Introduction
Total world fisheries and aquaculture from inland and marine in 2018 were 178.5 million tonnes, of which 87.6% were for human consumption (FAO, 2020). This number is estimated to rise each year due to the population growth, and changes in consumption habits due to the increasing knowledge about the health benefit effects of marine organisms. The fishing processing industries, however, create large amounts of processing by-products that include head, tail, skin, scale, viscera, and bone (Al Khawli et al., 2020; Zeller et al., 2018). It is estimated that 35% of the harvested fish is lost during the postharvest process, and then 70% of processed fish becomes by-products (FAO, 2020). The amount of marine processing by-products varies depending on species, size, season, and the fishing grounds (FAO, 2020; Villamil et al., 2017; Wang et al., 2019a). In salmon aquaculture, 41.5% of its biomass are by-products and only 15% of its by-products go to human consumption which has a full potential worth of almost 40 million USD (Stevens et al., 2018). Utilization of by-products can give more value to the fisheries and aquaculture industries such as biofilm packaging developed from skin collagen of a cartilaginous fish (Mustelus mustelus). This biofilm exhibited ultraviolet protecting properties and antioxidant activity which might be used to improve the shelf life of food or its perishable raw material (Azaza et al., 2022; Ben Slimane & Sadok, 2018). This can minimize environmental impact by reducing waste and pollution and also improve the economic gain of these industries (Marti-Quijal et al., 2020; Ucak et al., 2021).
Fish by-products have been utilized as raw material, not only in food industry, but also in pharmaceutical industries due to their bioactive compound (Abhari & Khaneghah, 2020; Dave & Routray, 2019; FAO, 2020). For many years, a great deal of interest has been developed by many research groups towards identification of bioactive materials from marine organisms including fish processing by-product. With this regard, it has been utilized in production of protein hydrolysate (Abhari & Khaneghah, 2020; Zamora-Sillero et al., 2018), fish oil (Jackson & Newton, 2016), therapeutants (Ashraf et al., 2020; Stevens et al., 2018) and even cosmetics (Nghia, 2020; Venkatesan et al., 2017). One of the valuable functional materials in marine processing by-products is the marine bioactive peptides, which have well documented beneficial health effect (Camargo et al., 2021; Daroit & Brandelli, 2021). However, there is a huge scope of using bioactive peptides from fish processing by-products as resources in the food and pharmaceutical industries; hence, further research is needed. Scientists should work out sustainable ways to refine fish by-products while governments and industries invest in using this abundant and cheap renewable resource. In this contribution, we focused on the current isolated bioactive peptides from marine processing by-products. In addition, their biological activities, as well as their potential as nutraceuticals were also discussed.
Development of Bioactive Peptides Derived from Fish By-Products
Protein plays various essentials parts, from structural and physiological support to improving health and body condition (Choy et al., 2021; Foegeding et al., 2017; Kumari et al., 2021; Ziegler et al., 2020). Nowadays greater recognition has been shown in developing proteins as dietary food since they provide a rich source of bioactive peptides (Chakrabarti et al., 2018; Foegeding et al., 2017; Gham et al., 2019; Karami & Akbari-Adergani, 2019). Bioactive peptides are peptide fragments of protein that have a beneficial impact on body function or conditions of living beings. That functional peptide fragments may contain from 2 to 20 amino acids residues and are encrypted within the protein sequence. Peptide sequences are released and then active after their parent protein is broken apart through protein hydrolysis reaction (Chakrabarti et al., 2018; Daroit & Brandelli, 2021; Karami & Akbari-Adergani, 2019). However, their function varies due to protein sources, a variation on amino acids sequence that builds those peptides, and even molecular weight (Chakrabarti et al., 2018; Karami & Akbari-Adergani, 2019; Pangestuti & Kim, 2017).
The oceans make up approximately 70% of earth’s surface and contain around 243,000 described species (Jo et al., 2017; Wang et al., 2017). However, those numbers are 16% of from marine species. In addition, the marine ecosystem is more complex and diverse than the terrestrial because it considers being four-dimensional (latitude, longitude, depth, and time) (Costello & Chaudhary, 2017). Thus, it remains the largest source of bio-functional compounds, including bioactive peptides. Also, marine organisms live in complex habitat and expose to more extreme condition than the terrestrial, which make the marine bioactive peptides have significant different amino acid compositions and sequences from land bioactive peptides (Atef & Mahdi Ojagh, 2017; Jo et al., 2017; Ucak et al., 2021; Wang et al., 2017).
Recently, fish by-products rise rapidly each year from discarded fish body parts, such as head, viscera, skin, bones, and scales (FAO, 2020). For instance, in salmon (finfish), approximately 41.5% of its biomass is by-products such as heads, frames and viscera, and other small portions including belly flaps, trimmings, blood and skins. Those by-products parts contain bioactive protein and peptides (Stevens et al., 2018). Regarding the increase in by-product volume, the trend in the development of bio-functional ingredients from fish by-products has become a new focus to maximize the value of fish processing waste (Atef & Mahdi Ojagh, 2017; Coppola et al., 2021; Zamora-Sillero et al., 2018). A considerable amount of research has been done in this area. Interestingly, many researchers highlight the recovery of bioactive peptides from fish waste/by-products due to their quality proteins and outline their potential as candidate raw material for bioactive peptide mining (Villamil et al., 2017; Zamora-Sillero et al., 2018).
Method in Bioactive Peptide Production
Protein hydrolysate production can be performed using two methods. These methods are chemical methods that include acid and alkali hydrolysis, and biochemical methods that involve internal or external proteolytic enzymes (Petrova et al., 2018; Zamora-Sillero et al., 2018). However, there are other notable extraction methods, for instance microbial fermentation and subcritical water hydrolysis (Guo et al., 2019; Marti-Quijal et al., 2020a; Melgosa et al., 2020; Zamora-Sillero et al., 2018). The advantages and drawbacks of these extraction process are summarized in Table 1.
