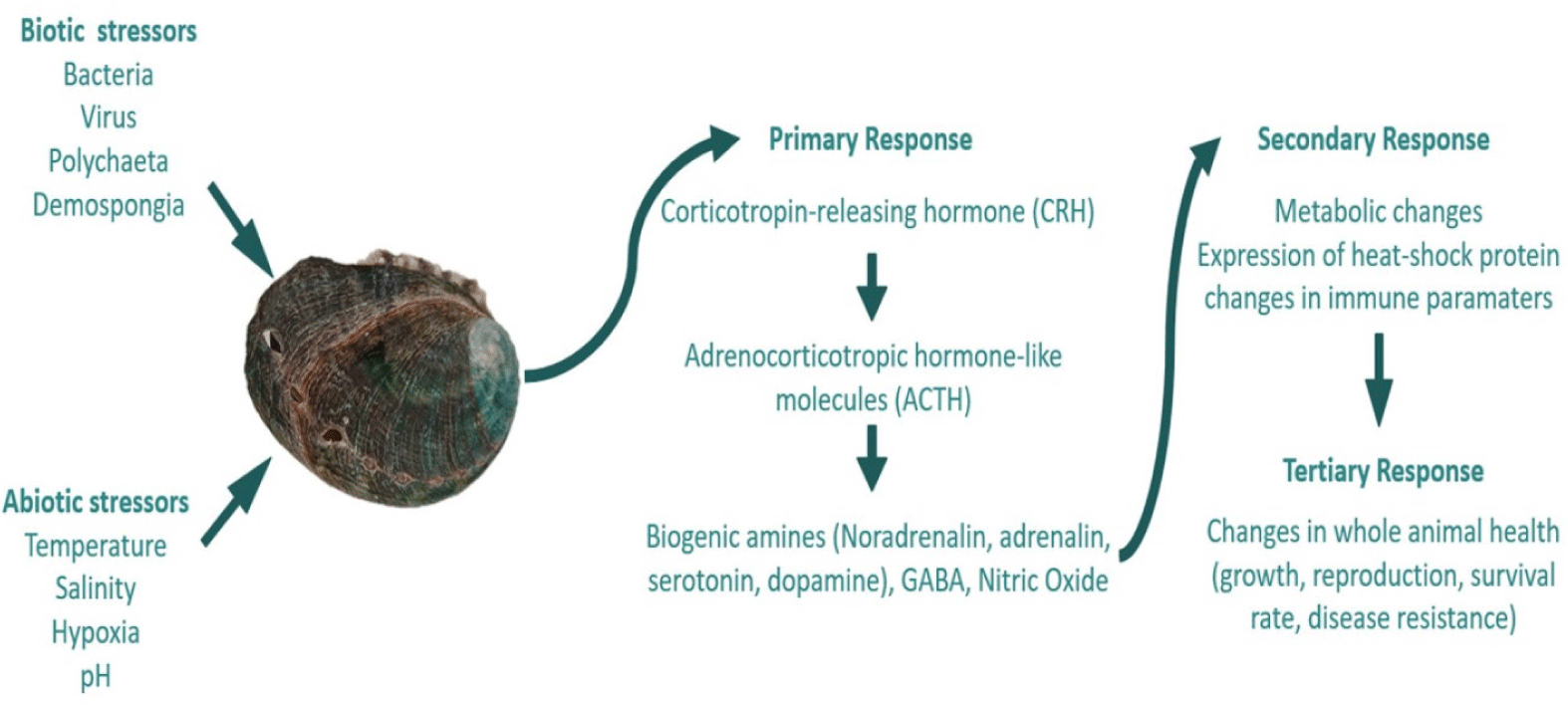
Stress responses are categorized into two types: primary responses, involving neuroendocrine activation, and secondary responses, characterized by alterations in blood constituents, such as metabolites and major ions, as well as the expression of heat-shock or stress proteins at the cellular level (Eissa & Wang, 2016). The corticotropin-releasing hormone (CRH) regulates the stress response in molluscs, similar to its function in vertebrates, by stimulating the release of adrenocorticotropic hormone-like molecules (Ottaviani et al., 1998). Molluscs subsequently release neurotransmitters (Ottaviani et al., 1991), including biogenic amines such as norepinephrine/noradrenaline, serotonin (5-HT), dopamine, and epinephrine/adrenaline (Kotsyuba & Dyachuk, 2023), as well as γ-aminobutyric acid (GABA), nitric oxide, and acetylcholine (Ach) Liu et al., 2018).
The secretion of stress hormones resulting from stress induction can alter immune function, occasionally leading to either an increase or decrease in certain immune parameters (Adamo, 2012). The defense system of abalone includes both cellular and humoral immunity. Cellular immunity is mediated by hemocytes through phagocytosis (Ding et al., 2016). Phagocytosis is followed by the generation of ROS, such as superoxide anion (SO), H2O2, and hydroxyl radicals (.OH) to eliminate pathogens. However, excessive ROS production may result in oxidative stress and cellular damage (Martemucci et al., 2022). Humoral immunity is characterized by non-cellular immunological responses such as lysozyme activity and phenoloxidase (PO). Lysozyme exhibits antibacterial properties. The PO system is a critical defense mechanism in numerous invertebrates, contributing to melanization (Al-Khalaifah, 2022). Antioxidant enzymes, such as CAT, protect cells against damage induced by ROS (Martemucci et al., 2022).
If the primary and secondary responses fail to maintain hemostasis, tertiary responses occur, resulting in alterations in the overall health of the animal (Eissa & Wang, 2016). The mechanism of stress responses is shown in Fig. 1. In this section, we describe how abalone respond to abiotic stressors and diseases and how these factors affect their health and survival.
Numerous abiotic factors can adversely affect abalone physiology and growth. However, in this section, we discuss the effects of four important abiotic factors that frequently fluctuate in the aquatic environment, namely temperature, salinity, O2, and pH, on abalone growth and several physiological parameters.
Abalone are thermoconforming poikilotherms, meaning their body temperature changes in response to external temperature variations (Kim et al., 2018). There are 56 valid species of abalone, along with 18 subspecies (Cook, 2023), inhabiting diverse marine environments ranging from cool temperate regions to tropical areas (Kim et al., 2018). Consequently, tolerance limits and optimal growth temperatures vary among species (Kim et al., 2018). For example, the suitable temperature range for Pacific abalone is 16°C–28°C (Kong et al., 2017), with an optimal growth temperature of 20°C (Ding et al., 2016). Yang & Min (2019) reported that the lowest temperature tolerance for Pacific abalone is 8°C. In contrast, the ideal temperature range for green abalone is from 20.5°C to 28.5°C, peaking at 24°C, with an upper thermal limit near 30°C (Lluch-Cota et al., 2023).
