Introduction
Burbot Lota lota are a holoarctic species adapted to cold-water rivers and lakes throughout Eurasia and North America. Although their exclusive freshwater life history differs from other species in the order Gadiformes, as adults, they share the piscivorous behavior of their marine cousins and often serve as an apex predator in the environments in which they live. Worldwide, many burbot populations have been extirpated or are in severe decline, attributed to habitat alterations or loss from dam development, invasive species, over-exploitation, and climate change (Stapanian et al. 2010). In response to these declines, several breeding and reintroduction programs have been initiated in both Europe and North America (Paragamian and Hansen 2011; Vught et al. 2007). One of the largest conservation reintroduction programs for burbot exists in Idaho as part of an effort to restore a transboundary population native to the Kootenai River basin in the USA and Canada. This population once supported a popular sport and commercial fishery and has been an important food resource for the Kootenai Tribe of Idaho for millennia (EPA 2016 and references within). However, as a result of dam development, which altered flow and temperature regimes and nutrient supply within the Kootenai River, the population crashed in the late 1970s (Paragamian et al. 2000). With the identification of fewer than 50 wild fish estimated in 2004 and little to no recruitment, the population was considered functionally extinct (Paragamian et al. 2008).
In an effort to rebuild the population, managers started experimenting with conservation aquaculture techniques for burbot in 2006 (Jensen et al. 2008) and the first stocking of hatchery-reared burbot in the Kootenai River occurred in 2009. Supplementation has continued annually and has involved the angling and spawning of wild burbot from Moyie Lake in British Columbia, Canada. Moyie Lake was chosen as a donor population because it is in the Kootenai River Basin and large enough to avoid impacting the spawning population. Following spawning on Moyie Lake, fertilized eggs are transported to hatchery facilities in Idaho for incubation, hatching, and rearing, prior to release into the Kootenai River. While the egg-collection program from Moyie Lake broodstock has been successful in increasing the burbot population in the Kootenai River, successful reproduction of hatchery-released fish has not yet been documented (Ross et al. 2018).
Given the logistical constraints (time, cost, and international transport) of using Moyie Lake broodstock, managers have been interested in collecting and incorporating adults that have survived and reached sexual maturity within the Kootenai River. Additionally, managers were interested in experimenting with spawning techniques that more closely mimic the reproductive behavior of wild burbot. In the wild, burbot are communal spawners, forming spawning balls consisting of many males surrounding one or two females (Cahn 1936). To mimic this behavior, managers have experimented with volitional spawning in tanks. Complicating these experiments is that burbot exhibit few sexually dimorphic characteristics and broodstock management requires segregating males and females prior to the spawning window before distributing to tanks in different sex ratios. To aid in broodstock management, our goal in this study was to identify sex-specific genetic assays in burbot. If successful, we would provide a tool that could assist in conservation aquaculture and population monitoring and provide evidence of the species’ sex determination system.
Methods
We extracted DNA from 18 phenotypic males and 18 phenotypic females used during spawning at Moyie Lake in 2015, using the nexttec™ Genomic DNA Isolation Kit from XpressBio (Thurmont, Maryland). To construct RAD libraries, we followed methods developed and described by Ali et al. (2016). Briefly, extracted DNA was quantified using a Qubit® 2.0 Fluorometer (Life Technologies) and the Qubit® dsDNA HS Assay Kit and normalized to 100 ng in a 10-ul volume. Digests were performed with the PstI restriction enzyme (New England Biolabs, Ipswich, MA, USA) at 37 °C for 60 min, then 85 °C for 30 min. BestRad adaptors were ligated to the digested DNA (SbfI cut sites), and the ligated DNA was sheared using a Q800R2 DNA Sonicator (Qsonica, LLC) for 4:30 min at 20% capacity and 4 °C. Resulting DNA fragments (~ 400 bp) were cleaned and isolated using micro-magnetic beads (Dynabeads, Life Technologies). Sequencing libraries were produced via PCR with P1 and P2 primers. Resulting libraries were sequenced on a NextSeq 500 platform (Illumina, San Diego, CA, USA) to generate raw sequencing paired-end reads of 150 base pairs.