| Extraction method | Chemical agent | Drawback or benefit | By-product source | Hydrolysate result |
|---|---|---|---|---|
| Chemical hydrolysis: • Acidic hydrolysis • Alkali hydrolysis |
Acidic hydrolysis: • Hydrochloric acid • Sulfuric acid |
• High temperature (138°C) • High pressure (310 MPa) • Time consuming (hours) • High amount of NaCl by-product • Protein solubility • Removing heavy metal (Melgosa et al., 2020; Petrova et al., 2018; Shen et al., 2021; Surasani, 2018) |
Mackerel whole body | Recovered protein 49.48% with HCl 0.1 M (Álvarez et al., 2018) |
| Baltic herring whole body | Protein yield 30.0% at pH 2.5 (Nisov et al., 2022) | |||
| Cod (head, tail, bones) | Protein yield 10%–30% at pH 2–3 (Abdollahi & Undeland, 2019) | |||
| Salmon (head, tail, bones) | Protein yield 30%–50% at pH 2–3 (Abdollahi & Undeland, 2019) | |||
| Herring (head, tail, bones) | Protein yield 30%–50% at pH 2–3 (Abdollahi & Undeland, 2019) | |||
| Yellowfin tuna liver | Protein yield 57.22% at pH 2 (Shen et al., 2021) | |||
| Alkali hydrolysis: • Calcium hydroxide • Sodium hydroxide • Potassium hydroxide |
• Warm temperature (54°C) • Toxic by-product • Combination acid-alkaline to improve protein extraction • Improve protein solubility • Reduced bioactivity • Removing heavy metal (Álvarez et al., 2018; Melgosa et al., 2020; Petrova et al., 2018; Shen et al., 2021; Surasani, 2018; Villamil et al., 2017; Zamora-Sillero et al., 2018) |
Mackerel whole body | Recovered protein 74.25% with NaOH 0.4 M (Álvarez et al., 2018) | |
| Baltic herring whole body | Protein yield 43.4% at pH 11.5 (Nisov et al., 2022) | |||
| Cod (head, tail, bones) | Protein yield 40%–60% at pH 11.5–12.5 (Abdollahi & Undeland, 2019) | |||
| Salmon (head, tail, bones) | rotein yield 60%–80% at pH 11.5–12.5 (Abdollahi & Undeland, 2019) | |||
| Herring (head, tail, bones) | Protein yield 40%–70% at pH 11.5–12.5 (Abdollahi & Undeland, 2019) | |||
| Yellowfin tuna liver | Protein yield 78.98% at pH 12 (Shen et al., 2021) | |||
| Biochemical: • Autolysis • Enzymatic hydrolysis |
Autolysis (endogenous enzyme) | • Warm temperature (40°C– 60°C) • 1–3 h • No additional enzyme • Various optimum working condition • Environmental friendly (de Silva & Senaarachchi, 2021; da Silva et al., 2017; Guo et al., 2019; Nikoo et al., 2021a; Nikoo et al., 2021b; Petrova et al., 2018; Vázquez et al., 2020) |
Discarded mud crab Scylla serrata | Carotenoprotein content 8.1% (de Silva & Senaarachchi, 2021) |
| Discarded tiger prawn Penaeus monodon | Carotenoprotein content 16.07% (de Silva & Senaarachchi, 2021) | |||
| Pacific white shrimp (head, shells, appendix and tails) | TCA-soluble peptide ~20% (Nikoo et al., 2021b) | |||
| Litopenaeus vannamei heads | Protein content 43.63% (da Silva et al., 2017) | |||
| Salmon viscera | Protein solubilization 80%–85% (Lapeña et al., 2018) | |||
| Rainbow trout (head, skin, bones and fins) | DH 12.6% (Nikoo et al., 2021a) | |||
| Enzymatic hydrolysis: • Alcalase • Papain • Trypsin • Flavorzyme • Neutrase • Pepsin • Prolyve BS • Protamex • Subtilisin (Bui et al., 2021; Mongkonkamthorn et al., 2020; Saidi et al., 2018; Zhang et al., 2019b) |
• High specificity • Lower to non-toxic chemical • Optimum working condition varied • Biological activity of extraction product varied between enzymes (Petrova et al., 2018; Villamil et al., 2017) |
Skipjack tuna head | DH 25.76% with GI digestion (pepsin-trypsin) (Zhang et al., 2019b) | |
| Sarda orientalis dark muscle | DH 5%–20% with Protamex (Bui et al., 2021) | |||
| Tuna Katsuwonus pelamis blood | • DH 26.5%–36.6% with Alcalase • DH 21.1%–41.7% with Neutrase • DH 21.7%–41.7% with Flavorzyme (Mongkonkamthorn et al., 2020) |
|||
| Salmon viscera | • DH 15%–20% with Pepsin • DH 15%–20% with Neutrase • DH ~15% with Alcalase • DH 10%–15% with Trypsin (Wang et al., 2020) |
|||
| Salmon frames | DH 10%–11.25% with Subtilisin (Valencia et al., 2021) | |||
| Salmon heads | Protein soluble 61.0 g/L with Alcalase (Vázquez et al., 2020) | |||
| Salmon frames and fins | Protein soluble 69.7 g/L with Alcalase (Vázquez et al., 2020) | |||
| Rainbow trout heads | Protein soluble 47.8 g/L with Alacalase (Vázquez et al., 2020) | |||
| Rainbow trout frames and fins | Protein soluble 53.9 g/L with Alcalase (Vázquez et al., 2020) | |||
| Fermentated hydrolysis | Lactic acid bacteria: • Anoxybacillus kamchatkensis • Bacillus licheniformis • Lactobacillus plantarum • Pediococcus acidilactici • Pseudomonas aeruginosa (Ghorbel-Bellaaj et al., 2018; Guo et al., 2019; Mechri et al., 2020; Nugroho et al., 2020; Rajendran et al., 2018) |
• Bacterial growth • Warm temperature • Biological activity of extraction product varied between fermentation system • Optimum working condition varied • Sustainable • Efficient (Guo et al., 2019; Rajendran et al., 2018) |
Metapenaeus monoceros by-product | Protein content 42% (Mechri et al., 2020) |
| Shrimp head | Protein content 66.7% (Guo et al., 2019) | |||
| Shrimp M. monoceros (head and appendix) | Protein content 47.01% (Ghorbel-Bellaaj et al., 2018) | |||
| Atlantic salmon (head and gut) | Protein content 57% (Rajendran et al., 2018) | |||
| Tuna viscera | Protein content 56.04% (Nugroho et al., 2020) | |||
| Subcritical water hydrolysis | Water | • High temperature (100°C–374°C) • High pressure (0.10–22.00 MPa) • Fast (< 60 min) • Protein degradation at higher temperature (Ab Rahman et al., 2019; Ahmed & Chun, 2018; Lee et al., 2021) |
Fish viscera | Protein content 1.705 g/L BSA at 180°C (Ab Rahman et al., 2019) |
| Tuna skin | DH 14.47% at 250°C (Ahmed & Chun, 2018) | |||
| Abalone viscera | Protein content 68.5% at 230°C (Hao et al., 2019) | |||
| Penshell viscera | Protein content 36.14 mg/g BSA at 230°C (Lee et al., 2021) | |||
| Sardine waste (heads, spines and viscera) | Protein content 73.2% at 190°C (Melgosa et al., 2020) |
Chemical methods are the conventional hydrolysis methods which utilize chemical agents to break apart protein molecules into several peptide fragments. Those chemical agents can be acidic for the acid hydrolysis or alkaline for the alkali hydro-lysis (Melgosa et al., 2020; Zamora-Sillero et al., 2018). Acidic hydrolysis employs hydrochloric acid or sulfuric acid under high temperature (up to 138°C) and high pressure (up to 310 MPa) for several hours during hydrolyzation (Melgosa et al., 2020; Petrova et al., 2018). After that, the mixture is neutralized and dehydrated. However, this process produces a high amount of NaCl that hinders the subsequent applications. In addition, due to intense heat and pressure, this process diminishes the product’s functionality (Petrova et al., 2018; Zamora-Sillero et al., 2018). On the other hand, alkali hydrolysis uses calcium, sodium, or potassium hydroxide as alkaline agents to divide protein molecules. In contrast to acidic hydrolysis, alkali hydro-lysis requires a less extreme working environment, for instance 54°C (Melgosa et al., 2020; Petrova et al., 2018). Despite lower temperature than the acidic process, this alkali method also has problematic issues, such as toxic by-products and reduced functionality (Petrova et al., 2018; Villamil et al., 2017; Zamora-Sillero et al., 2018). Nevertheless, this alkali hydrolysis is still considered in fish protein extraction to obtain bioactive protein hydrolysates (Petrova et al., 2018).
