Introduction
The family Stichaeidae comprises of about 70 species, consisting of 35 genera worldwide (Nelson et al., 2016), but only 25 species in 15 genera have been recorded in Korean waters (MABIK, 2022). Pricklebacks (family Stichaeidae) are elongated and slightly compressed and have numerous sharp spines in the dorsal fin (Nelson et al., 2016). This group of fish are mainly distributed in the North Pacific but are also found in North Atlantic regions (Froese & Pauly, 2022; Nelson et al., 2016). There have been reports of one species of Ernogrammus inhabiting Korean waters (i.e., Ernogrammus hexagrammus), characterized by four longitudinal lateral-line canals on each side of the body and no midventral canal (Follett & Powell, 1988).
Seven-lined prickleback, Ernogrammus zhirmunskii was first reported as a new species in 2011 in Peter the Great bay, eastern Russia (Markevich & Kharin, 2011). Further, Yamanaka et al. (2012) described its detailed morphology using sixteen specimens from the Pacific coast of Japan. With the exception of the above studies, scientific data including genetics, ecology, and morphology for the species are scarce. This study reports an additional record of Ernogrammus species (E. zhirmunskii) collected in Dokdo by describing its morphological characteristics and mitochondrial DNA cytochrome oxidase subunit I (mtDNA COI) sequences and updates its distribution in the south-western East Sea as well as the waters of Russia and Japan.
Materials and Methods
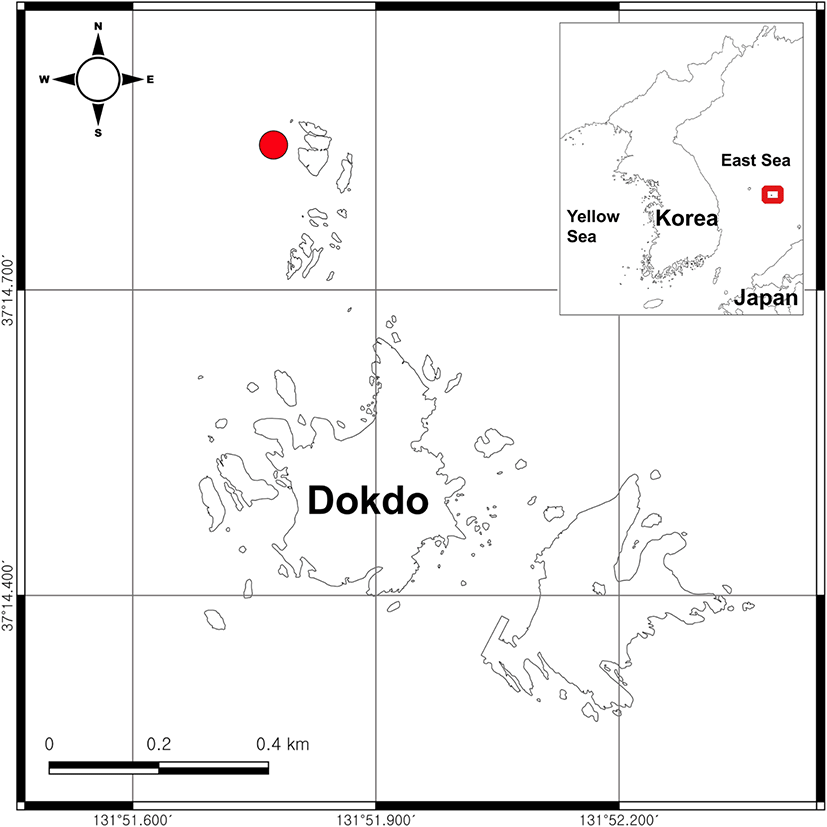
One specimen of E. zhirmunskii was collected from Dokdo (37°14’51.1”N, 131°51’48.1”E), Korea (Fig. 1). Sampling was conducted at a depth of approximately 23 m via scuba diving on July 26, 2021, during the daytime. Immediately after capture, the fish was transported to the laboratory in a living condition, using a small fishbowl with seawater. Protocol and procedures employed were ethically reviewed and approved by Ministry of Oceans and Fisheries, Korea government. The experiments were performed in accordance with the Animal Laboratory Ethics Committee guidelines and regulations for the care and use of laboratory animals.
In the laboratory, an image of the specimen was taken after being rendered insensible in the air, and then, meristic counts and body morphometrics were recorded following the method of Miki & Maruyama (1986) and Yamanaka et al. (2012). The body parts of the specimen were measured to the nearest 0.1 mm using digital vernier calipers under a stereo microscope (Leica EZ4E, Leica Microsystems, Wetzlar, Germany). The specimen was then preserved in 5% formalin for 48 h and later transferred to 70% ethanol for for depositing at the Marine Biodiversity Institute of Korea (MABIK).
