Background
Aquaculture has been the fastest-growing food-producing sector since 1970, with an average growth rate of ~9 % per year, compared with a 2.8 % growth rate of terrestrial farmed meat production over the same period (Bostock et al. 2010; Subasinghe et al. 2001). Worldwide, disease is considered to be a significant constraint on aquaculture; the economic losses caused by disease are estimated to be several billion US dollars per year (Subasinghe et al. 2001). Bacterial diseases are a major threat to aquaculture because bacteria can survive well and reach high densities in an aquatic environment independent of their hosts, which is generally not the case in terrestrial environments (Defoirdt et al. 2011; Pridgeon and Klesius 2013). In particular, the larval stages of several farmed aquatic animals are highly susceptible to bacterial diseases (Defoirdt et al. 2011). Major bacterial pathogens include Vibrio, Aeromonas, Edwardsiella, and Streptococcus species, which affect fish such as salmon, carp, and flat fish (Baeck et al. 2006; Han et al. 2006; Milton et al. 1996; Romalde 2002; Weinstein et al. 1997; Wiklund and Dalsgaard 1998; Won and Park 2008). Inactivation of microorganisms can be accomplished with light technologies, including ultraviolet C irradiation therapy, photodynamic therapy (PDT), and blue light therapy (Arrojado et al. 2011; Yin et al. 2013). Ultraviolet (UV) irradiation has an adverse effect on fish; it causes intensive skin lesions (Ghanizadeh and Khodabandeh 2010) and reduction of goblet cells in fish skin, resulting in less mucus production and, consequently, downregulation of innate immunity (Kaweewat and Hofer 1997). The use of blue light (400–500 nm) as a mono-therapy is gaining increasing attention because of its potential antimicrobial effect and because it does not require an exogenous photosensitizer (Yin et al. 2013). Blue light is much less harmful to mammalian cells than UV irradiation (Kleinpenning et al. 2010). Light treatment has been applied in aquaculture for many years. For example, European sea bass and sole larvae showed the fastest development and the lowest degree of deformity under blue light (half-peak bandwidth = 435–500 nm) than under other wavelengths of light (Villamizar et al. 2011). Also, another study found that retina from fish exposed to blue light revealed no signs of damage as assessed by extensive histological examination (Migaud et al. 2007). In spite of this potential, there is little information on light therapy as it applies to bacterial pathogens that threaten aquaculture. The aim of this study was to determine the extent of inactivation of bacterial fish pathogens, in particular, seven species including both Gram-negative and Gram-positive bacteria carried out in in vitro experiment. The effects of light-emitting diode (LED) on different bacterial densities and the effects of different light intensities were also evaluated.
Methods
Seven bacterial species were evaluated in this study. The bacterial strains were grown on tryptic soy agar (TSA) or brain and heart infusion agar (BHIA), supplemented with 1 % NaCl. A strain of Vibrio anguillarum isolated from diseased cod was purchased from the Korean collection for type cultures (KCTC), and Edwardsiella tarda KE1 and Aeromonas salmonicida RFAS1 originated from diseased olive flounder and black rockfish were previously used (Han et al. 2006, 2011). Vibrio harveyi Vh21FL, Photobacterium damselae Dae1-1L, Streptococcus iniae BS9, and Streptococcus parauberis SpOF3K obtained from diseased olive flounder were confirmed by polymerase chain reaction that was previously described (Table 1) (Mata et al. 2004; Osorio et al. 2000; Pang et al. 2006).