Biochemical extraction methods consist of autolysis and enzymatic hydrolysis. The autolysis process utilizes enzymes in the substrate to digest its protein molecules (Petrova et al., 2018; Zamora-Sillero et al., 2018). Thus, the autolysis does not require additional enzymes to put into the mixture. During hydrolysis warm working temperature between 40°C to 60°C for up to 3 h is milder than the chemical methods (Nikoo et al., 2021a ; Nikoo et al., 2021b; Vázquez et al., 2020). Moreover, this process results in better nutritional value. However, since this process utilizes its endogenous enzyme, thus the optimum condition and products might be inconsistent, driven by body part, species, and season (Guo et al., 2019; Nikoo et al., 2019; Nikoo et al., 2021b; Petrova et al., 2018; Vázquez et al., 2020). In contrast, enzymatic hydrolysis requires external enzymes to perform protein digestion, resulting in more specific functionality and desirable products (Petrova et al., 2018; Villamil et al., 2017). Moreover, both autolysis and enzymatic hydrolysis are considered to be environmentally friendly due to low energy expenditure to meet the working environment, effective, and less toxic chemical waste (Guo et al., 2019; Villamil et al., 2017).
Microorganisms can be used to obtain protein hydrolysate from fish by-products through microbial fermentation (Marti-Quijal et al., 2020a). During fermentation, lactic acid bacteria produce a proteolytic enzyme to digest protein molecules on the substrate into peptides (Kliche et al., 2017; Martí-Quijal et al., 2020b). Hydrolysis reaction improves over time with the accumulation of by-product fermentation, lactic acid, and reduction of pH environment. This condition gradually becomes more acidic and improves fermentation enzyme activity (Marti-Quijal et al., 2020a; Rajendran et al., 2018). Usually, this fermentation process is coupled with enzymatic hydrolysis during bioactive peptide extraction to improve the functionality of hydrolysate (Guo et al., 2019; Kang et al., 2020; Vázquez et al., 2020). Like enzymatic products, the fermentation products are more functional, and their process are more efficient, simple, and sustainable (Guo et al., 2019; Marti-Quijal et al., 2020a; Martí-Quijal et al., 2020b).
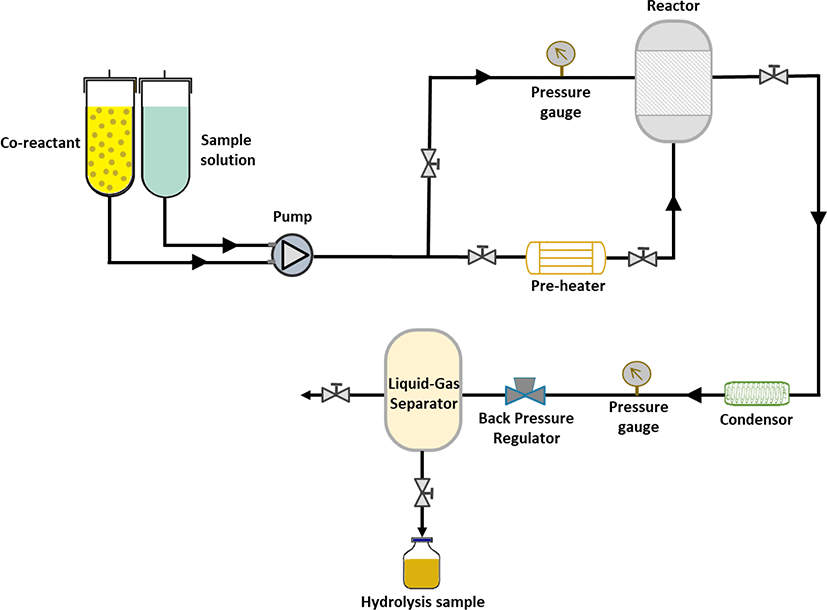
As illustrated in Fig. 1, subcritical water hydrolysis is one of the extraction techniques to obtain bioactive peptides. It is considered a green technology and safe for the environment, and yet still effective (Ahmed & Chun, 2018; Melgosa et al., 2020). This process employs superheated water or subcritical water, which is defined as water maintained in the liquid state at temperatures between the current boiling point of water (100°C) and the critical point of water (374°C) (Ahmed & Chun, 2018; Siahaan & Chun, 2020). Under this extreme condition, physicochemical water changes dramatically. The viscosity of water is declined, which improves water penetration and diffusion into the substrate (Melgosa et al., 2020). In addition, water forms hydronium ion (H3O+) and hydroxide ion (HO-), which perform as acid and based catalyst agents for protein digestion (Ahmed & Chun, 2018; Melgosa et al., 2020). Also, these ion concentrations increase at increased working temperatures. Thus, this process does not require additional proteolytic enzymes due to the existence of those ions in the working environment (Choi et al., 2017; Melgosa et al., 2020). The increased temperature enhances protein digestion rate and increases low molecular peptides (Ab Rahman et al., 2019; Ahmed & Chun, 2018; Lee et al., 2021). Moreover, the subcritical water hydrolysis might only demand up to 60 minutes during the hydrolysis process, faster than the enzymatic hydrolysis (Choi et al., 2017). However, these optimum working environments and periods are driven by objectives, targeted bioactive peptides products, body parts, and species. For example, the optimum condition to obtain the highest protein yield from gills and viscera of unspecified marine fish was at 180°C for 5 minutes (Ab Rahman et al., 2019). In contrast, the strongest functional activities from tuna skin can be obtained after hydrolysis at 280°C for 5 min (Ahmed & Chun, 2018). Meanwhile, the optimum condition for abalone viscera was at 170°C for 1 h to obtain the strongest antioxidant activities of the hydrolysate (Hao et al., 2019). Another study displays that the optimum condition was between 170°C to 230°C for 15 minutes to obtain maximum protein extract and low molecular peptides (< 1 kDa) from comb pen shell (Atrina pectinata) viscera (Lee et al., 2021).