To compare molecular data with other Stichaeidae species, total genomic DNA was extracted from the muscle tissue using 10% Chelex resin (Bio-Rad, Hercules, CA, USA). A portion of the mitochondrial COI gene was amplified using primers (Radchenko et al., 2010). PCR was performed in a 30 µL reaction tube containing 3 µL genomic DNA, 5 µL 10x PCR buffer, 4 µL 2.5 mM dNTP, 1 µL of each primer, 0.3 µL Ex-Taq DNA polymerase, and 15.7 µL sterile distilled H2O, using a thermal cycler (MJmini PTC-1148, Bio-Rad). The PCR profile consisted of initial denaturation at 95°C for 5 min, followed by 34 cycles of denaturation at 95°C for 1 min, annealing at 50°C, extension at 72°C for 1 min, and a final extension at 72°C for 5 min. PCR products were purified using ExoSAP-IT (United States Biochemical Corporation, Cleveland, OH, USA) and sequenced using an ABI PRISM BigDye Terminator v.3.1 Ready Reaction cycle sequencing kit (Applied Biosystems, Foster City, CA, USA), on an ABI 3730xl DNA analyzer (Applied Biosystems). We compared our molecular data with that of the mtDNA COI sequences (554 bp) from other Stichaeidae fish obtained from GenBank (National Center for Biotechnology Information, www.ncbi.nlm.nih.gov). Sequences were aligned using CLUSTAL W (Thompson et al., 1994) in BioEdit, version 7 (Hall, 1999). The genetic divergences were calculated using the Kimura 2-parameter (K2P) (Kimura, 1980) model with Mega 6 (Tamura et al., 2013). Phylogenetic trees were constructed using the neighbor-joining method (Saitou & Nei, 1987) in Mega 6 (Tamura et al., 2013), with confidence assessed, based on 10,000 bootstrap replications.
Results
Ernogrammus zhirmunskiiMarkevich & Kharin, 2011: 60 (type locality: Peter the Great bay, Russia); Yamanaka et al., 2012: 127 (Japan); Parin et al., 2014: 440 (Russia).
MABIK PI00051778, 1 specimen, 54.8 mm standard length (SL), Dokdo, Ulleung-gun, Gyeongsangbuk-do, Korea (37°14’51.1”N, 131°51’48.1”E).
The body counts, measurements, and proportions of body parts to SL are shown in Table 1. The body is elongated and compressed (Fig. 2A) with a round head and short snout. The mouth is relatively large; the posterior tip of the upper jaw does not reach vertically below posterior margin of eye. The anterior nostril has a short nasal tube, while the posterior nostril has no tube. The dorsal and anal fins are relatively longer, and the dorsal fin is originating from the vertical base of pectoral fin (Fig. 2D). The dorsal and caudal fins are connected by a membrane. The anal fin originates from the middle of body; the anterior part has two spines, and the posterior part has one spine (Fig. 2C); the entire margin of the anal fin is serrated, and the anal and caudal fins are separated. The pelvic fin originates from before the base of the pectoral fin. The pectoral fin is rounded and located vertically from the origin of the dorsal fin. The caudal fin is rounded at margins.
| Present specimen Dokdo (Korea) (n = 1) | Yamanaka et al. (2012) Japan (n = 16) | Markevich & Kharin (2011) Peter the Great bay holotype | |
|---|---|---|---|
| SL (mm) | 54.8 | 27.8–86.2 | 75 |
| Measurements (% SL) | |||
| Body depth at anal fin origin | 14.2 | 12.9–15.5 | 16.7 |
| Head length | 25.5 | 24.5–29.1 | 25.7 |
| Pre-dorsal fin length | 25.0 | 23.4–28.1 | 25.2 |
| Length of dorsal fin base | 75.4 | 75.6–79.4 | 75.3 |
| Length of 1st dorsal fin spine | 4.7 | 3.7–5.0 | – |
| Length of 10th dorsal fin spine | 7.8 | 7.4–9.7 | – |
| Pre-anal fin length | 50.0 | 47.9–53.1 | 53.3 |
| Length of anal fin base | 47.6 | 47.2–53.2 | 49.3 |
| Length of 1st anal fin spine | 2.0 | 1.6–4.7 | – |