Data analysis was primarily performed with Stacks v1.28 (Catchen et al. 2013). First, a custom Python script was used to evaluate paired reads and “flip” them as necessary so that the restriction enzyme cut site was present in read one. The Stacks programs process_radtags and clone_filter were then used with default settings to demultiplex and remove reads with ambiguous barcodes, no cut site, low quality scores, or PCR duplicates. The de novo Stacks pipeline (ustacks, cstacks, and sstacks) was then used with default settings (m, M, and n set to three, two, and one, respectively) to discover and genotype SNPs. After genotyping, candidate sex-linked SNPs were chosen based on having the pattern of one sex being only heterozygous and the other sex being fixed for one allele. We selected top candidates for further testing based on the total number of fish genotyped by Stacks for a given SNP.
Primers and fluorescently labeled hydrolysis probes were designed for the four top candidates. Additional samples were genotyped for each via PCR and endpoint quantification of fluorescence on an Applied Biosystems 7500 real-time PCR system with Taqman Universal PCR Master Mix (Thermo Fisher). The thermoprofile used for all markers was as follows: (1) initial denature at 95 °C for 10 min, (2) denature at 92 °C for 15 s, (3) anneal and extend at 62 °C for 1 min, and (4) repeat steps 2 and 3, 44 more times. The genotypes of these samples were evaluated for concordance with known phenotypic sex and for the absence of fish homozygous for the presumptive Y-linked allele (all candidates indicated male heterogamety).
Results
A total of 669,825,766 raw reads were acquired for all samples, and after removal of reads with ambiguous barcodes or cut sites, low quality, or PCR duplicates, a total of 567,127,714 reads (84.7%) remained for analysis. A total of 170,569 biallelic SNPs were found that were genotyped in at least 13 males and 13 females. Of these, none fit the pattern expected under female heterogamety (females heterozygous, males fixed). Twenty-two SNPs were found that fit the pattern expected under male heterogamety (males heterozygous, females fixed). Four of these SNPs were genotyped using Stacks at a minimum of 34 samples and were selected for Taqman assay development. Initial testing of the Taqman assays identified two (Llo186187_37 and Llo100864_67) that yielded scorable clusters and expected genotypes and were chosen for further testing and verification (Table 1, Fig. 1). The other two assays were discarded from further testing. The two chosen assays were subsequently screened on 445 mature phenotypic females and 475 mature phenotypic males (Table 2). Assay Llo186187_37 yielded an average genotyping success rate of 96% and accurately sexed all successfully genotyped samples. Assay Llo100864_67 yielded an average genotyping success rate of 98%. It accurately sexed all successfully genotyped phenotypic males and accurately sexed 440/445 of the phenotypic females (99%).