Health Benefit Effects of Bioactive Peptides Derived from Marine By-Products
Oxidation is a necessary metabolism in the vertebrate and the human body. However, it generates reactive oxygen species (ROS) and free radicals, which disrupt homeostasis that allows oxidative stress (Luan et al., 2020; Pisoschi et al., 2021; Rahman & Rahman, 2021). Oxidative stress can be excessively destructive for cells (Luan et al., 2020; Pisoschi et al., 2021; Rahman & Rahman, 2021), which can lead to several diseases over time, including heart disease (Cortassa et al., 2021; Vujic et al., 2021), stroke (He et al., 2020; Taira et al., 2020), arteriosclerosis (Car-mona-Maurici et al., 2020; Lian et al., 2019; Varona et al., 2017), diabetes (Michurina et al., 2020; Pal et al., 2020) and cancer (Shrivastava et al., 2021; Zahra et al., 2020). Furthermore, the oxidation of lipids by ROS is of great concern to the food industry and consumers because it causes food deterioration and the production of toxic compounds (Wang et al., 2016; Wang et al., 2018; Wang et al., 2019b; Wang et al., 2021). Lipid peroxidation is a problem in the food industry and human health (Peña-Bautista et al., 2019; Vijayan et al., 2021).
Antioxidants can protect the human body against free radicals and ROS effects. They may inhibit the progress of many chronic diseases and lipid peroxidation occurring in the human body by interrupting the radical chain reaction of oxidation (Lu et al., 2020; Luo et al., 2021; Vijayan et al., 2021; Zahra et al., 2020). Moreover, antioxidants protect foodstuffs against deterioration by lipid peroxidation. They are usually used as preservatives in food products by directly adding as additives or indirectly through diffusion from packaging material (Feki et al., 2021; Kongkaoroptham et al., 2021; Mancini et al., 2017; Sellimi et al., 2017). Several synthetic antioxidants such as butylated hydroxytoluene (da Cruz et al., 2019; de Jesus et al., 2020; Zhou et al., 2019), tert-butylhydroquinone (de Jesus et al., 2020; Salmanzadeh et al., 2018; Ye et al., 2018), butylated hydroxyanisole (Caleja et al., 2017; da Cruz et al., 2019; de Jesus et al., 2020), and propyl gallate (Gálico et al., 2015; Salmanzadeh et al., 2018), are generally used in the food products to inhibit deterioration. However, there is a strict safety standard for synthetic antioxidants application in food products (Salmanzadeh et al., 2018; Xu et al., 2021; Ye et al., 2018). Hence, natural antioxidants have gained consumer preferences regarding their potential health benefits since they have little or no side effects (Salmanzadeh et al., 2018; Xu et al., 2021).
Several studies have shown that peptides derived from marine by-products possess potent antioxidant properties (Li et al., 2021; Nikoo et al., 2021a; Nikoo et al., 2021b; Zamorano-Apodaca et al., 2020) (Table 2). The antioxidant can prevent oxidation by donating a hydrogen atom or an electron to radicals formed from unsaturated lipids and can interrupt these radical chain reactions by removing initiators or radical intermediates from the medium through the inactivation of metal catalysts (Neha et al., 2019; Pisoschi et al., 2021; Siddeeg et al., 2021). All functional properties of the peptides are highly dependent on their molecular weight. Shorter size peptides are believed to have higher inhibition activities of scavenging and lipid peroxidation when compared to the long-chain peptides (Hajfathalian et al., 2018; Zamorano-Apodaca et al., 2020). Several antioxidant peptides derived from marine by-products such as frame, skin, and viscera are summarized in Table 3.
| By product source | Bioactive peptide extraction | Benefits | References |
|---|---|---|---|
| Skate cartilage | Papain and thermal hydrolysis | Improve free radical scavenging activity | Li et al., 2021 |
| Pacific white shrimp mix by-products (cephalothorax, shells, and pleopods) | Trypsin autolytic hydrolysis | Inhibit oxidative reactions by scavenging hydroxyl radicals | Nikoo et al., 2021b |
| Various marine fish by-products (skin, heads, and skeletons) | Collagen hydrolysis | Potential antioxidant ingredients for functional foods and pharmaceuticals industry | Zamorano-Apodaca et al., 2020 |
| Abalone viscera | Enzymatic hydrolysis (papain, trypsin, neutral protease, alkali protease, pepsin) | Potential source of antioxidant | Zhou et al., 2012 |
| Squid pen | Trypsin hydrolysis | Enhance antioxidant activity | Shavandi et al., 2017 |
| Tuna dark muscle | Orientase and protease XXIII hydrolysis | Generate strong DPPH radical-scavenging activity and antioxidative activity | Hsu, 2010 |
| n-Hexane/ethanol extraction | Inhibit the growth of obesity related diseases through the suppression of hepatic triacylglycerol and cholesterol accumulation | Maeda et al., 2017 | |
| Stripped weakfish by-products (skin and bone) | Alcalase and protamex hydrolysis | Potential natural antimicrobial and antioxidant preservatives in food | Lima et al., 2019 |
| Bycatch shrimp Oratosquilla woodmasoni waste | Thermolysin hydrolysis | Produce ACE-I inhibition peptide that could be utilized as anti-hypertentsive and free radicals prevention | Joshi et al., 2020 |
| Pearl oyster shell | Orientase hydrolysis | Potent ACE inhibitory activity | Sasaki et al., 2019 |
| Squid skin | Pepsin hydrolysis | Good source of ACE inhibition peptide | Lin et al., 2012 |
| Tuna blood | Enzymatic hydrolysis (alcalase, neutrase, flavourzyme) | Exhibit strong antioxidant and ACE inhibitory activity | Mongkonkamthorn et al., 2020 |
| Shrimp shell | Protease hydrolysis | Demonstrate higher ACE inhibitory activity compared to hypertension drug (captopril) | Mechri et al., 2020 |
| Marine catfish skin | Chemical extraction | Possess anticancer activity against human colon cancer line | Raja et al., 2020 |
| Octopus ink | Dichloromethane extraction | Potential immunomodulatory and anti-proliferative against colorectal and breast cancer | Hernandez-Zazueta et al., 2021 |
| Cuttlefish posterior salivary gland toxin | SDS-PAGE extraction | Exhibit great cytotoxicity against breast cancer and inhibit penetration of metastatic cells | Karthik et al., 2017 |
| Rainbow trout skin | Flavourzyme and alcalase hydrolysis | Possess antioxidant and anticancer activities | Yaghoubzadeh et al., 2020 |