Currently, the global sea surface temperature (SST) between 1950 and 2020 has increased by 0.11°C (0.19 °F) (Venegas et al., 2023). Data on SST fluctuations obtained from the Jeongseon Ocean Observation Point in Korea by the National Institute of Fisheries Science (NIFS) indicates that the rate of SST increase around the Korean Peninsula has increased by 95% over the past 52 years (1968–2019) (Choi et al., 2023). From 1968 to 2022, the rise in seawater temperature resulted in the mean SST in Korea being approximately 2.6 times higher than the global average (Han et al., 2023). The Intergovernmental Panel on Climate Change (IPCC)’s 2013 report predicts a mean worldwide ocean temperature rise of 1°C–4°C by 2100 (Alfonso et al., 2021). The increase in seawater temperature also exhibits fluctuations, particularly in summer (Kang et al., 2019).
Changes in seawater temperature can induce stress in poikilothermic organisms and may even cause mortality if the temperature exceeds their tolerance limits (Pedroso, 2017). In summer, abalone can experience mass mortality as temperature changes affect seawater chemistry, including pH, dissolved oxygen levels, salinity, ammonia concentration, and nutritional availability (Botwright et al., 2021). Water temperature regulates nearly every aspect of on-farm production and physiological processes in abalone, including metabolism (Kang et al., 2019), growth, survival (Pedroso, 2017), and immune system function (Ding et al., 2016). Table 1 provides a summary of several published studies on the effect of temperature on abalone physiology.
| Species | Stressor levels (°C) | Duration | Parameters | General findings | References |
|---|---|---|---|---|---|
| Blacklip abalone (Haliotis rubra) |
18 (control), 21, and 24 | 7 d | SO, THC, antibacterial activity against Vibrio anguillarum and antiviral activity against herpes simplex virus type 1. | THC and antiviral activity increased, antibacterial activity decreased, and there was no significant difference in SO levels. | Dang et al., 2012 |
| Pacific abalone (Haliotis discus hannai) |
8, 14, 20 (control), and 26 and Vibrio anguillarum injection | 30 d and 24 h for bacterial challenge. | THC, hemocyte population characteristic, hemocyte mortality, Phagocytic capacities, ROS production, and temporal expression of immune-related genes. | Hemocyte phagocytic capability and ROS generation were more responsive to cold temperatures. High temperature affected Spi gene expression more. Higher and lower water temperatures greatly altered Cpi expression. | Ding et al., 2016 |
| Pacific abalone (Haliotis discus hannai) |
Temperature 16, 18, 20, 22, 24 (Control), 26, 28, 30 and 32 | 77 d | SGR, survival rate, MSAP, DNA methylation states, and epigenetic structure. | Juveniles can grow well at 23°C–25°C. There are no significant variations in global methylation levels. Temperature can cause epigenetic differentiation. | Kong et al., 2017 |
| Donkey ear’s abalone (Haliotis asinina) |
Ambient (29), +2 (31), and +4 (33) | Larvae: 7 d Breeder: 3 mon |
Hatching rate, survival rate of larvae and breeder, larvae shell length, SGR and daily consumption rate of breeder. | The hatching rate, survival rate, and shell length of larvae were all decreased at 33°C. Female and male breeders all died after 2 and 3 months, respectively. | Pedroso, 2017 |
| Pacific abalone (Haliotis discus hannai) |
Stable temperature experiment (3, 8, 13, 18, 23, and 28) and fluctuation temperature experiment (range between 20 to 26). | 24 h for stable experiment and for 144 h for fluctuating experiment. | Oxygen consumption, proteomic analysis, ammonia excretion, O:N ratio, and metabolic pathway analysis. | In the steady warm summer experiment, metabolic rates surged and varied in response to brief temperature fluctuations experiment (20°C–26°C). Both experiments had similar acute ammonia excretion rates. In the fluctuation temperature experiment, enzyme activity decreased, and structure-related protein expression increased. | Kang et al., 2019 |
| Tropical abalone (Haliotis Squamata) |
28, 30 (control), 32 and 34. | 96 h | Mucus production, muscle hardness, survival rate, CAT, SOD, phenoloxidase, HSP70 and HSP90 gene expressions, and foot histology. | Temperature stress increases muscle hardness, foot muscle abnormalities, and mucus production. Lower and higher temperatures enhanced abalone enzyme activity. Abalone did not survive at 34°C after 12 hours. After high-temperature stress, SOD, CAT, and phenol oxidase activity were altered. After 6–12 h above 30°C, we observed an increase in HSP70 and HSP90 proteins. | Yasa et al., 2019 |
| Red Abalone (Haliotis rufescens) and Blackfoot Paua (Halioti iris) | +5 over ambient temperature | 6 weeks | Respiration rates, Digestive enzyme assays, organic matter digestibility, and algal consumed. | Both abalone species maintained digestive functions in species-specific ways. | Frederick et al., 2022 |
Energy balance is crucial for organisms to adapt to temperature fluctuations (Xu et al., 2023). Some ectothermic organisms adapt by increasing metabolic energy demands at high temperatures until their thermal limit is reached (Kang et al., 2019; Morash & Alter, 2016). Conversely, other organisms adjust their physiological rates to reduce metabolic energy demand during short-term temperature fluctuations, enhancing their thermal tolerance (Kang et al., 2019). For instance, the respiration rate of the slipper snail (Crepidula fornicata) increased consistently as the temperature increased from 18°C to 34°C over 12 h, under both normal and high pCO2 conditions (Noisette et al., 2015). In contrast, the South American mussel (Mytella strigata), subjected to daily heat air treatment at 40°C for 6 h over 5 consecutive days, maintained its energy acquisition while reducing its respiration rate (Xu et al., 2023).
The impact of temperature on the metabolic rate of abalone has also been extensively studied. Red abalone and blackfoot paua (Haliotis iris) exhibited elevated metabolic rates following exposure to temperatures 5°C higher than ambient conditions for 6 weeks, with Q10 values of 1.73 for red abalone and 2.46 for blackfoot paua. Algal consumption is also elevated by red abalone and blackfoot paua under high-temperature conditions. Both abalone species maintained dietary digestibility throughout increased metabolic demands induced by heat stress in distinct manners (Frederick et al., 2022). Similarly, the metabolic rate of Pacific abalone increased with elevated temperatures under stable warm summer conditions (3°C, 8°C, 13°C, 18°C, 23°C, or 28°C) and fluctuated in response to short-term temperature variations (20°C–26°C) (Kang et al., 2019). These findings suggest the metabolic costs of Pacific abalone are highly dependent on temperature and the duration of exposure (Kang et al., 2019). During the initial stages of thermal stress, abalone require substantial energy to combat the stress, leading to an increase in energy metabolism. However, prolonged stress inhibits aerobic metabolism, reduces energy supply, and downregulates certain energy-intensive metabolic pathways, ultimately prompting the organism to adopt a strategy of lower energy consumption (Yu et al., 2023).