Bacterial strains | Isolation source | Primers | References |
|---|---|---|---|
Gram-negative bacteria | |||
Vibrio harveyi Vh21FL | Diseased olive flounder | Tox F: 5'-GAAGCAGCACTCACCGAT-3' Tox R: 5'-GGTGAAGACTCATCAGCA-3' | Pang et al. (2006) |
Vibrio anguillarum KCTC 2711a | Diseased cod | – | – |
Photobacterium damselae Dae1-1L | Diseased olive flounder | Car 1: 5'-GCTTGAAGAGATTCGAGT-3' Car 2: 5'-CACCTCGCGGTCTTGCTG-3' Ure -3': 5'-CTTGAATATCCATCTCATCTGC-3' Ure -5': 5'-TCCGGAATAGGTAAAGCGGG-3' | Osorio et al. (2000) |
Edwardsiella tarda KE1 | Diseased olive flounder | – | Han et al. (2006) |
Aeromonas salmonicida RFAS1 | Disease black Rockfish | – | Han et al. (2011) |
Gram-positive bacteria | |||
Streptococcus iniae BS9 | Diseased olive flounder | Lox-1: 5'-AAGGGGAAATCGCAAGTGCC-3' Lox-2: 5'-ATATCTGATTGGGCCGTCTAA-3' | Mata et al. (2004) |
Streptococcus parauberis SpOF3K | Diseased olive flounder | Spa F: 5'-TTTCGTCTGAGGCAATGTTG-3' Spa R: 5'-GCTTCATATATCGCTATACT-3' | |
aType strain
The 405- and 465-nm LEDs, each composed of 120 individual LEDs, were kindly provided by the LED-Marine Convergence Technology R&D Center (Pukyong National University). The spectra of the 405- and 465-nm LEDs as measured by a temperature-controllable integrating system (Withlight Co. Ltd., Korea) are shown in Fig. 1. The maximum irradiation of the 405- and 465-nm LED array were 250 and 516 μ mol m−2 s−1, respectively, as calculated using a laboratory radiometer (Biospherical Instruments Inc., USA). Photosynthesis photon flux density (PPFD; μ mol m−2 s−1) was converted to radiant flux density (mW cm−2) by using the following formula:
Approximately 105 CFU ml−1 of each culture was suspended in phosphate buffered saline (PBS; pH 7.2–7.4). Each bacterial suspension (10 ml, with a depth of 5 mm) was plated on a 30-mm petri dish on TSA (V. anguillarum, V. harveyi, P. damselae, E. tarda, and A. salmonicida) or BHIA (S. iniae and S. parauberis) supplemented with 1 % NaCl, exposed to 250 μ mol m−2 s−1 of the 405- or 516 μ mol m−2 s−1 of the 465-nm LED light, and placed in a 25 °C incubator for 0, 1, 3, 6, 12, 24, or 48 h. Each lamp was placed 3.5 cm above open plates containing the bacterial cultures and positioned perpendicularly. Temperature was routinely monitored during irradiation. The cultures were stirred with a sterile magnetic bar for a few seconds just before being plated, and bacterial counts were performed. A method slightly modified from a previous study (Maclean et al. 2009) was used to express the inactivation data: log10 (N/N0) was plotted as a function of exposure time, where N0 is the initial bacterial population in CFU ml−1 prior to inactivation and N is 10 CFU ml−1. Thus, the mean bactericidal efficiency (BE) was defined as the log10 reduction in a bacterial population [log10(10/N0)] by inactivation per unit dose in J cm−2. Exposure time was deduced from the time at which bacterial populations reached 10 CFU ml−1.
In order to determine the effects of initial bacterial density on the antibacterial activity of LEDs, 200 μl of six 10-fold serial dilutions (103, 104, 105, 106, 107, and 108 CFU ml−1, in BHIB supplemented with 1 % NaCl) were inoculated in a 96-well microplate. The plates were exposed to a 405- or 465-nm LED at 25 °C. Optical density (OD) was measured at 630 nm after 24 h irradiation using a Sunrise™ spectrophotometer (TECAN Austria), and data was analyzed using OD of 24 h exposure group/OD of 24 h non-exposure group × 100 (%) formula.