| Flathead by-products (head, backbone, skeleton) | AFP hydrolysis | Potent antioxidant and cancer cells cytotoxic agents | Nurdiani et al., 2017 |
| Blue mussel by-product | Protamex hydrolysis | Exhibit inhibitory activity against cancer cells | Beaulieu et al., 2013 |
| Sardine by-products (viscera, heads, skins, and edges) | Isoelectric precipitation | Improve LCAT activity and reduce complications related to obesity | Affane et al., 2018 |
| Skate skin | Collagen extraction | Demonstrate anti-obesity impact | Woo et al., 2018 |
| Squid by-products (viscera and ink sacs) | Protease hydrolysis | Suppress the activity of gram-negative and gram-positive bacteria | Jiang et al., 2018 |
| Salmon by-products (bones, fins, and tails) | Protease hydrolysis | Enhance copper-binding capacity | Vo & Pham, 2020 |
| Giant croaker skin | Neutral protease hydrolysis | Potential immunomodulatory agent | Yu et al., 2020 |
| Organism | Body part | Antioxidant peptides | Reference |
|---|---|---|---|
| Sardinella aurita | • Head | • Gly-Gly-Glu (263.08 Da) | Bougatef et al., 2010 |
| • Viscera | • Leu-His-Tyr (431.2 Da) | ||
| • Gly-Ala-Trp-Ala (403.1 Da) | |||
| • Leu-Ala-Arg-Leu (471.3 Da) | |||
| • Gly-Ala-Leu-Ala-Ala-His (538.2 Da) | |||
| Thunnus tonggol | Dark muscle | • Pro-Met-Asp-Tyr-Met-Val-Thr (756 Da) | Hsu, 2010 |
| • Leu-Pro-Thr-Ser-Glu-Ala-Ala-Lys-Tyr (978 Da) | |||
| Cynoscion guatucupa | • Skin | • IELIEKPMGIF (1,288.71 Da) | Lima et al., 2019 |
| • Bone | • RADLSRELEEISERL (1,814.95 Da) | ||
| Raja kenojei | Skin | • Pro-Gly-Pro-Leu-Gly-Leu-Thr-Gly-Pro (975.38 Da) | Lee et al., 2011 |
| • Gln-Leu-Gly-Phe-Leu-Gly-Pro-Arg (874.45 Da) | |||
| Gadus macrocephalus | Skin | • Thr-Cys-Ser-Pro (388 Da) | Ngo et al., 2011 |
| • Thr-Gly-Gly-Gly-Asn-Val (485.5 Da) | |||
| Haliotis discus hannai | Whole body | ATPGDEG (752 Da) | Qian et al., 2018 |
| Oratosquilla woodmasoni | Muscle | Asn-Gly-Val-Ala-Ala (431 Da) | Joshi et al., 2020 |
| Pinctada fucata | Shell | Gly-Val-Gly-Ser-Pro-Tyr (578.7 Da) | Sasaki et al., 2019 |
| Dosidicus gigas | Skin | DPVAPGGPQP | Alemán et al., 2013 |
A study on the antioxidant activity of protein hydrolysate (Table 4) from abalone viscera revealed that the hydrolysis process using pepsin, papain, trypsin, and neutral protease had similar strength for scavenging activity (half maximal inhibitory concentration [IC50] 500 μg/mL) of 2,2-diphenyl-1-picrylhydrazyl (DPPH) (Zhou et al., 2012). Meanwhile, the antioxidant activity of protein hydrolysate was higher when squid pen was hydrolyzed with bacterial endopeptidase high-throughput (40%–45%) and trypsin (40%–45%) rather than pepsin (35%– 40%) (Shavandi et al., 2017). Protein hydrolysate of tuna had higher antioxidant activity after its dark muscle was hydrolyzed with protease XXIII (41.0%) rather than orientase (31.5%) (Hsu, 2010). Lastly, protein hydrolysate of stripped weakfish by-product obtained with alcalase had higher DPPH activity (60%–70%) rather than with protamex (50%–60%) (Lima et al., 2019).
| By product source | Protease / peptide extraction | Scavenging activity (Activity value or IC50 at certain condition i.e. sample concentration or certain degree hydrolysis) |
|
|---|---|---|---|
| DPPH | Hydroxyl radical | ||
| Abalone Haliotis discus hannai viscera (Zhou et al., 2012) | Alkali protease | IC50; 7 mg/mL | IC50; 10 mg/mL |
| Neutral protease | IC50; 4 mg/mL | IC50; 11 mg/mL | |
| Papain | IC50; 4 mg/mL | IC50; 17 mg/mL | |
| Pepsin | IC50; 4 mg/mL | IC50; 5 mg/mL | |
| Trypsin | IC50; 4 mg/mL | IC50; 23 mg/mL | |
| Arrow squid Nototodarus sloanii pen (Shavandi et al., 2017) | Bacterial endopeptidase (HT) | 40%-45%; 4 mg/mL | NA |
| Trypsin | 40%-45%; 4 mg/mL | NA | |
| Pepsin | 35%-40%; 4 mg/mL | NA | |
| Squid Ommastrephes bartrami (Song et al., 2016) | Endogenous protease | IC50; 0.231 mg/mL | IC50; 0.74 mg/mL |
| Salmon Salmo salar skin (Zhang et al., 2022) | Alcalase | NA | 70%-80%; 4 mg/mL |
| Neutrase | NA | 50%-60%; 4 mg/mL | |
| Flavourzyme | NA | 30%-40%; 4 mg/mL | |
| Protamex | NA | 40%-50%; 4 mg/mL | |
| Rainbow trout Oncorhynchus mykiss skin (Yaghoubzadeh et al., 2020) | Alcalase | 40%-45%; 0.8 mg/mL | NA |
| Flavourzyme | 45%-50%; 0.8 mg/mL | NA | |
| Tuna dark muscle (Saidi et al., 2018) | Prolyve BS | 30%-35%; 2 mg/mL | 35%-40%; 2 mg/mL |
| Tuna Thunnus tonggol dark muscle (Hsu, 2010) | Orientase | 31.5%; 3 mg/mL | NA |
| Protease XXIII | 41.0%; 3 mg/mL | NA | |
| Striped weakfish Cynoscion guatucupa skin and bones (Lima et al., 2019) | Alcalase | 50%-60%; hydrolysis at 10% DH | 70%-80%; hydrolysis at 10% DH |
| 60%-70%; hydrolysis at 15% DH | 40%-50%; hydrolysis at 15% DH | ||
| Protamex | 50%-60%; hydrolysis at 10% DH | 30%-40%; hydrolysis at 10% DH | |
| 50%-60%; hydrolysis at 15% DH | 40%-50%; hydrolysis at 15% DH | ||
IC50, half maximal inhibitory concentration; DPPH, 2,2-diphenyl-1-picrylhydrazyl; HT, protein/protease prepared from bacteria using high-throughput preparation; NA, not available; BS, Prolyve BS. A neutral enzyme composed of metalloprotease produced by fermentation using a selected strain of Bacillus subtilis.; DH, degree of hydrolysis.
Cardiovascular disease (CVD) is one of four noncommunicable diseases that took the biggest number of casualties around the globe. There were 17.9 million people that sadly passed away due to this disease in 2016. The major risk factor for CVD is blood pressure elevation or hypertension (World Health Organization, 2020). Renin and angiotensin-converting enzyme (ACE) are the two key enzymes associated with the renin-angiotensin system, the important endocrine system which regulates blood pressure (Te Riet et al., 2015). ACE plays an important role in raising blood pressure since it regulates the inactivation of bradykinin. ACE can raise blood pressure by converting angiotensin I released from angiotensin by renin into biologically active angiotensin II (Abdelhedi & Nasri, 2019; Pujiastuti et al., 2019; Te Riet et al., 2015). Therefore, inhibiting ACE and renin may positively contribute to hypertension treatment (Abachi et al., 2019; Pujiastuti et al., 2019). Many synthetic inhibitors of ACE and renin such as aliskiren, captopril, enalapril, lisinopril, and alcacepril have been widely used. However, the natural antihypertensive is more desirable for future prevention and hypertension treatment due to its low adverse side effects (Abachi et al., 2019; Abdelhedi & Nasri, 2019).