Environmental stress, particularly temperature stress, may cause animals to divert energy from growth to self-defense mechanisms against environmental challenges, resulting in slower growth rates (Stapleton & Donovan, 2024). Vosloo et al. (2013) assert that an increase of +3°C above the ambient temperature (19°C) approaches a long-term thermal threshold for winter-acclimated South African abalone (Haliotis midae) juveniles. These organisms endure such conditions by reallocating energy towards survival mechanisms rather than growth. A different study involving Pacific abalone also demonstrated that temperature stress may decrease survival rates and growth. After four days at 32°C, juvenile Pacific abalone died completely. The growth and survival rates of juvenile Pacific abalone at temperatures ranging from 16 to 28°C were significantly higher than at 30°C (Kong et al., 2017). Exposure to +2°C and +4°C (31°C and 33°C) above ambient temperatures in donkey’s ear abalone (Haliotis asinina) resulted in a decline in survival rates and growth of both larvae and breeders. Complete mortality was observed in female breeders at 33°C after 2 months and in male breeders after 3 months (Pedroso, 2017). The decrease in growth and increase in mortality are believed to result from a reduction in daily feed consumption at elevated temperatures (Pedroso, 2017). Temperature tolerances and optima may also result from variations in size and genetic background (Kong et al., 2017).
An increase in temperature outside the optimal temperature range may also potentially increase susceptibility to microbial infections (Dang et al., 2012). In blacklip abalone (Haliotis rubra) cultured at 24°C, THC and SO production increased on day 1 but returned to control levels by day 7 (Dang et al., 2012). The decline in total hemocytes is believed to result from cell lysis or increased movement of cells from the hemolymph into tissues. The increase in total hemocytes may result from either cell proliferation or the migration of cells from tissues into the hemolymph (Ding et al., 2016). The antibacterial activity of blacklip abalone cultured at 24°C decreased on day 7, with values lower than in the control group (Dang et al., 2012). This decrease in antibacterial activity is likely caused by a reduction in lysozyme, a crucial antimicrobial agent in the hemolymph (Ding et al., 2016). The decrease in both antibacterial activity and total hemocyte renders abalone more vulnerable to bacterial infection. Additionally, elevated temperatures can accelerate bacterial growth and increase viral production (Dang et al., 2012).
Temperature stress induces oxidative stress, characterized by increased production of ROS and elevated antioxidant enzyme activity (Kim et al., 2023). However, antioxidant enzyme activity can fluctuate during the initial and later stages of exposure (Gao et al., 2024). For example, catalase activity in tropical abalone decreased at 32°C at 12 h compared to the control temperature of 30°C and the initial measurement (day 0). However, CAT activity increased at 24 h, followed by a decline at 48, 72, and 96 h. In contrast, SOD activity increased after 12 h of treatment compared to the control and day 0 but decreased at 48 hours (Yasa et al., 2019). Laboratory experiments with hybrid abalone Pacific abalone and green abalone (Haliotis fulgens) also revealed that catalase significantly increased after 24 h of exposure to +1°C, followed by a decline between 48 and 96 h. Meanwhile, SOD activity significantly increased at the 96-h treatment temperature of +1°C. These results contrast with field test results. The catalase activity of hybrid abalone at +1°C treatment decreased over time, from 24 h to 96 h, but showed no significant difference between the time group and the control group. SOD activity in abalone under field conditions also differed from laboratory results, exhibiting a reduction at 96 h compared to the control group (Gao et al., 2024). Field research may yield different outcomes than laboratory experiments due to variable and unstable temperature fluctuations. The challenging environmental temperature variations may induce such tendencies within a significantly shorter duration. The reduction in antioxidant enzyme activity may result from elevated ROS, potentially culminating in the failure of the antioxidant defense system (Gao et al., 2024).
Temperature also plays a crucial role in abalone gonadal maturation and spawning during the breeding season, along with nutrient availability and photoperiod. The optimal temperature for gonad maturation varies depending on the abalone species. When temperature and nutrient availability are favorable, all abalone will recondition their gonads throughout the spawning season. However, constant reconditioning through repeated spawning may increase mortality risk. For instance, during high summer temperatures, European abalone can spawn multiple times, a process that demands substantial energy, ultimately depleting the abalone and compromising their immune system, making them more vulnerable to opportunistic diseases (Botwright et al., 2021).
Salinity in the open sea is generally stable. However, in intertidal zones and estuarine areas, it can fluctuate due to seasonal heavy rainfall, evaporation, tides, and human activities (Cao et al., 2022). The average salinity in the ocean is approximately 35 psu, but this can vary depending on the location. The Baltic Sea, situated adjacent to a water body with high river inflow, exhibits low salinity, approximately 7.5 psu (Fundacja Mare, 2024). In Hakata Bay, salinity at the river mouth can drop to 15 psu one day after heavy rain, while surface water salinity in the inner part of the Bay remains below 28 psu after rainfall (Fukuda et al., 2021). On abalone farms, salinity fluctuations may occur due to water inlets and tidal movements of coastal waters (Morash & Alter, 2016). Variations in salinity can significantly affect the survival and growth of abalone (Morash & Alter, 2016). Table 2 summarizes published literature on the effects of salinity on abalone physiology.