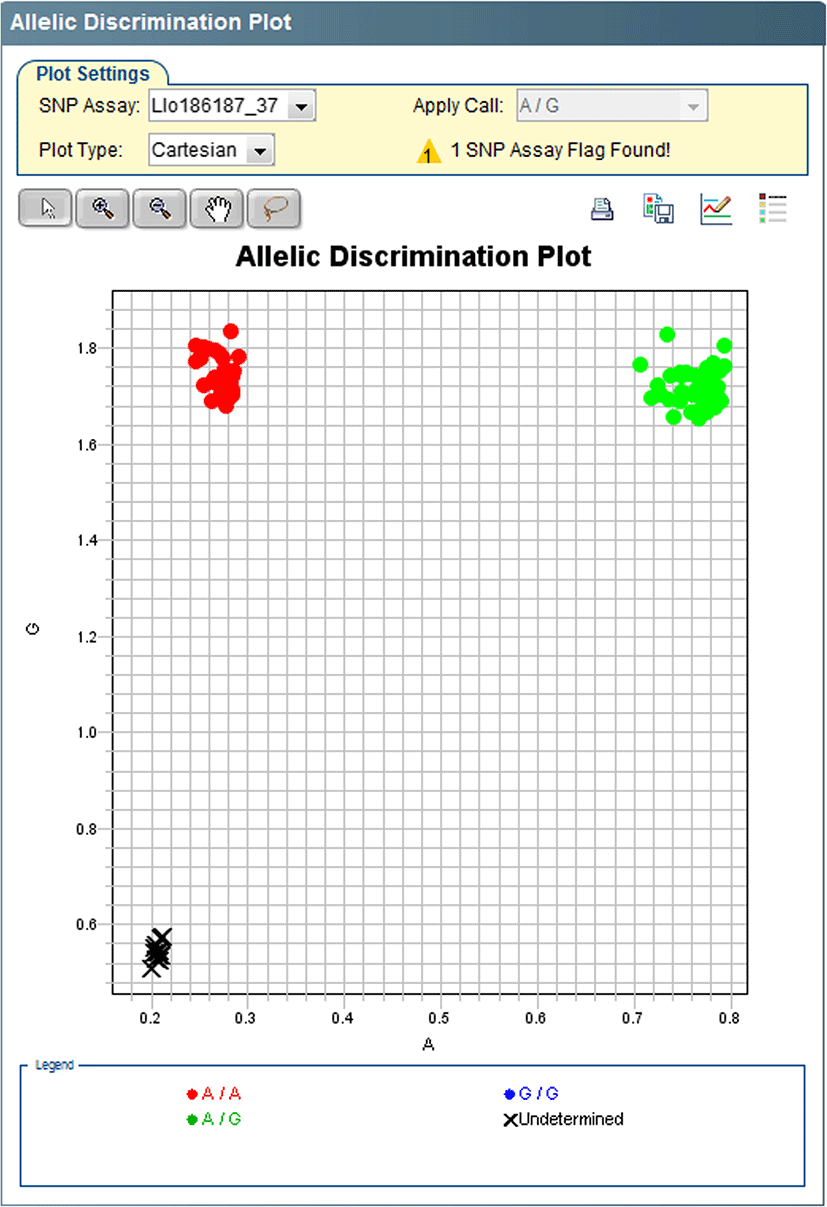
Discussion
Systems of sex determination vary widely among fish species, with examples of both environmental sex determination (Struussmann et al. 1996) and genetic sex determination. Within the category of genetic sex determination, systems of male heterogamety (male-determining allele is dominant (Chourrout and Quillet 1982, Komen et al. 1991)) and female heterogamety (female-determining allele is dominant (Dabrowski et al. 2000, Glennon et al. 2012)) are most common. However, isolated examples of polygenic systems can also be found (Vandeputte et al. 2007, Delomas and Dabrowski 2018). The identification of a SNP with genotypes that are predictive of phenotypic sex demonstrates genetic sex determination in burbot. Males and females were observed to be heterozygous and homozygous for the major allele, respectively, at this locus, and so it can be inferred that burbot have a system of male heterogamety (males are XY and females are XX). This system has been observed in the Atlantic cod Gadus morhua (Whitehead et al. 2012), which is in the same order, Gadiformes, as burbot, but sex determination systems have been observed to vary even among species in the same genus (Cnaani et al. 2008), and so this is not necessarily due to conservation of the sex-determining mechanism.
The successful development of two sex-specific genetic assays for burbot should be of immediate use to managers working on reintroduction and supplementation efforts aimed at recovering burbot populations. Immediately, it will help managers of conservation supplementation efforts in the Kootenai River basin, as they experiment with volitional spawning techniques. In addition, these assays should greatly benefit future conservation and management efforts by providing a tool to assist in estimates of sex-specific migration, growth, and mortality of this species. These monitoring and evaluation efforts will be necessary in the Kootenai River and for other supplemented populations worldwide, aimed at providing sustainable subsistence and recreational harvest of burbot into the future.
Conclusion
This study is the first to identify sex-specific genetic markers in burbot and the first to provide evidence that burbot have a genetic sex-determining system of male heterogamety. The two sex-specific SNP genetic markers we developed were both robust (~ 98% genotyping completeness) and accurate (~ 99% concordance with known phenotypic sex). This study provides another example of the utility of RAD sequencing for the identification of sex-specific genetic assays and the system of sex determination in non-model organisms. The successful development of sex-specific genetic assays for burbot will benefit both conservation and management of this species.