In addition, the whole body of Oratosquilla woodmasoni from the by-product of marine captured fisheries had antioxidant activities that give nutraceutical industries more benefits (Joshi et al., 2020). A study on pearl oyster (Pinctada fucata) revealed hexapeptide that has ACE inhibitory properties (82.4%) from its shell (Sasaki et al., 2019). Another study also showed that blood from tuna and squid skin from Dosidicus eschrichitii have similar ACE inhibitory properties as well (IC50 0.28 mg/ mL and 0.33 mg/mL, respectively) (Lin et al., 2012; Mongkonkamthorn et al., 2020). Moreover, protein hydrolysate from shrimp captured by-product, Metapenaeus monoceros, had strong ACE inhibitory activities (IC50 71.52%) than the hyper-tension drug, captopril (IC50 85.33%) (Mechri et al., 2020).
Cancer is the second largest non-communicable diseases worldwide that had caused 9 million deaths in 2016 (World Health Organization, 2020). Cancer is complex because it is several diseases caused by uncontrolled growth of cells. Genetic mutation in normal cell alters cell function and growth that turns into a cancer cell, and thus it is considered a genetic disease or cellular disease. Moreover, this cellular alteration also causes disruption and even deterioration to an adjacent cell, tissue, and organ in the body (Miller, 2018; Papaccio et al., 2017; Wellenstein & de Visser, 2018). Recently, there have been four treatments for cancer such as mechanical treatment (surgery), physical treatment (radiotherapy), chemical treatment (chemotherapy), and biological treatment (immunotherapy) (Link, 2019). A natural product can be used for cancer treatments (Link, 2019). Previous studies show that protein extract from marine by-products, such as skin and even visceral organs, had anticancer activities. For example, protein-rich extract from Tachysurus dussumieri skin revealed anticancer properties against the proliferation of colon cancer cells. Moreover, it also induced cell cycle arrest and even apoptosis of the cells (Raja et al., 2020). Anticancer properties were also observed from Octopus vulgaris ink extract that constrained colorectal cancer cell proliferation by inducing apoptosis (Hernández-Zazueta et al., 2021).
The marine natural product has a wide range of molecular size and chemical diversity that could not be chemically synthesized and could give more benefits for treatments due to the complex physiological process of cancer (Link, 2019; Lu et al., 2021; Patra et al., 2020). For instance, hemocyanin from marine gastropods shows antitumor and immunogenic properties (Mora Román et al., 2019; Nigam et al., 2019). This immunomodulatory activity is important for biological treatment because the tumor microenvironment greatly alters non-tumor adjacent cells and matrix phenotypes that hinder the natural body immune system (Kumar, 2020). In the other case, protein extract from salivary gland of cuttlefish Sepia pharaonis inhibited cell proliferation and induced apoptosis, and suppressed cancer cell infiltration to adjacent cells. Thus, the salivary extract becomes an anti-metastasis agent (Karthik et al., 2017).
As previously mentioned, anticancer activities from protein hydrolysate of marine by-products disregard the molecular size of protein extract. However, it shows that small molecular size tends to have stronger bioactivities. For instance, low molecular weight peptide (< 3kDa) retained from protein hydrolysate of rainbow trout (Oncorhynchus mykiss) skin has stronger cytotoxicity (IC50 0.249 mg/mL) on colon cancer cells than higher molecular weight (> 3 kDa) (IC50 2.738 mg/mL) that hindered cell proliferation (Yaghoubzadeh et al., 2020). A similar result shows that small molecular size peptides (< 3 kDa) isolated from Flat-head by-products were reported to inhibit the growth of HT-29 colon cancer cells up to 91.04% (Nurdiani et al., 2017). On the other hand, 50 kDa fraction from protein hydrolysate of Mytilus edulis by-product had been reported to have anticancer activities on 85% of colon cancer cells (Beaulieu et al., 2013).
Obesity is a significant threat to human health worldwide, and its global prevalence has increased up to 1.5 times since 2000 (World Health Organization, 2020). This epidemic poses a risk for several diet-related chronic diseases, including type II diabetes mellitus, CVD, hypertension and stroke, and certain forms of cancer. The health consequences of obesity range from increased risk of premature death to severe chronic conditions that reduce the overall quality of life (Blüher, 2019; Chooi et al., 2019; World Health Organization, 2020). The proposed mechanisms to prevent and treat overweight and obesity such as increased satiety and thermogenesis, accretion of fat-free mass, and lowering food intake (World Health Organization, 2010).
Relating to dietary intake, natural product from marine based by-product that has benefits to ameliorate overweight and obesity is shown in Table 5. Protein hydrolysate from Sardina pilchardus by-products (viscera, head, skin, and fins) has been reported to reduce food intake and body weight gain (Affane et al., 2018). The essential amino acids in fish protein hydrolysate alter adipogenesis by suppressing the genetic expression of key genes for preadipocytes differentiation and lipid accumulation in adipose tissue (from 3.14% reduced to 2.58%) (Lee et al., 2017). Moreover, a previous study shows that protein hydrolysate from dark muscle tuna increased fatty acid oxidation in mitochondria, promoted lipid excretion, limited lipid absorption by the intestine wall, and suppressed lipid deposition in adipocytes tissue (from ~0.35 mg/g decreased to ~0.25 mg/g) (Maeda et al., 2017). Similarly, a peptide from skate (Raja kenojei) skin shows anti-obesity activities by restraining lipogenesis and adipocyte differentiation and allowing more fatty acid oxidation. Adipose tissue size reduced from 3% (control) to 1.5% (skin) (Woo et al., 2018).