| Species | Stressor levels | Duration | Parameters | General findings | References |
|---|---|---|---|---|---|
| Many-colored abalone (Haliotis diversicolor) |
20, 25, 30 (control) and 35‰ and injection with 1.6×105Vibrio parahaemolyticus | 120 h | Mortality rate, THC, phenoloxidase activity, respiratory burst activity, phagocytic activity, and bacterial clearance. | Immunity and resistance to Vibrio parahaemolyticus decreased when salinity increased from 30‰ to 20‰, 25‰, and 35 ‰. | Cheng et al., 2004a |
| Disk abalone (Haliotis discus discus) |
25, 30, 33 (control), and 35 psu | 48 h | Suvival rate, THC, CAT, SOD, respiratory burst, phenoloxidase activity, lysozyme activity, HSP70 expression in gills. | Low salinity stress weakens the immune system in Pacific abalones by decreasing the activity of phenoloxidase and lysozyme. | Jwa et al., 2009 |
| Disk abalone (Haliotis discus discus) |
25‰ and 32‰ (control) | 24 h | Gene expressions of MnSOD, CAT, CuZnSOD, SeGPx, TPx, TRx-2, SOCS-2, and Mx. | At low salinity, the gene expressions of MnSOD, CAT, CuZnSOD, SeGPx, TPx, TRx-2, SOCS-2, and Mx all increased. | de Zoysa et al., 2009 |
| Pacific abalone (Haliotis discus hannai) |
Changing the salinity at 3 different temperature Control 35 psu for 7 days From 35→30→25→20→15 psu for 7 days From 35 →25→15 psu seven days From 35 →15 psu |
7 d | Survival rate, and oxygen consumption. | Salinity of 25–35 PSU is ideal for abalone production. Salinities below 20 PSU lower survival and oxygen consumption. Sudden salinity changes and high water temperatures enhance mortality. | Lim et al., 2014 |
| Pacific abalone (Haliotis discus hannai) |
16, 18, 20, 22, 24, 26, 28, 30, 32 (control), 34, 36, 38, 40, 42, 44 and 46 ppt | 77 d | SGR (length, width, weight), survival rate, DNA methylation, and epigenetic structure. | The optimal salinity was 30–36 ppt. There are no significant variations in global methylation levels. Salinity can cause epigenetic differentiation. | Kong et al., 2017 |
| Many-colored abalone (Haliotis diversicolor) |
20, 25, 31 (control), 35 and 40 ppt | 2 mon | SGR (weight and length), density and sizes of mucous cells (digestive gut, gills, lips). | Growth was highest around 25–35 ppt. Lip and gill mucous cells had the greatest density and smallest cell size at 40 ppt, while digestive gut cells were at 20 ppt. | Creencia & Noro, 2018 |
| Pacific abalone (Haliotis discus hannai) |
26, 30, and 34 psu combined with 4°C, 6°C, 8°C, and 10°C | 7 d | Survival rate, antioxidant enzyme, THC, hemocyte mortality | The Pacific abalone has the lowest tolerances for salinity and water temperature, at 30 psu and 8°C, respectively. | Yang & Min, 2019 |
| Pacific abalone (Haliotis discus hannai) and Giant abalone (Haliotis gigantea) |
34 (control), 30, 26, 22, 18 and 14 psu | 3 h salinity exposure and the culture for 7 days at normal salinity | Hatching, survival rate, and abnormality. | Hatching, abnormalities, and survival rates were not significantly different between 30 and 34 PSU for both species. The hatching and survival rates of both species were reduced by short-term low salinity stress, while abnormality rates were elevated. | Phan et al., 2022 |
SGR, specific growth rate; THC, total hemocyte count; CAT, catalase; SOD, superoxide dismustase; HSP, heat shock protein; MnSOD, manganese superoxide dismutase; CuZnSOD, copper zinc superoxide dismutase; TPx, thioredoxin peroxidase; SeGPx, selenium-dependent glutathione peroxidase; TRx-2, thioredoxin-2 (TRx-2); SOCS-2, cytokine signaling-2; Mx, myxovirus resistance.
Abalones are osmoconformers, which regulate the osmotic pressure of body fluids to match the external environment within a specific salinity tolerance range (Burke et al., 2001). Due to their limited mobility, they are susceptible to fluctuations in salinity (Lim et al., 2014). Osmoconformers exhibit the ability to regulate inorganic ions, including potassium and sodium, in response to salinity stress (Burke et al., 2001). Water is either absorbed or released, altering the osmotic pressure, which subsequently activates Na+/K+-ATPase (Gao et al., 2017). Optimal growth in aquatic organisms is achieved when they can maintain osmotic pressure in their body fluids equal to that of their surrounding environment. This equilibrium requires minimal energy expenditure and maximizes energy conversion efficiency (Sun et al., 2021). However, variations in salinity beyond tolerance limits can impair osmotic pressure regulation, growth, and survival rates in aquatic animals (Huang et al., 2023).
Kong et al. (2017) analyzed the impact of various salinities on juvenile Pacific abalone for 77 days at a temperature of 20°C. The findings indicated that the specific growth rate in length, width, and weight at salinities of 18, 20, 22, 42, and 44 ppt significantly decreased compared to the control at 32 ppt. No abalone survived 16 and 46 ppt. The decrease in growth rate may also be associated with feeding behavior, which is influenced by salinity stress (Creencia & Noro, 2018). Salinity also affects the survival rate, with each species and life stage exhibiting different tolerance limits. The duration of exposure to salinity levels below the species’ lethal physiological threshold also influences mortality (Peteiro et al., 2018). Juvenile many-colored abalone can tolerate salinity ranging from 20–40 ppt (Creencia & Noro, 2018). Meanwhile, juvenile greenlip and blacklip abalone have a tolerance of 25–40 ppt, with deviations of 2 ppt beyond this range resulting in mortality (Edwards, 2003). The 7-day median lethal salinity (7-day LS50) of adult Pacific abalone cultured at 24°C ranged from 20.1–28.2 psu, with confidence limits of 20.1–28.2 psu (Shin et al., 2011). According to Shin et al. (2011), 100% of Pacific abalone died after 24 hours at 12.8 psu and within 6 h at 19.8 psu. Another study found that juvenile Pacific abalone cultured at salinities of 18, 20, and 22 psu at a constant temperature of 20°C survived until the 77th day but exhibited significantly lower survival rates compared to the 32 psu control. However, juveniles experienced 100% mortality when cultured at 16 and 46 psu (Kong et al., 2017). Lim et al. (2014) study the effect of gradual and rapid salinity changes on the survival rate and oxygen consumption rate of juvenile Pacific abalone. The results show that decreasing salinity from 35 to 15 psu gradually by 5 psu every 24 hours still produces a high survival rate. However, gradually decreasing the salinity by 10 psu from 35 to 15 psu every 24 hours results in a drastic decrease in the survival rate. Even juvenile abalone had complete mortality if there was a rapid change in salinity from 35 to 15 psu. This occurs because changes in salinity also affect the oxygen consumption rate and cause metabolic imbalance, thus reducing the survival rate.