The data points shown in Fig. 2 and in Table 3 are expressed as mean values with standard deviations. Two-tailed Student’s t tests and ANOVA Tukey’s test were used to determine statistically significant differences (P < 0.05 or P < 0.01) between groups exposed to blue light and controls.
Results
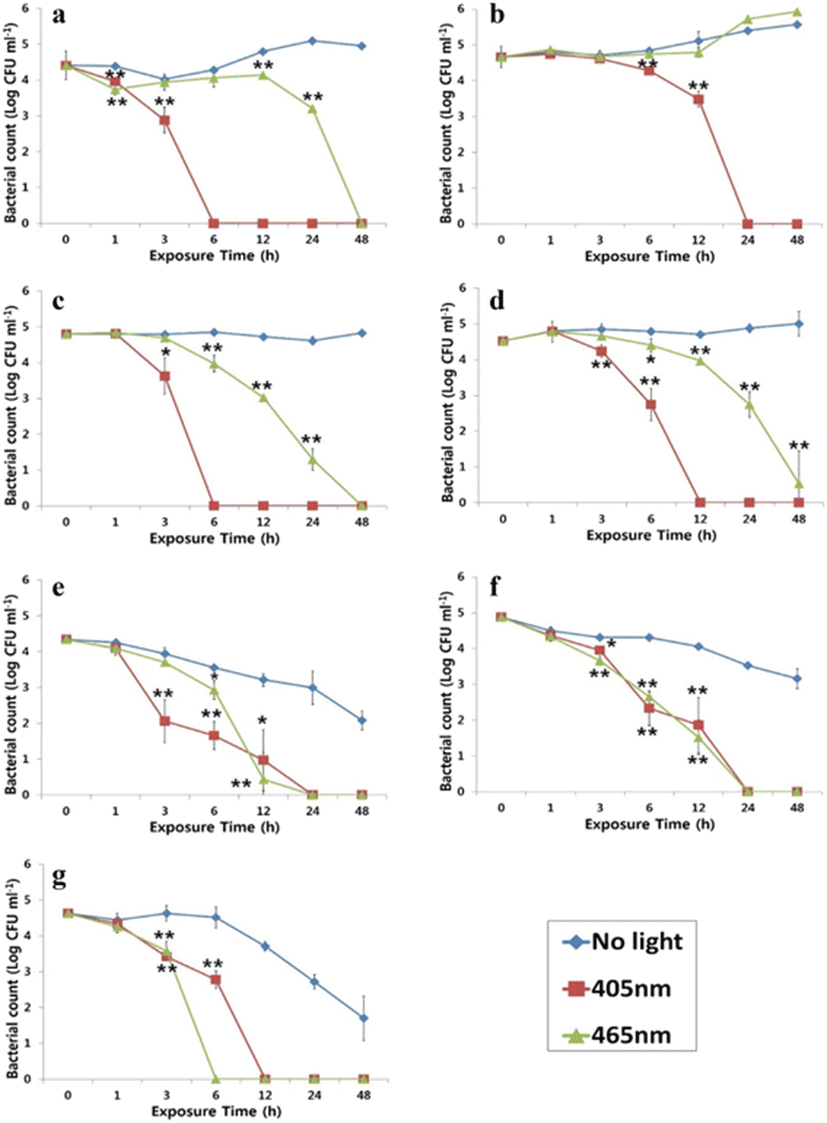
This study successfully demonstrates the bactericidal effects of 405- and 465-nm LEDs on selected bacterial fish and shellfish pathogens. As shown in Fig. 2, growth of the pathogens evaluated was clearly inactivated by exposure to either a 405- or 465-nm LED, although the degree of inactivation varied depending on bacterial species and sampling time point. The one exception was that a 465-nm LED was unable to inactivate V. harveyi, but that strain was inactivated by a 405-nm LED. Complete inactivation of A. salmonicida and S. parauberis was seen 24 h after irradiation with a 405-nm LED, whereas only 6 h were required for complete inactivation of V. anguillarum and P. damselae under the same conditions. Although S. iniae was more rapidly inhibited by a 465-nm LED, overall, there were no differences between 405 and 465 nm LEDs in the inactivation rate of S. parauberis.