| Protein source | BW gain or final BW | Food intake | Adipose tissue | Total cholesterol | Fecal total lipid | |
|---|---|---|---|---|---|---|
| Sardines Sardina pilchardus (Affane et al., 2018) | Viscera, heads, skins and edges | 0.17 g/day/rat | 16.13 g/day/rat | 2.58 | 1.51 mmol/L | 76.8 mg/day/rat |
| Fillet | 0.64 g/day/rat | 20.49 g/day/rat | 2.87 | 1.60 mmol/L | 68.5 mg/day/rat | |
| Control (Casein) | 1.10 g/day/rat | 25.27 g/day/rat | 3.14 | 2.20 mmol/L | 56.9 mg/day/rat | |
| Tuna Thunnus orientalis (Maeda et al., 2017) | Dark muscle | 0.50 g/day | 129 g | 4.19 | ~0.25 mg/g | NA |
| Control (Casein) | 0.45 g/day | 124 g | 3.95 | ~0.35 mg/g | NA | |
| Tuna (Lee et al., 2017) | Skin | 0.216 g/day/rat | 33.24 g/day/rat | Smaller cell size | 1,670 mg/L | NA |
| Control (high fat diet) | 0.365 g/day/rat | 35.30 g/day/rat | Bigger cell size | 2,245 mg/L | NA | |
| Skate Raja kenojei (Woo et al., 2018) | Skin | 33.3 g | 1.5-2 g/day | 1.5%-2% | ~400 mg/L | NA |
| Control (high fat diet) | 36.6 g | 2-2.5 g/day | 2.5%-3% | ~400 mg/L | NA |
Protein hydrolysate obtained from marine by-products has a wide range of biological activities due to various compounds and metabolites of marine organisms. Antibacterial activity is another biological activity from protein hydrolysate that can inhibit bacterial growth (Kang et al., 2019a). A previous study shows that protein extract from squid by-products (viscera and ink sac) has bioactivities to inhibit gram-negative and gram-positive bacteria (Jiang et al., 2018). White shrimp (Litopenaeus vannamei) cara-paces has antibacterial activities after the conjugation with glucosamine (Djellouli et al., 2020). Furthermore, the application of protein hydrolysate from shrimp by-products in food processing has been studied in the bakery to underline improved shelf-life period, palatability, and bread quality. It is suggested that glutamic acid in protein hydrolysate is responsible for the better taste and low molecular weight peptide for the growth of fermentative bacteria (Karimi et al., 2020). Meanwhile, protein hydrolysate obtained from yellowfin tuna (Thunnus albacores) by-products (viscera) has similar biological activity against bacterial growth. Moreover, its bioactivities are inversely related to molecular weight. It is suggested that the low molecular weight of peptides has better interaction and penetration to disrupt bacterial cell wall structure (Pezeshk et al., 2019).
Protein hydrolysate also possesses anti-inflammation and immunomodulatory activities. For instance, protein hydroly-sate from sardine (S. pilchardus) by-products of the canning industry has anti-inflammation properties. Its low molecular weight fraction (< 10 kDa) hinders inflammation regulation in endothelial cells (Vieira et al., 2018). Meanwhile, protein hydro-lysate from giant croaker (Nibea japonica) skin promotes the immune system through cell-mediated immunity, such as increased splenocyte proliferation, and humoral immunity, such as elevated immunoglobin level (Yu et al., 2020). Together with antibacterial properties, marine organisms are potential sources for pharmaceutical industries (Kang et al., 2019a).
As indicated previously, marine by-products have a wide range of biological activities. Its protein hydrolysate also has a metal chelating ability due to the aromatic ring of specific amino acids in its chain (Lima et al., 2021). For instance, protein hydrolysate from salmon by-products has a copper-binding capacity which is essential to delivering copper into the human body (Vo & Pham, 2020). It is crucial to attenuate copper deficiency which will cause anemia, fetal death, and Menkes disease (Chen et al., 2020a). Moreover, iron and calcium are essential minerals for respiration, cellular communication, and body movement. Overload or deficiency of these metals results in dire conditions. Thus, a metal-chelating peptide is crucial to ameliorate the effects by transporting the metal into the body or absorbing metal ions to remove the excess metal in the body (Walters et al., 2018). In this regard, calcium-binding peptides have been extracted from Pacific cod (Gadus macrocephalus) by-product (bone) (Zhang et al., 2019a), and iron-binding peptides as well from cod skin (Wu et al., 2017).
Potential Applications of Bioactive Peptides Derived from Fish By-Products
A significant number of research on producing functionally active peptides from marine waste have been carried out. To date, antioxidant, antihypertensive, anticancer, antimicrobial, and mineral binding peptides from marine by-products have been most identified for their beneficial health effects. A successfully finding of bioactive peptides from marine waste suggests that they can potentially be used as valuable ingredients for a comprehensive array of products, especially in biomedicine. Fishbone peptides from Johnius belengerii has been studied for their bioactive potential in the biomedical application of osteoporosis treatment. Its peptides promote osteogenesis through increased genetic expression for osteoblastic differentiation and mineral accumulation (Heo et al., 2018). Meanwhile, collagen and gelatine are essential compounds in connective tissue and extracellular matrix (He et al., 2019; Nurilmala et al., 2020). Collagen has numerous applications in the biomedical and pharmaceutical industries such as for wound healing and skin regeneration (Jang et al., 2018; Sghayyar et al., 2020; Wei et al., 2019), a drug delivery (Kang et al., 2019b; Song et al., 2018), orthopedics (Gao et al., 2020; Han et al., 2021), angiogenesis (Chen et al., 2020b; Kook et al., 2018), the medical implant (Knopf-Marques et al., 2019; Liu et al., 2020; Versteegden et al., 2018). Fish gelatine from hydrolytic degradation of collagen can be obtained from marine by-products such as skin, scales, and bones of bigeye snapper (Priacanthus hamrur) (Radhika Rajasree et al., 2020), tuna (Thunnus albacares) (Nurilmala et al., 2020), skipjack tuna (Katsuwonus pelamis) (Yang et al., 2019), salmon (Dave et al., 2019), barred mackerel (Scomberomorus commerson) (Mirzapour-Kouhdasht et al., 2020) and pacific cod (Gadus macrocephalus) (Wu et al., 2017). The utilization of fish skin collagen and gelatin has gained a greater preference from industries, especially for medical uses, since the marine product does not contradict any religious issues (Nurilmala et al., 2020; Yuswan et al., 2021).
The optimal exploitation of marine bioactive peptides from by-products for human consumption may bring an exciting scientific and technological challenge while at the same time offering the potential for successful commercialization. Many scientific, technological, and regulatory aspects must be resolved before these marine by-product peptides can be optimally utilized. Firstly, it is necessary to treat the by-products as a valuable raw material. Best handling and sorting of the by-product are essential to reduce enzymatic degradation and microbial spoilage. Secondly, since the production of bioactive peptides from marine by-products may be scaled up to an industrial level, there is a need to develop an industrial-friendly technology. Methodologies for pilot-scale extraction and the purification of the bioactive peptides are critical for the commercial exploitation of peptides. Furthermore, to isolate the desired molecular weight and functional properties of the peptides, suitable extraction, and purification methods are needed. Thirdly, a clinical test of the marine by-product peptides on the human body is a critical aspect. Up to now, most of the biological effects of the peptides have been tested in animal models. In addition, the safety and quality standards of the peptide product should be assessed before the peptide product can be handed to the market.
Potential Resources of Marine By-Product Bioactive Peptides from Indonesia
Indonesia is one of the central fish-producing countries globally. The total production volumes of capture and aquaculture fish-eries in 2017 were 7,071,453 tons and 16,114,991 tons, respectively (Ministry of Marine Affairs and Fisheries, 2017). Fisheries by-products that have been produced by fisheries industries, including capture, aquaculture, and processing activities in Indonesia, have not been used optimally (Irianto et al., 2014). A marine by-product from the fisheries industry is raw materials that can be used to generate value-added products such as marine bioactive peptides. Those raw materials are underutilized fish parts such as cut-offs, fishbone, skin, viscera, and blood (Hayes & Flower, 2013).