Low salinity during the rainy season has detrimental effects on embryo development. Embryo hatching rate and survival rate at 7 days post-hatching in disk abalone and giant abalone (Haliotis gigantean) significantly decrease compared to the control (34 psu) when exposed at salinities of 14, 18, and 22 psu for 3 h. Abnormality rate was increase significant at 14, 18, 22, and 26 psu for both species. These findings suggest that early life stages are more susceptible, as even short-term exposure (3 h) to low salinity can adversely affect embryo development (Phan et al., 2022).
Salinity stress can induce oxidative stress in abalone, altering the activity of antioxidant enzymes (Yang & Min, 2019). In adult Pacific abalone hemolymph, exposure to 26 psu resulted in an increase in SOD and glutathione levels compared to exposure at 30 and 34 psu (Yang & Min, 2019). Jwa et al. (2009) evaluated the impact of salinity stress (25, 30, 33, and 35 psu) on the hemolymph of juvenile disk abalone (Haliotis discus discus) for 48 h. Low salinity levels of 25 and 30 psu led to an increase in SOD and CAT activity compared to the experimental group at 33 psu. However, PO and lysozyme activity decreased under these conditions. These findings indicate that low salinity stress suppresses the immune system by reducing PO and lysozyme activity in disk abalone (Jwa et al., 2009). Several genes are upregulated in Pacific abalone gills when exposed to 25‰ of salinity stress for 24 h. These genes include copper zinc SOD (CuZnSOD), manganese SOD (MnSOD), CAT, selenium-dependent glutathione peroxidase (SeGPx), thioredoxin-2 (TRx-2), cytokine signaling-2 (SOCS-2), thioredoxin peroxidase (TPx), and myxovirus resistance (Mx; de Zoysa et al., 2009).
Salinity also affects THC and phagocyte activity in many-colored abalone (Haliotis diversicolor; Cheng et al., 2004a). THC significantly decreased over time at 20 and 25 psu, remained unchanged at 30 psu, and increased at 35 psu for 5 days of exposure (Cheng et al., 2004a). Elevated THC levels may result from various factors, including cell proliferation, the transfer of cells from tissues into the circulation, and water loss from the hemolymph to the medium for osmotic regulation. Meanwhile, the phagocytic activity and clearance efficiency of Vibrio parahaemolyticus by hemocytes of many-colored abalone at 20‰, 25‰, and 35‰ decreased significantly (Cheng et al., 2004a). In a study using Pacific abalone, exposure to 26 psu for 7 days at temperatures of 4°C and 6°C reduced THCs and increased apoptotic and necrotic cells compared to 30 or 34 psu and the control groups (Yang & Min, 2019). The health status of abalone can be assessed through THCs and hemocyte mortality. Low THC levels and high apoptotic and necrotic cells indicate that 4°C, 6°C, and 26 psu are not optimal conditions for the physiology of Pacific abalone (Yang & Min, 2019).
Hypoxia can result from coastal upwelling, global warming (Kim et al., 2013a), and eutrophication (Shen et al., 2022) driven by anthropogenic activities such as industrial wastewater discharge, agricultural runoff, and nutrient enrichment (Nam et al., 2020). Therefore, coastal areas frequently experience seasonal or episodic hypoxic conditions (Nam et al., 2020). Although hypoxic conditions typically last only a few hours, they can occur regularly, ranging from 50 to 200 times per year in certain coastal areas, particularly in ecosystems influenced by upwelling, such as the California Current and Humboldt Current large marine ecosystems (Kim et al., 2013a). Hypoxia has also been reported in industrial and agricultural areas, including the natural Jinhae Bay (JB) and the artificial Gamak Bay, Cheonsu Bay, Shihwa Bay, and Yeongsan Bay (Lee et al., 2018). Dissolved oxygen levels tend to decline in summer as temperatures rise, increasing mortality rates (Bansemer et al., 2023). Hypoxia poses a significant risk to aquaculture and natural abalone populations, as most abalone farms are located along the coast. Water becomes hypoxic when DO concentrations fall to 2.8 mg O2/L, with chronic low DO levels potentially causing significant economic losses (Nam et al., 2020).
The availability of oxygen in seawater is an important source of energy for the growth and physiological processes of abalone and other marine organisms (Shen et al., 2022). Table 3 summarizes published literature on the effects of hypoxia on abalone physiology. Under normal oxygen levels, the right gill is primarily responsible for oxygen uptake, facilitated by the movement of cilia on the gill lamellae, increased water flow over the shell, and enhanced blood circulation through the branches. The epipodium also contributes to oxygen uptake. During hypoxia, the left gill becomes involved in oxygen uptake by increasing its surface area, thereby enhancing oxygen intake (Morash & Alter, 2016). In hypoxic conditions, organisms allocate energy to maintain homeostasis and survival rather than growth. Prolonged exposure to low DO levels can adversely affect metabolism and growth (Nam et al., 2020; Shen et al., 2022). When Pacific abalone were reared in seawater with 2.5 mg O2/L of oxygen for 4 months, shell length and width did not exhibit an increase, and meat weight, survival rate, oxygen consumption rate, and metabolic waste removal (ammonia) were significantly lower compared to those reared in control seawater with 8 mg O2/L of oxygen. This indicates a reduced metabolic rate, which forces the abalone to utilize remaining energy resources for survival, thereby inhibiting homeostasis and growth. Additionally, hypoxia conditions lead to a decline in food intake (Nam et al., 2020). Similarly, another study reported that hypoxia during early developmental stages slows juvenile growth in Pacific abalone (Shen et al., 2022).
