Background
Marine polyphenol phlorotannins are produced from secondary metabolites via the acetate-malonate pathway in brown seaweeds (Shibata et al., 2004; Isaza Martínez & Torres Castañeda 2013). They have fundamentally different structures than the polyphenols of terrestrial plants (Isaza Martínez & Torres Castañeda 2013; Shibata et al., 2002). Terrestrial plant polyphenols are based on gallic acids or flavones, whereas phlorotannins are only derived from oligomers and polymers of phloroglucinol (1,3,5-trihydroxybenzene) (Koivikko et al., 2007). Thus far, many phlorotannins, such as dieckol, eckol, triphlorethol A, bieckol, fucol, fucophlorethol, have been identified (Isaza Martínez and Torres Castañeda 2013; Kim et al., 2014; Cho et al., 2012).
Over the past 10 years, studies on the biological activities of phlorotannins have increased exponentially (Isaza Martínez & Torres Castañeda 2013). They have a wide range of biological properties, such as antioxidant (Zou et al., 2008), anti-inflammatory (Kim et al., 2009), anti-allergic (Li et al., 2008), and neuroprotective effects (Ahn et al., 2012). Recently, Cho et al. (2014) reported that a phlorotannin preparation and its constituent, eckstolonol, promoted non-rapid eye movement sleep via the benzodiazepine site of gamma-aminobutyric acid type A receptors. Therefore, phlorotannins are considered a promising material for the development of functional foods and supplements.
The standardization of phlorotannin preparations is required for the development of functional foods (Hwang et al., 2009). However, a validation for the standardization of phlorotannin products has not been reported. Dieckol is generally the most abundant compound in phlorotannin preparations, and is used as an indicator compound (Shibata et al., 2004; Cho et al., 2012; Goo et al., 2010). Therefore, we developed and validated an HPLC method for the determination of dieckol for the commercialization of phlorotannin preparations.
Methods
Phlorotannin preparations (PRT) were obtained from S&D Co., Ltd. (Cheongwon-gun, Korea). The PRT were prepared from the brown seaweed, Ecklonia cava, using a macroporous adsorption resin, as described in our previous work (Kim et al., 2014). The total phlorotannin content (TPC) of the PRT was assessed by the Folin-Ciocalteu method (Slinkard and Singleton, 1977), which determined that there were 905 mg phloroglucinol equivalents/g. Dieckol, a standard compound, was isolated using silica gel and Sephadex LH-20 column chromatography. All of the reagents used were of HPLC grade and purchased from Sigma-Aldrich Co. (St. Louis, MO, USA).
A dieckol stock solution was prepared by dissolving 5 mg in 2 mL of dimethyl sulfoxide (DMSO) and 3 mL of methanol. Analytical working solutions were prepared by diluting the stock solution with methanol to seven concentrations, i.e., 12.5, 25, 50, 100, 200, 300, and 400 μg/mL. The phlorotannin extract (50 mg) was added to a volumetric flask, and was then dissolved in 10 mL of DMSO and 40 mL of methanol using ultrasonication at ambient temperature. The PRT sample solution was filtered through a 0.45 μm PTFE syringe filter paper (Whatman, Maidstone, UK), and was used as a sample solution for the HPLC analysis.
We evaluated chromatographic variables, such as flow rate, mobile phase, solvent ratio, and column oven temperature to optimize the chromatographic conditions. The determination of dieckol, an indicator compound in PRT, was performed using an Agilent system (Agilent, Palo Alto, CA, USA) consisting of an autosampler, pump, degasser, and column oven. Shideido capcellpak C18 UG (4.6 mm × 250 mm, 5 μm particle size; Shiseido, Tokyo, Japan), Cadenza C18 (4.6 mm × 150 mm, 3 μm particle size; Imtakt, Kyoto, Japan), and Supelco Discovery C18 (4.6 mm × 250 mm I.D., 5 μm particle size; Supelco, Bellefonte, PA, USA) columns were used for the separation of dieckol. The analytical conditions (mobile phase composition, flow rate, oven temperature, and injection volume) are summarized in Table 1. The temperature of the column oven was fixed at 25 °C, and the UV detection wavelength for the phlorotannins was set to 230 nm.