| Length of 2nd anal fin spine | 3.8 | 2.3–5.8 | – |
| Length of posterior most anal fin spine | 3.8 | 3.7–6.5 | – |
| Depth of caudal peduncle | 7.3 | 6.3–7.7 | 8.7 |
| Length of pectoral fin base | 7.1 | 5.9–7.4 | 6.9 |
| Length of longest pectoral fin ray | 18.1 | 16.9–22.1 | 17.3 |
| Pre-pelvic fin length | 23.0 | 18.6–23.0 | 21.9 |
| Pelvic fin length | 9.9 | 10.3–13.1 | 9.9 |
| Length of pelvic fin spine | 5.1 | 2.0–5.1 | – |
| Caudal fin length | 14.8 | 15.4–21.6 | – |
| Ventral lateral line canal length | 22.8 | 17.0–21.7 | – |
| Snout length | 4.9 | 4.8–6.5 | 8.7 |
| Upper jaw length | 10.0 | 10.1–11.5 | 14.6 |
| Lower jaw length | 9.7 | 9.9–11.2 | – |
| Width of fleshy part of interorbit | 5.1 | 3.3–6.5 | 5.4 |
| Width of bony part of interorbit | 1.5 | 0.4–1.5 | – |
| Orbital length | 5.7 | 5.4–8.6 | 5.7 |
| Postorbital length of head | 15.1 | 14.6–17.5 | – |
| Counts | |||
| Dorsal fin rays | XL | XL–XLI | XLI |
| Anal fin rays | II, 25, I | II, 26–27, I–II | II, 27, I |
| Pectoral fin rays | 14 | 14–15 | 14 |
| Pelvic fin rays | I, 3 | I, 3 | I, 3 |
| Caudal fin rays | 6 + 6 | 6 + 5–6 | 6 + 6 |
| Vertebrae (= abdominal + caudal) | – | 44–45 (14–15 + 29–31) | 44 (15 + 30) |
| Branchiostegal rays | 6 | 6 | – |
| Nasal pores | 3 | 3 | 3 |
| Interorbital pores | 9 | 9 | 9 |
| Occipital pores (main canal + posterior canal) | 9 + 6 | 7–8 + 4–8 | 8 + 5 |
| Infraorbital pores | 11 | 11 | 11 |
| Preopercular pores | 6 | 6 | 6 |
| Mandibular pores | 4 | 4 | 4 |
| Postorbital pores | 13 | 10–13 | 13 |

The sensory canal is developed on the head and body. The body has seven longitudinal lateral-line canals, upper, middle, lower lateral-line canals on each side, and unpaired ventral canals (Fig. 2E). The origin of the upper lateral-line canal is located above the operculum and runs slightly under the base of the dorsal fin. The middle lateral-line canal originates vertically below the anterior dorsal fin margin extending to the caudal fin base. The origin of the lower lateral-line canal is located on the anterior base of the pelvic fins and extending to near base of the caudal fin. The ventral lateral-line canal is short and originates from the posterior base of the pelvic fins; it extends to before the anus and connects with the lower lateral-line canals on each side before anus.
The head is brown with white spots on the lower part, and the body is brown with 10 light brown spots (Fig. 2A and 2B). Light brown patterns are scattered throughout the body. Small white spots are found along the lower lateral-line canal. The dorsum (above the upper lateral-line canal) has nine white transverse bands, which start front of dorsal fin base, and one white band on the front and back of the inter orbital. The dorsal fin is blown with irregular bright gray blotches, black spots occurring between the 4th and 8th dorsal spines, and yellow spots in front of the black spots. The anal fin is brown with light gray horizontal stripes, darker towards the edge, and the tip is white. The pectoral fin has 6–7 black bands on a white background, with darker insides and lighter outsides. The pelvic fin is transparent with a black base (Fig. 2E). The causal fin is dark yellow, with three transparent bands; the posterior part is dark, and the tip of the fin is yellow.
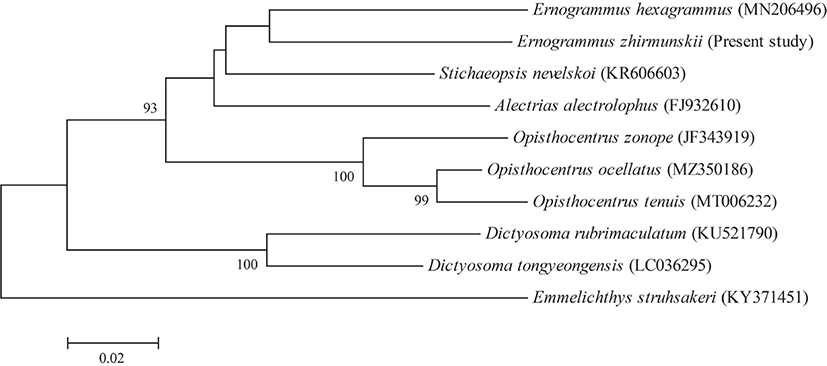
Based on an analysis of the COI gene sequence (554 bp), the present specimen was different from other Stichaeidae species with genetic distances of 0.097–0.191 (Fig. 3). In addition, this study analyzed mtDNA of E. zhirmunskii and registered the information in NCBI under the accession number of “OP062269”.