| Species | Stressor levels | Duration | Parameters | General findings | References |
|---|---|---|---|---|---|
| Many-colored abalone (Haliotis diversicolor) | 7.70, 5.61, 3.57 and 2.05 mg/L. | 96 h | THC, respiratory burst, phenoloxidase activity, clearance efficiency, and phagocytic activity. | Concentrations as low as 2.05 and 3.57 mg/L induce immunosuppression in Haliotis diversicolor supertexta, enhancing its vulnerability to Vibrio parahaemolyticus infection. | Cheng et al., 2004b |
| Disk abalone (Haliotis discus discus) | 95% dissolved oxygen (control) and without oxygenation | 8 h | Gene expressions of MnSOD, CuZnSOD, CAT, SeGPx, TPx, SOCS-2, TRx-2, and Mx. | MnSOD, Tpx, Cat, SOCS-2, SeGPX, and Mx were upregulated. | de Zoysa et al., 2009 |
| Pacific abalone (Haliotis discus hannai) | 2.5, 4, and 8 mg O2/L (control) | 4 mon | Shell length and width, meat weight, oxygen consumption and ammonia-N excretion rates, survival rate, THC, antibacterial activity, lysozyme activity, MDA, GSH, CAT, SOD. | Severe hypoxia (2.5 mg O2/L) dramatically altered all measured parameters. | Nam et al., 2020 |
| Pacific abalone (Haliotis discus hannai) | Eggs were fully aerated and moderate hypoxia (4 mg/L) until trochopore. Then juvenile from these group were exposed to 0.56 O2 mg/L. | 27 h | SGR (length, width, weight), survival rate, Whole-Genome Resequencing and Transcriptome Sequencing of larval Abalone. | Juvenile abalones subjected to hypoxia at early developmental stages exhibited increased hypoxia tolerance but with reduced weight growth. Abalone hypoxia tolerance is differentiated through both genetic selection and epigenetic control of lncRNAs. | Shen et al., 2022 |
| Pacific abalone (Haliotis discus hannai) | Air exposure at different temperature | 30 h | THC, hemocyte mortality, and ROS | THC decreased, and ROS and hemocyte mortality increased after 30 h at 5°C and 10°C. | Sawangwong et al., 2019 |
SGR, Specific growth rate; THC, total hemocyte count; CAT, catalase; SOD, superoxide dismustase; MnSOD, manganese superoxide dismutase; CuZnSOD, copper zinc superoxide dismutase; TPx, thioredoxin peroxidase; SeGPx, selenium-dependent glutathione peroxidase; TRx-2, thioredoxin-2 (TRx-2); SOCS-2, cytokine signaling-2; Mx, myxovirus resistance; MDA, Malondialdehyde; GSH, glutathione.
Hypoxia induces oxidative stress, resulting in a reduction in antioxidant capacity. Malondialdehyde is a key biomarker of oxidative stress. Aquatic organisms are typically rich in lipids containing polyunsaturated fatty acids (PUFAs), which are highly susceptible to peroxidation. The peroxidation of PUFAs generates malondialdehyde as a byproduct. In juvenile Pacific abalone, exposure to hypoxia at 2.5 mg O2/L for 1 month led to an increase in malondialdehyde levels in the hemolymph. This treatment also suppressed antioxidant capacity, characterized by a decrease in levels of glutathione, catalase, and SOD activities (Nam et al., 2020). Conversely, adult Pacific abalone gills subjected to hypoxia for 8 h exhibited upregulation of the expression of antioxidant enzyme genes (MnSOD, TPx, CAT, SOCS-2, SeGPx, Mx; de Zoysa et al., 2009).
Hypoxia treatment (2.5 mg O2/L) also reduced THC and inhibited antibacterial and lysozyme activities in Pacific abalone. This suppression is likely due to the critical role of oxygen in cell cycle regulation and cell division. The observed decline in antibacterial activity may be attributed to the reduced efficiency of bacterial cell wall-degrading enzymes, such as chitinase and muramidase. Chitinase hydrolyzes chitin into N-acetyl glucosamine (Nam et al., 2020). Other investigations utilizing Pacific abalone demonstrated a significant reduction in THC following 30 hours of air exposure at 5°C and 10°C and after 24 hours at 20°C. Hemocyte mortality and ROS levels were significantly raised. They reached their highest levels after 30 hours at 5°C and 10°C, followed by a progressive decline during the next 24-hour recovery phase (Sawangwong et al., 2019). Furthermore, hypoxic conditions at 3.57 and 2.05 mg O2/L increased the susceptibility of many-colored abalone to Vibrio parahaemolyticus infection. This increased vulnerability is likely associated with diminished phagocytic activity, reduced THCs, and impaired clearance efficiency against Vibrio parahaemolyticus under hypoxic conditions (Cheng et al., 2004b).
Ocean acidification is projected to decrease ocean pH by 0.3–0.4 units by 2100 and by 0.7–0.8 units by 2300 (Li et al., 2023). However, pH levels in coastal areas can fluctuate more significantly than in the open ocean, with variations exceeding 1 unit (Li et al., 2023). This is attributed to several factors, such as coastal upwelling, river inputs (Li et al., 2023), increased CO2 absorption and other anthropogenic influences (Guo et al., 2023). For example, in Saint Joseph Bay, Florida, monthly pH values range from 7.36–8.28 (a difference of 0.92 units), and the daily pH values range from 7.70–8.06 (a difference of 0.36 units; Challener et al., 2016). Another study in three bays of northeastern New Brunswick (Canada) reported highly fluctuating pH values ranging from 7.31 to 8.90 (Mayrand & Benhafid, 2023).
Several studies have investigated the effects of reduced pH on abalone physiology (Table 4). Incubating Pacific abalone larvae at pH 7.3 and 7.6 resulted in shorter larval length, increased abnormalities, and decreased settlement rates (Li et al., 2013). Exposure to seawater pH 7.85 and 7.65 decreased shell length and increased the malformation rate of the donkey ear’s abalone larvae compared to the control pH 8.15. Shell abnormalities were even visible as early as 2 hours after treatment. These findings may signify high risk to environmental factors and suggest physiological impacts (Santander-De Leon & Sayno, 2018). Another experiment demonstrated that tissue formation, shell formation, calcification and the length of 72-hour-post-fertilization larvae of European abalone were significantly reduced at pH 7.7 compared to pH 8.0 (Kavousi et al., 2022).