The method was validated in terms of its specificity, linearity, accuracy, recovery, and precision according to the guidelines of the International Conference on the Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) (Jeong et al., 2013; International Conference on Harmonization ICH 1997).
Linearity was tested at seven concentrations, i.e., 12.5, 25, 50, 100, 200, 300, 400 μg/mL. The linear regression equation was calculated from the calibration curve as Y = Ax + B, where A and B are the slope and intercept of the calibration curve, respectively, x is the concentration of dieckol, and Y is the peak area (Goo et al., 2010; Kim et al., 2013). The correlation coefficient (R2) values were determined for the calibration curve.
The accuracy was measured by the recovery, which was determined by the addition of a known amount of dieckol (Ariffin et al., 2014; Moussata et al, 2014). Phlorotannin samples were mixed with three different concentrations of dieckol, and the percent recoveries were calculated using the following equation:
The precision was determined using the intermediate (inter-day) and intra-day assays and reported as the percent relative standard deviation (%RSD) (Mittal and Parmar, 2010; Sarkar et al., 2006). The intermediate assay was performed by two different analysts over four days, and the intra-day assay was analyzed through six replicate injections of each sample solution. The %RSD was calculated using the following equation (Jeong et al., 2013):
Results and Discussion
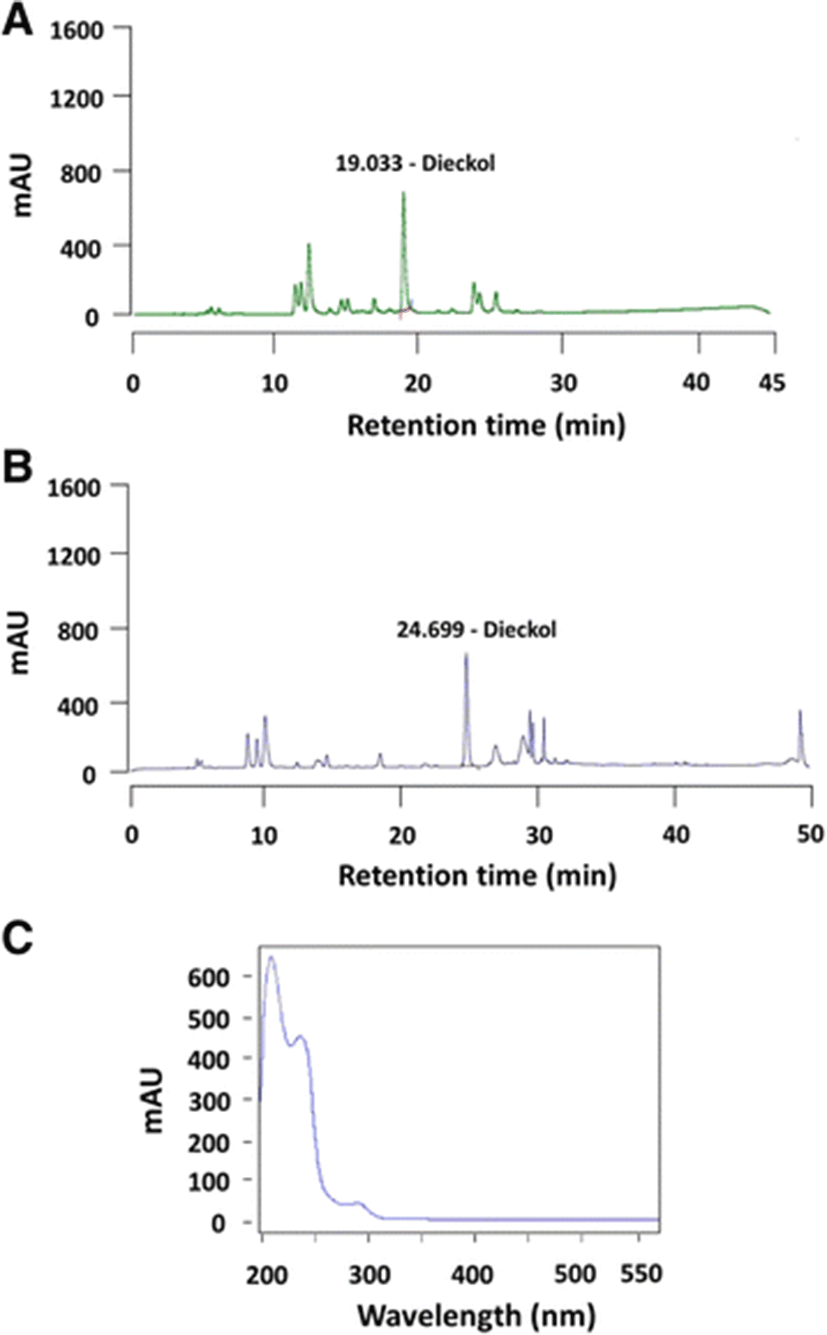
Different chromatographic conditions (flow rate, solvent ratio, elution time, and oven temperature) were varied to optimize the HPLC analysis of dieckol in PRT (Table 1). Using the Set 1 conditions, the dieckol peak was separated from the other minor peaks (19 min), but had an unstable