BE was measured in this study using a method modified from one that was previously described (Maclean et al. 2009). Details of the inactivation parameters for all bacterial species are listed in decreasing order of BE in Table 2. We calculated BE using exposure time, which was deduced from the time at which bacterial populations reached 10 CFU ml−1. P. damselae, V. anguillarum, and E. tarda were the most susceptible bacteria, while S. parauberis was the least susceptible, to exposure to a 405-nm LED. Our results show that Gram-negative bacteria, such as P. damselae (36.1 J cm−2), V. anguillarum (41.2 J cm−2), and E. tarda (68.4 J cm−2), seem to be more sensitive to a 405-nm LED light than are Gram-positive bacteria like S. parauberis (153.8 J cm−2) and S. iniae (90.4 J cm−2) (Table 2). However, some Gram-negative bacteria such as A. salmonicida (98.7 J cm−2) and V. harveyi (126.4 J cm−2) have lower susceptibility than S. iniae.
aBactericidal efficiency, calculated as log10 (10/N0) per radiant flux (J cm−2)
N0 initial bacterial population in CFU ml−1, NA not applicable, BE bactericidal efficiency
The degree of inactivation of bacterial suspensions with varying initial population densities in BHIB + 1 % NaCl following exposure to a 405- or 465-nm LED for 24 h is displayed in Table 3. In general, the OD values indicate that the higher starting bacterial densities were associated with lower inactivating efficacies. However, there were exceptions: unlike the other bacterial species, P. damselae exposed to a 405- or 465-nm LED and V. harveyi exposed to a 465-nm LED were not affected by their initial concentrations. P. damselae was able to survive a 405- or 465-nm light exposure in BHIB + 1 % NaCl, but it was much more susceptible when suspended in PBS.
Different letters (b, c, and d) in the superscripts indicate significant differences (P<0.05)
aRatio of optical density (OD) of control to OD of LED treatment group (treatment OD630 nm/control OD630 nm)
Discussion
Antimicrobials are commonly used in aquaculture to prevent and treat bacterial infections in fish. Significant increases in the bacterial resistance to various antibiotics, such as oxytetracycline, quinolones, and amoxicillin, have been repeatedly found in proximity to fish farms (Defoirdt et al. 2011; Guardabassi et al. 2000; Schmidt et al. 2000). Excessive use of antimicrobials may significantly reduce their effectiveness and their usefulness in aquaculture. More importantly, studies have demonstrated that resistance plasmid for some antibiotics can be shared between bacterial fish pathogens, aquatic bacteria, and human pathogens, and some of them appear to have originated in the aquatic environment (Cabello et al. 2013). Thus, non-antibiotic therapies for infectious diseases are receiving considerable attention (Jori et al. 2006; Maisch 2009). It was previously demonstrated that blue light has a broad-spectrum bactericidal effect on both Gram-negative and Gram-positive bacteria (Dai et al. 2012; Maclean et al. 2009). In this study, growth of the bacterial fish and shellfish pathogens evaluated was clearly inactivated by exposure to either a 405- or 465-nm LED light. Inactivation was dependent on light intensity and exposure time. Overall, our results show that Gram-negative bacteria, such as P damselae (36.1 J cm−2), V. anguillarum (41.2 J cm−2), and E. tarda (68.4 J cm−2), seem to be more sensitive to a 405-nm light than are Gram-positive bacteria like S. parauberis (153.8 J cm−2) and S. iniae (90.4 J cm−2). This result does not agree with a previous study which showed that Gram-positive bacteria such as Staphylococcus, Clostridium, and Streptococcus species were more susceptible to LED light than Gram-negative bacteria. Exceptions have been reported; Enterococcus faecalis suspensions exposed to 10 mW cm−2 light for up to 120 min experienced negligible inactivation (Maclean et al. 2009). Another study also found that the Gram-positive Listeria monocytogenes was more resistant to a 405-nm light than was the Gram-negative