| Species | Stressor levels and duration | Duration | Parameters | General findings | References |
|---|---|---|---|---|---|
| Pacific abalone (Haliotis discus hannai) | pH 7.3, 7.6, 7.9, and 8.2 (control). | Eggs: 15 h Hatched larvae: 48 h |
Hatching rate and time, shell length, malformation and metamophosis rate. | Reductions of 0.6–0.9 units in pH level had a negative impact on abalone early development. | Li et al., 2013 |
| Donkey ear’s abalone (Haliotis asinina) | pH 8.15, 7.85, and 7.65 | 24 h | Shell length and malformation rate. | Shell length decreases and malformation increases at low pH. | Santander-De Leon & Sayno, 2018 |
| European abalone (Haliotis tuberculata) | pH 8.0 (460 µatm pCO2, control) and 7.7 (1,000 µatm pCO2) | 5 mon | Survival, growth, phagocytosis, pHT of hemolymph, metabolism, gene expression, shell strength, nano-indentation measurements, SEM. | The hemolymph pH was reduced. Immunity and metabolism were not impacted. Mechanical characteristics, calcification, and shell growth were all negatively impacted. | Avignon et al., 2020 |
| European abalone (Haliotis tuberculata) | pH 7.9, 7.7, and 7.4 | 15 d | Survival and growth, Hemolymph pH, total alkalinity, pCO2, HCO3−, Saturation state (Ω), and total protein content of hemolymph. | Survival, growth, and protein content were not significantly different. The other parameters were reduced at pH 7.4. | Auzoux-Bordenave et al., 2021 |
| European abalone (Haliotis tuberculata) | pH 8.0 (control) and 7.7 | 5 d | Hatching rate, % swimming larvae, malformation rate, shell length, shell birefringence, SEM, swimming behavior, respiration rate, and larval settlement. | Low pH significantly impacts the shell and tissue formation, shell length, and birefringence. | Kavousi et al., 2022 |
| Pacific abalone (Haliotis discus hannai) and Many-colored abalone (Haliotis diversicolor) | 560 μatm (control), 880 μatm (pH 7.9) and 1,600 μatm (pH 7.7). | 1 y | Shell surface, growth, crushing force, microstructure, hardness, composition, and Calcium carbonate (CaCO3) morphology. | Ocean acidification (OA) altered many-colored abalone shell CaCO₃ morphology, microstructure, and hardness, reducing shell breaking force. Pacific abalone had unchanged CaCO₃ morphology and microstructure, and OA did not diminish nacre layer hardness. Due to reduced calcification, shell resistance to crushing force was still inadequate. | Guo et al., 2023 |
| Pacific abalone (Haliotis discus hannai) | pH 8.1 and 7.5 combination with temperature 15°C, 20°C, and 25°C | 5 d | Hemolymph H2O2 and MDA level, gene expression of CAT, SOD, and caspase-3 in hepatopancreas, in situ hybridization for SOD mRNA expression, and apoptosis level in the hepatopancreas. | H2O2, MDA, SOD, CAT, and caspase-3 increased at low pH and/or low/high water temperature. Apoptosis level was also high at high temperatures and low pH. | Kim et al., 2023 |
Shell growth in adult many-colored abalone was also reduced when cultured in seawater with 1600 μatm pCO2 for 1 year compared to control seawater with 560 μatm pCO2 (Guo et al., 2023). The reduction in abalone shell growth under low pH conditions induced by elevated pCO2 may result from decreased concentrations of CO32– ions, which are essential for the formation of calcium carbonate (CaCO3) in the shell. Carbonic acid (H2CO3) is formed when carbon dioxide dissolves in seawater. H2CO3 then dissociates into hydrogen ions (H+) and bicarbonate ions (HCO3–). HCO3– further break down into H+ and CO32– ions (Guo et al., 2023).
A decrease in pH can also enhance the dissolution of CaCO3. Two distinct types of CaCO3 constitute the abalone shell. The exterior prismatic layer may consist of aragonite, calcite, or a combination of both, while the inner shell (nacre) is composed of aragonite (Cummings et al., 2019). The formation of mollusc shells commences during the trochophore stage in abalone. The larval shell is secreted by a specialized group of ectodermal cells that constitute the shell gland and the organic periostracum (Wessel et al., 2018). The initial deposition of CaCO3 occurs during the early veliger stage, involving the deposition of amorphous calcium carbonate (ACC), which rapidly transforms into crystalline aragonite. However, both ACC and aragonite are more soluble than calcite under acidic conditions, rendering larval shells more vulnerable to dissolution than juvenile or adult shells (Wessel et al., 2018).
The reduction in pH due to elevated pCO2 can also alter the hemolymph pH. The pH of the hemolymph in adult European abalone significantly decreased under pH 7.7 treatment during the first 2 months compared to the control pH but not significantly after 4 months (Avignon et al., 2020). If the treatment lasted only 15 days, exposure to pH 7.7 also did not change the hemolymph pH of adult European abalone, but a reduction in hemolymph pH only occurred in the pH 7.4 treatment. The limited capacity of abalone to maintain acid-base balance is thought to contribute to the reduction in hemolymph pH (Auzoux-Bordenave et al., 2021). After 15 days at a pH level of 7.7, the survival and growth, hemolymph pH, total alkalinity, pCO2, HCO3−, saturation state (Ω), and total protein content of hemolymph in European abalone showed no significant changes. However, exposure to pH 7.4 for 15 days lowered hemolymph pH, total alkalinity, pCO2, HCO3−, and Ω (Auzoux-Bordenave et al., 2021). Exposure to pH 7.7 for 5 months also did not impact the acid-base equilibrium and overall metabolism of European abalone; however, it decreased shell growth and strength (Avignon et al., 2020).
The pH stress alone or combined with other environmental factors can also induce oxidative stress and lead to cell death. Kim et al. (2023) studied the combined effect of pH (pH 8.1 and pH 7.5) with temperature (15°C, 20°C, and 25°C) on adult Pacific abalone for 5 days. The results show that oxidative stress parameters (H2O2 and MDA) and antioxidant enzyme activities (SOD and CAT) increased at low pH and/or low/high water temperature. These environmental stresses also increase caspase-3 expression in the hepatopancreas and cause apoptosis (Kim et al., 2023). The increase in SOD and CAT occurs as a mechanism for abalone to reduce the negative impact of increased H2O2 and MDA. However, the rise in caspase-3 expression shows that the increase in SOD and CAT cannot counteract the higher levels of H2O2 and MDA, leading to cell death.
The expansion of the aquaculture sector has been accompanied by the translocation of animals from various regions, potentially facilitating the transmission of infections alongside the intended host species (Wetchateng et al., 2010). Pathogens capable of causing disease in abalone include the bacterium Candidatus Xenohaliotis, which indices Withering syndrome (WS) (Lafferty & Ben-Horin, 2013); Vibrio spp., which cause vibriosis (Cardinaud et al., 2014); the herpes virus, which causes ganglioneuritis (Lafferty & Ben-Horin, 2013); and the spionid Polychaeta and sponge Cliona spp., which damage abalone shells (McDiarmid et al., 2004).