baseline (Fig. 1a). The Set 2 conditions did not show superior chromatographic results (data not shown). However, the Set 3 conditions had the most stable baseline near the dieckol peak (25 min), and the peaks were well resolved (Fig. 1b). The peak stabilities of dieckol and the other phlorotannin constituents in the PRT were considerably affected by the chromatographic conditions. To confirm the detection wavelength for dieckol, its UV spectrum was recorded between 200–550 nm, and it strongly absorbed at 230 nm (Fig. 1c). The PRT sample also showed same spectrum (data not shown). Henry and Alstyne (2004) reported that phlorotannins absorbed in the UV-C region between 195–265 nm. These results showed that the optimum conditions for the detection of dieckol in PRT could be found through the use of various conditions in the HPLC system.
The validation provides reliable documentation for standardization through regulated experiments (Shabir, 2003; Epshtein 2004). According to the United States Food and Drug Administration (FDA) and Pharmacopeia, validation is a critical factor in the development of the functional food industry (Epshtein 2004; Kazakevich and Lobrutto 2007). Therefore, the validation of the analytical HPLC conditions was conducted in terms of specificity, linearity, accuracy, recovery, and precision (Satinder and Henrik 2011; Meyer, 2010).
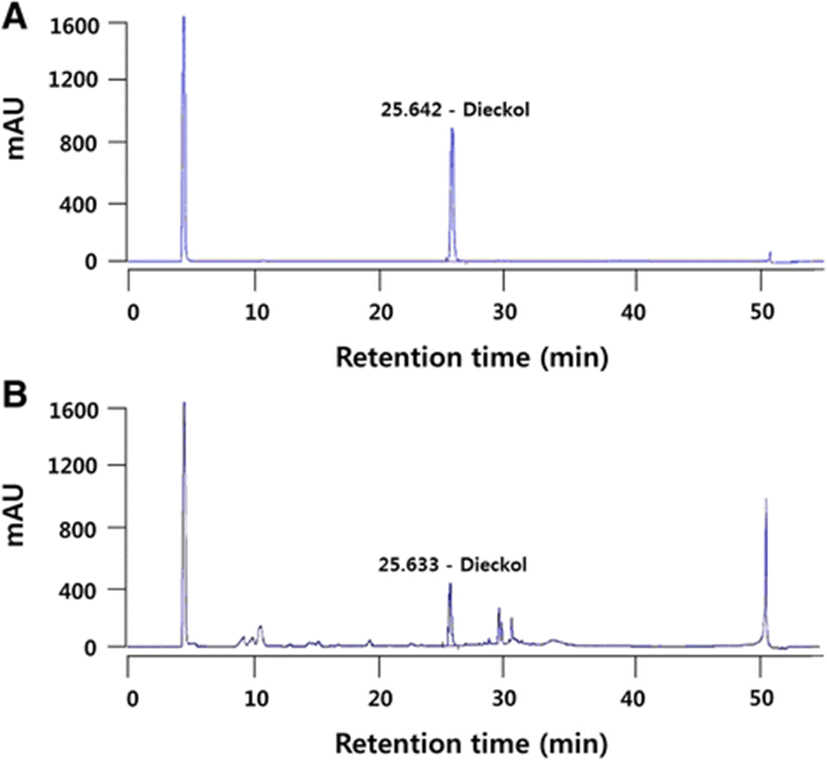
Specificity is the ability to separate the target compound from interference in a mixture of components (Ariffin et al., 2014; Shabir, 2003). The chromatograms show the absence of any interfering peak near the retention time of dieckol (25 min) (Fig. 2a and b). This indicated that the developed chromatographic parameters (Set 3) were adequate for the acceptable resolution of dieckol in the PRT during detection (Shabir, 2003).