Salmonella enterica on acrylic and PVC surfaces (Murdoch et al. 2012). Taken together, it appears that Gram-positive bacteria are not always more rapidly inactivated than Gram-negative bacteria. The BE observed in this study are much lower than those seen in a previous study. This is because it took bacterial counts nine times over 200 min, which was much more frequent than in our study, where sampling was done only seven times over 48 h (Maclean et al. 2009). In addition, we used 250 μ mol m−2 s−1 (approximately 7.4 mW cm−2) and 516 μ mol m−2 s−1 (approximately 13.3 mW cm−2) intensities of 405- and 465-nm light, respectively, which are approximately 1.5–10 times lower than those used in previous studies (e.g., 19.5 mW cm−2 of 415 nm, 100 mW cm−2 of 415 or 455 nm, or 10 mW cm−2 of 405 nm) (Dai et al. 2013; Lipovsky et al. 2010; Maclean et al. 2009). This is one likely explanation as to why inactivation of pathogens in this study took longer than in previous studies. The precise mode of action of the antimicrobial effect of blue light is not yet fully understood. The commonly accepted hypothesis is that blue light excites endogenous intracellular porphyrins, which then behave as photosensitizers; photon absorption leads to energy transfer and, ultimately, the production of highly toxic reactive oxygen species (ROS) (Ashkenazi et al. 2003; Hamblin et al. 2005; Maclean et al. 2008). The differences in inactivation kinetics found in this study may be caused by organism-specific differences in porphyrin levels or porphyrin types, as suggested previously. The peak absorption wavelengths of different bacterial porphyrins may differ, and varying wavelengths may be required for their maximum photostimulation (Maclean et al. 2010). The degree of inactivation of bacterial suspensions with different initial densities was determined in order to assess LED activity on pathogens in the presence of nutrients mimicking a natural aquatic environment. P. damselae was able to survive a 405- or 465-nm light exposure when cultured on nutrient-enriched environment but was much more susceptible when suspended in PBS, as shown in Fig. 2. Several studies have reported that bacterial pathogens, including Escherichia coli, A. salmonicida, Streptococcus pneumoniae, and V. harveyi, produce different superoxide dismutase (SOD) and catalase isozymes inducible under certain growth conditions (Barnes et al. 1996; Flint et al. 1993; Vattanaviboon and Mongkolsuk 2001; Yesilkaya et al. 2000). However, P. damselae is not able to produce different SOD or catalase isozymes when exposed to oxidative stress induced by hydrogen peroxide, or under iron-depleted conditions (Díaz-Rosales et al. 2006). Also, P. damselae, possessing a high-affinity iron uptake system, grown under iron-limited conditions have a reduced amount of capsular material covering the cells (Do Vale et al. 2001; Naka et al. 2005). These indicate that P. damselae grown under nutrient-enriched conditions would be more resistant to oxidative stress (ROS) induced by LED irradiation than when grown under iron-limiting conditions (e.g., PBS). As it has been already demonstrated that blue light has caused no or very little damage to teleost (Migaud et al. 2007; Villamizar et al. 2011), it might be an alternative method to treat and prevent bacterial diseases in fish farm.
Conclusions
To the best of our knowledge, this study is the first to demonstrate that blue light is capable of inactivating major aquatic pathogens without requiring any external photosensitizer. As it is generally accepted that blue light is much less harmful to animal cells than is UV irradiation, and caused little damage to teleost that have already been demonstrated in previous studies (Migaud et al. 2007; Villamizar et al. 2011), application of blue light might be alternative to the use of antibiotics in aquaculture and would also have safety benefits. We hope our results will inspire further experiments to explore practical applications of blue light to fish and shellfish.