Indonesia, consisting of 18,110 islands, has high species richness and endemicity of marine biodiversity, and it is part of the coral reef triangle (Asaad et al., 2018; Hutomo & Moosa, 2005). This high marine biodiversity has become essential for various marine bioactive natural products, including protein and peptides (Chasanah, 2008; Fawzya & Irianto, 2020; Putra & Murniasih, 2016). Nine major marine fish obtained from one of the main fishing ports in Indonesia contained up to 86.56% total protein (Priatni et al., 2018). Moreover, its marine by-products, such as fish skin obtained from red snapper, contained easily digested collagen peptides (Wibawa et al., 2015). Similarly, collagen peptides obtained from tuna skin waste from the processing industry have antioxidant properties (IC50 251.23 ppm) and antiglycation (4.36%) (Nurilmala et al., 2019; Nurilmala et al., 2020).
Several studies revealed that some marine fisheries by-products from Indonesia were utilized to retrieve their bio-active peptides, components, and activity (Table 6). Dolphin fish (Coryphaena hippurus) roe is another by-product of the fishing industry in Indonesia that consists of 19.16% of protein. Seventeen amino acids have been discovered, such as proline (3.32%), arginine (3.01%), and serine (2.85%) (Thenu et al., 2017). Protein hydrolysate from fish by-products could be utilized as peptone, a component of bacterial growth medium. For instance, the by-product of the mackerel fishing industry has improved the bacterial growth of Escherichia coli to 76.85% in the positive control (Setijawati et al., 2019). Meanwhile, grouper and parrotfish heads have been documented to improve the bacterial growth of E. coli from control of 42.81% to 64.63% in grouper heads and 25.87% to 50.33% in parrotfish heads (Jaziri et al., 2020). Other parrotfish heads showed high antioxidant activity around 58.2% of its protein hydrolysate that contained 69.15% of protein (Prihanto et al., 2019). Milkfish skin hydro-lysate has not only antioxidant activity (2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid [ABTS] 80%–90%) but also antifungal activity of Candida albicans (5 Log CFU/mL) (Kusumaningtyas et al., 2019). Moreover, fermented shrimp from shrimp by-products has been reported to have bioactive peptides and improved taste (Hajeb & Jinap, 2012).
| By product source | Biological activity and value | Protein, bioactive peptides, amino acids or nitrogen content of hydrolysate |
|---|---|---|
| Milkfish skin (collagen extraction) (Wibawa et al., 2015) | NA | Protein (0.407 mg/mL) |
| Dolphin fish Coryphaena hippurus (Thenu et al., 2017) | NA | Protein (19.16%), proline (3.32%), arginine (3.01%), serine (2.85%), phenylalanine (2.65%). Cystine, histidine, valine and methionine (< 2%) |
| Mackerel Scomberjaponicus heads (Setijawati et al., 2019) | Alternative peptone for bacterial growth medium. Improve biomass of Escherichia coli (from control 61.04% to 76.85%), Staphylococcus aureus (from control 48.24% to 56.08%), Salmonella thypi (from control 59.46% to 71.04%), and Aeromonas hydrophila (from control 49.83% to 54.15%) | Nitrogen (11.53%) |
| Grouper Epinephelus fuscoguttatus head (Jaziri et al., 2020) | Alternative peptone for bacterial growth medium. Improve biomass of E. coli (from control 42.81% to 64.63%) and S. aureus (from control 35.74% to 59.38%) | Peptone yield (4.61%-5.70%), protein (85.08%-86.43%), glycine (21.48%), glutamic acid (10.67%), alanine (10.26%), proline (8.99%), arginine (8.16%) and aspartic acid (7.60%). Tyrosine, and histidine were less than 3% |
| Parrotfish Scarus javanicus head (Jaziri et al., 2020) | Alternative peptone for bacterial growth medium. Improve biomass of E. coli (from control 25.87% to 50.33%) and S. aureus (from control 33.20% to 55.63%) | Peptone yield (3.27%-3.45%), protein (83.80%-86.67%), glycine (21.34%), glutamic acid (10.12%), alanine (8.97%), proline (8.94%), aspartic acid (7.76%), and phenylalanine (6.73%). Tyrosine and histidine were less than 3% |
| Parrotfish Chlorurussordidus head (Prihanto et al., 2019) | Antioxidant (DPPH 58.2%) | Protein (69.15%), glutamic acid (14.43%), aspartic acid (11.06%), leucine (8.48%), lysine (8.3%), glycine (7.63%) and alanine (7.41%). Serine and histidine were less than 3% |
| Milkfish Chanos chanos skin (Kusumaningtyas et al., 2019) | Antioxidant (ABTS 80%-90%) and antifungal (Candida albicans ~5 Log CFU/mL) | NA |
| Grouper E. fuscoguttatus swim bladder (collagen hydrolysate) (Trilaksani et al., 2020) | Collagen extract suitable for cosmetic material | Protein (75.20%), glycine (102.04%), proline (48.20%), alanine (41.11%), glutamic acid (35.68%), aspartic acid (19.98%), serine (18.02%) |
| Bigeye tuna Thunnus obesus skin (collagen) (Devita et al., 2021) | Antioxidant (DPPH 0.18-0.46 mg AAE/g) | Protein (6.58%-16.37%) |
| Yellowfin tuna Thunnus albacares skin (collagen hydrolysate) (Nurilmala et al., 2019) | Antioxidant (IC50 251.23 ppm), antiglycation (4.36%) | Glycine (175.75 mg/g), proline (70.44 mg/g), arginine (64.10 mg/g), glutamic acid (58.37 mg/g), alanine (54.72 mg/g), aspartic acid (27.66 mg/g) |
| Mackerel by-product (Setijawati et al., 2020) | Alternative peptone for bacterial growth medium | Soluble protein (0.98-2.10 g/L), lysine (16.73%), glutamic acid (12.74%), alanine (9.32%), aspartic acid (8.74%), proline (8.73%), arginine (6.91%) |
| Mackerel S. japonicus head (Nurdiani et al., 2022) | Antioxidant (DPPH 15.64%-36.95%) | Serine (21.16%), glycine (13.27%), histidine (7.99%), aspartic acid (6.82%), alanine (6.74%), glutamic acid (6.17%) |
| Blue swimming crab Portunus pelagicus hepatopancreas (Fadilah et al., 2020) | NA | Protein (49.21%), glutamic acid (3.75%), leucine (2.41%), arginine (2.13%), aspartic acid (1.96%), valine (1.84%), glycine (1.65%) |
Conclusion
The increasing of marine by-products rich in protein gives concern to obtaining bioactive peptides to minimize waste and increase economic value in marine industries. Bioactive peptides recovery from marine by-products can be performed through several types of protein hydrolysis that will break protein molecules apart into the small molecular size of peptides. Subcritical water hydrolysis is more superior hydrolysis process by having a fast hydrolysis process and no additional digestion enzymes. Recovered peptides from marine by-products show a wide range of biological activities favorable for biomedicine application, for instance, in cancer treatment. Thus, valorization of marine by-products through obtaining bioactive peptides gives benefits to the environment and human health as well. However, scale-up applications should be considered and prepared together with stakeholders to optimize this marine bioactive peptide exploitation from marine by-products.