Linearity is the ability to elicit results through the analysis of diverse concentrations within a given range (Shabir, 2003; Shah et al., 2014). A linear regression analysis (n = 3) was measured over seven different concentrations (12.5–400 μg/mL), and its regression equations are given in Table 2. The seven point calibration curves for dieckol were generated using the appropriate peak area ratios (data not shown). The slope ranged from 32.9–34.8 with a correlation coefficient (R2) value of 0.999. The R2 indicated an acceptable linearity over each concentration (12.5–400 μg/mL) (Shabir, 2003; Epshtein 2004). Shabir (2003) reported that R2 > 0.999 is generally considered an acceptable value for a regression line. Because each R2 was >0.998, the regression suggested good linearity between the peak areas and the compound concentrations over the tested range (Goo et al., 2010).
Parameters | Regression equationsb | ||
|---|---|---|---|
Concentration (μg/mL) | 12.5–400 | ||
Slope | 34.575 | 32.992 | 34.864 |
Intercept | 30.112 | 1.297 | −24.646 |
Correlation coefficient (R2) | 0.9994 | 1.000 | 0.9999 |
aValues were calculated using linearity analyses (n = 3)
bRegression equation is Y = Ax + B, where Y is the peak area and x is the dieckol concentration (μg/mL)
The accuracy was determined nine times at a minimum of three concentrations (13.45, 20.17, and 26.90 μg/mL) and the results are given in Table 3. The recovery of dieckol ranged from 98.8–103.1 % and the %RSD was 1.2–2.0. Shabir reported that the recovery (%) for an accuracy criterion should be 100 ± 2 % at each added concentration (Shabir, 2003). Our results showed recoveries >98 %, which indicated that the method was acceptable for the quantification of dieckol in PRT.
RSD relative standard deviation of recovery
aValues were calculated using accuracy (n = 9) analyses
The intermediate and intra-day (repeatability) assay precision must be evaluated in terms of dieckol content (mg/g) and %RSD. Therefore, we tested the intermediate and intra-day assays through intra-lab variations, such as different days, analysts, equipment, and six replicate measurements for each sample (Shabir, 2003). As shown in Table 4, the intermediate assay results of the dieckol content and %RSD were 73.0–75.3 mg/g and 1.2, respectively. The intra-day assay results of the dieckol content and %RSD were 72.7–75.0 mg/g and 1.0, respectively. According to the International Conference on Harmonization (ICH), the precision criterion for the %RSD should be ≤2 % (International Conference on Harmonization ICH 1995; César et al., 2006). Based on our results, we confirmed that both %RSD values were <1.5 %, which indicated a good precision for the analysis of dieckol.
SD standard deviation, RSD relative standard deviation
aValues were calculated using intra-day analyses (n = 6)
Conclusions
We successfully developed an HPLC method for the determination of dieckol in PRT. In addition, we systematically investigated the validation of this analysis. Because information regarding the standardization of phlorotannin preparations or brown seaweed extracts is limited, our study could be useful for the development and commercialization of phlorotannins.