Candidatus Xenohaliotis, a Rickettsia-like organism, induces WS, a serious disease that leads to high mortality rates in abalone (Lafferty & Ben-Horin, 2013). The disease derives its name from the withered appearance of the abalone’s foot muscles, which lose the ability to adhere to rocks or substrates (Lafferty & Ben-Horin, 2013). The clinical symptoms of WS are marked by structural alterations in the digestive gland, including metaplasia and the deterioration of gastrointestinal tissue. These changes are characterized by tubular atrophy, connective tissue proliferation, and inflammation, which impair digestive function. The disease also alters the structural proteins of the pedal muscle, replacing them with connective tissue, thereby preventing the abalone from adhering to substrates (González et al., 2012). WS is characterized by atrophy of the pedal musculature, mantle retraction, epipodial discoloration, hepatopancreas damage, and reduced response to tactile stimuli. These symptoms hinder the abalone’s ability to recognize and absorb food, leading to malabsorption, weakness, body mass constriction, lethargy, and ultimately death. Infected abalone demonstrated significant suppression of protein expressions related to stress responses, Cu/Zn-SOD, and actin, which play a crucial role in muscle contraction and regulation (Di et al., 2016). In experiments involving red abalone and Pacific abalone, juveniles infected with Candidatus Xenohaliotis californiensis for 130 days exhibited varying responses. Candidatus Xenohaliotis californiensis infects juvenile red abalone more easily compared to juvenile Pacific abalone. Ninety-two percent of juvenile red abalone exposed to the bacterium acquired the infection, as confirmed by histological analysis. Conversely, none of the Pacific abalone juveniles tested positive for WS-RLO (Withering Syndrome Rickettsiales-like Organism) by histology, although 23% tested positive according to PCR. Juvenile red abalone exhibited a reduction in ingestion rate, basal metabolism, and feces production. Disruptions in energy balance may reduce the energy available for growth by up to 49% (González et al., 2012). Many-colored abalone affected by WS showed muscle necrosis and organ damage and decreased alkaline phosphatase (AKP) and T-SOD in pedal mucus (Di et al., 2016). The susceptibility of abalone to whitering syndrome is also influenced by temperature. Crosson & Friedman (2018) found that different species of abalone died at different temperatures when affected by WS: pinto abalone died at the lowest temperature (17.32 ± 0.09°C), red abalone at a middle temperature (17.96 ± 0.16°C), and pink abalone at the highest temperature (18.84 ± 0.16°C).
Vibriosis is another disease capable of causing mass mortality in abalone populations. It is characterized by white spots on the feet, inflammation of the pericardial tissue, and septicemia (Cardinaud et al., 2014). Vibrio carchariae infection caused mass mortality of European abalone along the French coast in 1997, 1998, and 1999 (Nicolas et al., 2002). The bacterial infection process can be divided into three stages. The first stage involves colonization, during which the pathogen adheres to the host surface, begins initial multiplication, and penetrates the host’s body through one or more entry points. The second stage is marked by bacterial invasion of the host’s circulatory system or internal organs. The third stage involves the exit of the pathogen from the host and subsequent transmission of the disease (Cardinaud et al., 2014). Vibrio infections can progress rapidly within the host. For instance, V. harveyi can adhere to and penetrate the entire gill-hypobranchial gland tissue of European abalone within 1 h of contact, subsequently invading the abalone hemolymph within 3 h (Cardinaud et al., 2014). This rapid infection process can result in mass mortality in abalone populations. Laboratory trials demonstrated that the first deaths occurred two days after a 24-h exposure, with cumulative mortality reaching 93% after nine days. The invasion and proliferation of V. harveyi in the abalone circulatory system were observed 24 h post-exposure (Cardinaud et al., 2015). A significant decline in hemocyte viability and ROS production was detected after 9 h of exposure, and hemocyte concentration and phagocytic activity considerably decreased after 24 h of exposure to V. harveyi. Acid phosphatase (AP), PO, and SOD activities remained unaffected by abalone exposure to V. harveyi; however, glutamine synthase (GLS) and cytochrome c oxidase (CCO) activities exhibited significant reductions 24 h post-exposure (Cardinaud et al., 2015). The vulnerability of abalone to V. harveyi may escalate with alterations in abiotic factors, such as high temperatures. Lee et al. (2023) demonstrated that the viability of hemocytes in Pacific abalone exposed to V. harveyi considerably declined at 25°C, although no such decline occurred at 20°C. Exposure to V. harveyi at 25°C additionally induces the overexpression of pro-inflammatory and apoptosis-related genes in abalone hemocytes. Evidence indicates that elevated temperatures enhance Vibrio pathogenicity and abalone stress responses, highlighting their susceptibility to global warming.
The AbHV, also known as Haliotid herpesvirus-1 (HaHV-1) is a spherical virus with an icosahedral core and an envelope, responsible for causing abalone viral ganglioneuritis (AVG; Corbeil et al., 2016; Gu et al., 2019). AVG is characterized by rapid death and necrosis of the cerebral ganglia and nerve bundles in the foot muscle (Corbeil et al., 2016). This virus can infect multiple organs in animals across various developmental stages, leading to diminished vitality and growth rates, increased mucus production, and abdominal hardening and darkening. The infection can result in rapid mortality, with rates reaching up to 100% (Gu et al., 2019). Between 2005 and 2006, abalone mortalities in both farmed and wild populations along the coast of Victoria, Australia, were attributed to AbHV infection (Corbeil et al., 2016).
Shell Boring Disease is a condition affecting abalone shells caused by Polychaeta or the demosponge Cliona spp. Abalone with weakened shells may experience reduced growth, increased vulnerability to predators, and mass mortality (McDiarmid et al., 2004). During the larval stage, polychaetes examine the surface of abalone shells until they identify a suitable location to settle. Once settled, they undergo metamorphosis and burrow into the abalone shells (David, 2021). Cliona spp. can penetrate CaCO3 shells both chemically and mechanically, creating a network of cavities and tunnels within the substrate. Although molluscs generate additional shell material to counteract the inward advancement of the sponge, this defensive process may compromise somatic growth and reproductive capacity (Carroll et al., 2015).
Conclusion
Molluscs, particularly abalone, secrete CRH to release adrenocorticotropic hormone-like molecules and neurotransmitters during stress, leading to secondary physiological changes. All environmental challenges (temperature, salinity, hypoxia, and pH) and also disease can alter energy metabolism, reduce immunity, and induce other physiological stress responses, leading to reduced growth and survival rate of abalone. Although the available information about individual or combined effects of two environmental stressors on physiological responses is quite a lot. However, sometimes the changes that occur in seawater in nature and aquaculture farms involve more than one or two factors. Therefore, future work analyzing the combined effect of multiple stress factors will help to understand the mechanistic control and its perturbation evoked by environmental factors in abalone. Several mechanisms can be implemented to reduce the impact of environmental change on abalone. Aquaculture farms need to modify infrastructure, such as increasing water flow, oxygenating the water, more efficient ammonia scavenging, and salinity and temperature control. CO2 reduction can be done by using carbon capture utilization and storage. Development of feed containing probiotics or using phage therapy as a mechanism to increase abalone resistance to disease pathogens. Aquaculture farms also need to monitor seawater regularly.