Discussion
In this study, a single specimen of Stichaeidae fish was collected from Dokdo with an elongated and compressed body, spinous dorsal fin, and body with seven longitudinal lateral-line canals. The body morphometrics of the specimen are well matched with the original description of E. zhirmunskii, especially with seven lateral-line canals and one spine at the end of the anal fin (Markevich & Kharin, 2011).
The specimen shows differences in some morphometric measurements compared to previous listings, that is, slightly shorter pelvic fin, caudal fin, upper jaw, and lower jaw lengths compared to the specimens collected from Japan (Table 1). The specimen reported in this study has longer ventral lateral-line canal length (22.8% of SL) than that of the Japanese specimen (21.7% of SL) (Table 1). In addition, two meristic characters are slightly different between the current and the Japanese specimens in number of anal fin rays (25 vs. 26–27) and occipital pores (9 vs. and 7–8; Table 1). These differences can be considered as intraspecific variation. Despite the differences, all the morphometrics were well matched with the previous records (Markevich & Kharin, 2011; Yamanaka et al., 2012). Therefore, this study documents the first record of E. zhirmunskii in Korean waters and suggests the new Korean name of ‘Il-gob-jul-be-do-la-chi’ for the species based on their morphological characteristic (seven lateral lines on the body) and common name (i.e., seven-lined prickleback).
In total, three Ernogrammus species, E. zhirmunskiiMarkevich & Kharin, 2011, E. hexagrammus (Schlegel in Temminck & Schlegel, 1845), and E. walkeriFollett & Powell, 1988, have been reported worldwide. The E. zhirmunskii has a body morphology much similar to that of E. hexagrammus, but the two species distinctly different in the number of ventral lateral-line canal (one vs. two on the belly in E. zhirmunskii vs. E. hexagrammus) and a spine at the end of the anal fin (one spine vs. absence; Fig. 2C) (Markevich & Kharin, 2011; Yamanaka et al., 2012). In addition, the result of molecular analysis indicated that the genetic distance of mtDNA COI sequences between the two species was 0.111, showing species-level difference (Zemlak et al., 2009; Fig. 3). E. walkeri shows a distinct difference in black band through eye compared to the former two species (without black band vs. with black band) (Markevich & Kharin, 2011; Yamanaka et al., 2012).
E. zhirmunskii was first reported in 2011 and thereafter in 2012. This study documents the third record of the species worldwide, updating additional distribution in Korean waters together with the waters around Russia and Japan. Efforts of recording the distribution range of a marine species become an important basic data for future protection and management plans of the population (Bellucci et al., 2021). If unrecorded species occur in a certain place, they are often misidentified as morphologically similar cogeneric species (Bellucci et al., 2021; Morii et al., 2018). For example, many fish biologists in Korea have often determined all Ernogrammus specimens as E. hexagrammus, because they believed that there was only one Ernogrammus species in Korean waters, even if E. zhirmunskii as well as E. walker was collected. Therefore, updating the new distribution range can be one of the important works on accurately identifying marine species.
In Korea, studies on first or new records of marine fishes, e.g., Brachaluteres ulvarum and Chaetodon vagabundus (Lee & Kim, 2021; Myoung et al., 2021c), have been frequently reported from the southern sea including Jeju island, because recent climate change introduces warm water species under the influence of Tsushima warm water and Yellow Sea warm water (Chen, 2009). The change in water temperature between the past and the present shows that the marine ecosystem in Jeju island is gradually changing to a subtropical sea area (Suh et al., 2011). Consequently, new occurrences of tropical or subtropical marine species have been increasingly recorded in the southern Korean sea (Jung, 2008; Lee et al., 2021; Sakamoto et al., 2021). Efforts of discovering unrecorded species, e.g., Neoclinus chiriroe (Dokdo) and Neoclinus lacunicola (Ulleung island), in the East Sea only have been made recently (Myoung et al., 2021a, Myoung et al., 2021b). With the exception of the above newly recorded species (truly temperate fishes), there likely remain several unrevealed warm water species in the East Sea, because recent northward trend of the Tsushima Warm Current facilitates range expansions of subtropical marine organisms to the East Sea (Son et al., 2020).
Most unrecorded species so far have been documented as larger specimens, collected by commercial fishing gears (Al Mabruk et al., 2021; Lombarte et al., 2021). While small fish species are relatively unrevealed due to difficulty of sampling, this group of species is mostly hard to find or collect because of their small size and hiding behavior between crevices of rocks. Recently, direct collection through scuba diving is suggested as an alternative method for discovering such unrecorded small fish species (Myoung et al., 2021a, Myoung et al., 2021b). Application of various methods will be one of the important works to discover hidden diversity by reporting unrecorded or new species inhabiting Korean seas.








